Abstract
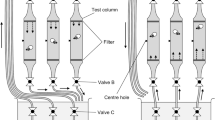
This study examined the effect of warm temperature on the survival of paralarvae of Japanese common squid Todarodes pacificus and on their swimming behavior as they ascended to the surface. Observations were conducted on paralarvae in Petri dishes and in 85-cm-tall, cylindrical tanks that had a warmer upper layer and cooler lower layer separated by a small thermocline. Paralarvae were obtained through artificial fertilization and reared in Petri dishes at six experimental temperatures between 20.9 and 30.4 °C. Paralarvae reared at lower temperatures survived longer than those reared at warmer temperatures, and survival decreased at temperatures above 24 °C. When the mean temperatures in the upper layer of the tanks were 24.4–26.0 °C, the paralarvae ascended through the thermocline to the surface, but when the mean temperatures in the upper layer were 29.7–29.8 °C, paralarvae stopped ascending at the thermocline. These results show that paralarvae have a temperature preference but ascend to the surface in the unfavorable temperature range. The results suggest that increasing surface temperatures at spawning grounds will negatively affect both the survival and behavior of T. pacificus paralarvae.





Similar content being viewed by others
References
Levitus S, Antonov JI, Boyer TP, Locarnini RA, Garcia HE, Mishonov AV (2009) Global ocean heat content 1955–2008 in light of recently revealed instrumentation problems. Geophys Res Lett 36:L07608. doi:10.1029/2008GL037155
IPCC (2007) Climate Change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. IPCC, Cambridge, New York
Doney SC, Ruckelshaus M, Emmett Duffy J, Barry JP, Chan F, English CA, Galindo HM, Grebmeier JM, Hollowed AB, Knowlton N, Polovina J, Rabalais NN, Sydeman WJ, Talley LD (2012) Climate change impacts on marine ecosystems. Ann Rev Mar Sci 4:11–37. doi:10.1146/annurev-marine-041911-111611
Brierley AS, Kingsford MJ (2009) Impacts of climate change on marine organisms and ecosystems. Curr Biol 19:R602–R614. doi:10.1016/j.cub.2009.05.046
Roper CFE, Nigmatullin C, Jereb P (2010) Family Ommastrephidae. In: Jereb P, Roper CFE (eds) Cephalopods of the world. An Annot. Illus. Cat. species known to date, vol 2. Myopsid Oegopsid squids. FAO species cat fish purp. No. 4, vol. 2. FