Abstract
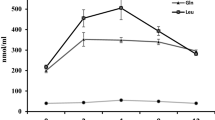
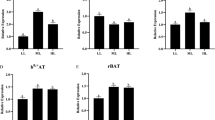
This study aimed to investigate the effects of Gln and its precursors on Gln anabolism and ammonia excretion to determine the role of Gln in protein synthesis in Cyprinus carpio. The growth performance, glutamine synthetase (GS) activity, blood ammonia level, and gene expression of GS, rhesus glycoprotein (Rhag, Rhbg and Rhcg), TOR and 4E-BP1 of fish were measured. Seven diet treatments including glucose (control), glutamine (Gln), glusate (Glu), α-ketoglutarate (AKG), l-ornithine-α-ketoglutarate (OKG), l-arginine-α-ketoglutarate (AAKG), and α-ketoglutarate sodium (2Na-AKG) were conducted. All were substituted for glucose at 1.5% of the dry diet. The results showed the feed conversion ratios (FCRs) of the AKG group and AAKG group were significantly lower (P < 0.05) than that of the control group. The expression levels of the Rhbg gene in the gills of the AKG, AAKG and 2Na-AKG groups were significantly higher than that in the control group (P < 0.05). The expression levels of the TOR gene in the gut of the fish in the AKG group and the Glu group were significantly higher than that in the control group (P < 0.05). Therefore, the addition of AAKG in feed can significantly reduce the FCR of Cyprinus carpio and significantly improve the weight gain rate (WGR) and protein efficiency of the fish. Gln can reduce ammonia release in gills, and AKG can effectively promote the excretion of ammonia. The addition of Gln, Glu, AKG and AAKG in diets can effectively promote protein synthesis. The Gln, Glu, AKG and AAKG can significantly up-regulate GS gene expression in the gut; however, the expression level of the GS gene is not significantly correlated with GS activity.
Similar content being viewed by others
References
Krebs H, Baverel G, Lund P (1980) Effect of bicarbonate on glutamine metabolism. Int J Biochem 12(1/2):69–73
Newsholme E, Carrie A (1994) Quantitative aspects of glucose and glutamine metabolism by intestinal cells. Gut 35(1 Suppl.):S13–S17
Wu G, Knabe D, Flynn N (1994) Synthesis of citrulline from glutamine in pig enterocytes. Biochem J 299(Pt 1):115–121
Bai Y, Yu M, Liu Z (2006) Immunoloregulation effect of glutamine on intestines. Chin J Clin Rehabil 10(4):153–155 (in Chinese with English abstract)
Chen J, Zhou X, Feng L, Liu Y, Jiang J (2009) Effects of glutamine on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells of Jian carp (Cyprinus carpio var. Jian). Aquaculture 288(3/4):285–289
Lin Y, Zhou X (2006) Dietary glutamine supplementation improves structure and function of intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 256(1/2/3/4):389–394
Xu Q, Wang C, Xu H, Zhen Q, Ma J (2009) Effects of l-glutamine on growth performance and intestine of rainbow trout juveniles (Oncorhynchus mykiss). J Chin Cereals Oils Assoc 24(4):98–102 (in Chinese with English abstract)
Brito G, Carneiro-filho B, Oria R, Destura R, Lima A, Guerrant R (2005) Clostridium difficile toxin A induces intestinal epithelial cell apoptosis and damage: role of Gln and Ala-Gln in toxin A effects. Dig Dis Sci 50(7):1271–1278
Kim S, Koo O, Kwon D, Kang J, Park S, Gomez M, Atikuzzaman M, Jang G, Lee B (2013) Replacement of glutamine with the dipeptide derivative alanyl-glutamine enhances in vitro maturation of porcine oocytes and development of embryos. Zygote 22(2):186–289
Blomqvist B, Hammarqvist F, Alexandra D, Jan W (1995) Glutamine and α-ketoglutarate prevent the decrease in muscle free glutamine concentration and influence protein synthesis after total hip replacement. Metabolism 4:1215–1222
Cynober L (1991) Ornithine alpha-ketoglutarate in nutritional support. Nutrition 7:313–322
Kalefarenzos F, Spiliotis J, Melachrinou M, Katsarou C, Spiliopoulou I, Panagopoulus C, Alexandries T (1996) Oral ornithine α-ketoglutarate accelerates healing of the small intestine and reduces bacterial translocation after abdominal radiation. Clin Nutr 15:29–33
Nordgren A, Karlsson T, Wiklund L (2002) Glutamine concentration and tissue exchange with intravenously administered α-ketoglutaric acid and ammonium: a dose–response study in the pig. Nutrition 18:496–504
Song F, Wang L, Xu Q (2016) Effects of glutamine and its precursors on tissue antioxidant capacity and serum biochemical indices of Songpu mirror carp. Chin J Anim Nutr 28(2):627–634 (in Chinese with English abstract)
Xu Y, Zhang X, Zheng X, Kuang Y, Lu C, Cao D, Yin S, Li C, Sun X (2013) Studies on quantitative trait loci related to super oxide dismutase in mirror carp (Cyprinus carpio L.). Aquac Res 44:1860–1871
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–256
Livak K, Schmitgen T (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCt method. Methods 25:402–408
Yang Q, Zhou Q, Tan B, Chi S, Dong X, Du C, Wang X (2008) Effects of dietary glutamine on growth performance, feed utilization and anti-disease ability of hybrid tilapia (Oreochromis niloticus × O. naureus). J Fish Sci China 15(6):1016–1023 (in Chinese with English abstract)
Gao J, Ai Q, Mai K (2010) Effects of dietary glutamine on growth, survival and activities of selected digested enzymes of large yellow croaker (Pseudosciaena crocea) larvae. J Ocean Univ China 40(Sup 1):049–054 (in Chinese with English abstract)
Ye S, Zhang J, Lu H, Zhou C, Zhu F (2016) Effects of glutamine on growth performance, intestinal morphology and non-specific immune related gene expression of juvenile yellow catfish (Pelteobagrus fulvidraco). Chin J Anim Nutr 28(2):468–476 (in Chinese with English abstract)
Wei Y, Xu Q, Li J, Wang C, Luo L, Zhao Z (2013) Effects of α-ketoglutarate supplementation in different protein level diets on growth performance, body composition and serum biochemical indices of Songpu mirror carp. Chin J Anim Nutr 2(12):2958–2965 (in Chinese with English abstract)
Wei Y (2015) Effects of α-ketoglutarate supplementation on nitrogen metabolism, antioxidant capacity and intestinal mucosal morphology and function of Songpu mirror carp (Cyprinus carpio). Master’s thesis. Shanghai Ocean University, Shanghai
Chen D (2015) Effects of dietary α-ketoglutarate supplementation on growth performance, antioxidant capacity, digestive enzyme activity and gene expression of hybrid sturgeon (Acipenser schrenckii♀ × A. baeri♂). Master’s thesis. Shanghai Ocean University, Shanghai
Li J, Xu Q, Wei Y, Wang Y, Wang C, Luo L, Zhao Z (2013) Effect of glutamine precursors on growth performance, body composition and plasma biochemical parameters of Songpu mirror carp (Cyprinus carpio specularis). J Northeast Agric Univ 44(12):1–4 (in Chinese with English abstract)
Handlogten M, Hong S, Westhoff C, Weiner I (2004) Basolateral ammonium transport by the mouse inner medullary collecting duct cell (ml MCD-3). Am J Physiol Renal Physiol 287(4):628–638
Klein J, Sands J, Qian L, Wang X, Yang B (2004) Up-regulation of urea transporter UT-A2 and water channels AQP2 and AQP3 in mice lacking urea transporter UT-B. J Am Soc Nephrol 15(5):116–1167
Huang C, Liu P (2001) New insights into the Rh super family of genes and proteins in erythroid cells and nonerythroid tissues. Blood Cells Mol Dis 27(1):90–101
Marini A, Vissers S, Urrestarazu A, Ander B (1994) Cloning and expression of the MEP1 gene encoding an ammonium transporter of Saccharomyces cerevisiae. EMBO J 13(15):3456–3463
Ninnemann O, Jauniaux J, Frommer W (1994) Identification of a high affinity NH4 + transporter from plants. EMBO J 13(15):3464–3471
Patrica A, Wrighe C, Wood M (2009) A new paradigm for ammonia excretion in aquatic animals: role of rhesus (Rh) glycoproteins. J Exp Biol 212:2303–2312
Hung C, Tsui K, Wilson J, Nawata C, Wood C, Wright P (2007) Rhesus glycoprotein gene expression in the mangrove killifish Kryptolebias marmoratus exposed to elevated environmental ammonia levels and air. J Exp Biol 210(14):2419–2429
Nawata C, Hung C, Tsui T, Wilson J, Wright P, Wood C (2007) Ammonia excretion in rainbow trout (Oncorhynchus mykiss): evidence for Rh glycoprotein and H+-ATPase involvement. Physiol Genom 31(3):463–474
Dong X, Wei Y, Xu Q (2013) Cloning and expression of rhesus glycoprotein genes in tissues in common carp (Cyprinus carpio). Chin J Fish 26(5):6–10 (in Chinese with English abstract)
Dong X, Wei Y, Xu Q (2014) Effects of glutamine and its precursors on Rh genes expressions and content of blood ammonia of in common carp (Cyprinus carpio). Chin J Fish 27(5):19–23 (in Chinese with English abstract)
Finger D, Blenis J (2004) Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell cycle progression. Oncogene 23:3151–3171
Wullschleger S, Loewith R, Hall M (2006) TOR signaling in growth and metabolism. Cell 124:471–484
Gingras A, Raught B, Sonenberg N (2001) Control of translation by the target of rapamycin proteins. Prog Mol Subcell Biol 27:143–174
Nelson D, Cox M (2005) Lehninger principles of biochemistry. WH Freeman, New York
Xiao Y, Hong Q, Liu X, Liu R, Zhao X, Chen A, Yang C (2012) Effects of glutamine on growth performance, apparent digestibility of nutrients, jejunal alkaline phosphatase activity and expression of genes related to intestinal health in weaner piglets. J Anim Nutr 24(8):1438–1446 (in Chinese with English abstract)
Wang L (2009) The effects of α-ketoglutarate on growth and metabolism of muscle and intestinal mucosa with reference to the molecular mechanism in piglets after Lip polysaccharide challenge. Master’s thesis. Wuhan Polytechnic University, Wuhan
Jiang J (2009) lEes GLS and TOR gene cDNA clone and Gin to IECs protein synthesis influence and mechanism research in Carp Cyprinus carpio. Ph. D. thesis. Sichuan Agricultural university, Ya-an
Eid T, Behar K, Dhaher R, Bumanglag A, Lee T (2012) Roles of glutamine synthetase inhibition in epilepsy. Neurochem Res 37:2339–2350
Kirsten E, Wanles I (2013) Glutamine synthetase expression in activated hepatocyte progenitor cells and loss of hepatocellular expression in congestion and cirrhosis. Liver Int 33:525–534
Singh K, Ghosh S (2013) Regulation of glutamine synthetase isoforms in two differentially drought-tolerant rice (Oryza sativa L.) cultivars under water deficit conditions. Plant Cell Rep 32:183–193
Dong X, Wei Y, Yu J, Wang Y, Xu Q (2014) Glutamine precursor supplementation increases glutamine synthetase gene expression in intestine of common carp (Cyprinus carpio). Aquac Res 45:1559–1566
Acknowledgements
This study was funded by the earmarked fund for the China Agriculture Research System (CARS-46), and the Central Public-interest Scientific Institution Basal Research Fund, CAFS (No. 2016HY-ZD0602), and Special Scientific Research Funds for Central Non-profit Institutes, Chinese Academy of Fishery Sciences (2014A08XK03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, Z., Song, F. & Xu, Q. Effects of glutamine and its precursors on the growth performance and relevant protein synthesis pathway of mirror carp Cyprinus carpio . Fish Sci 83, 1019–1026 (2017). https://doi.org/10.1007/s12562-017-1124-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-017-1124-y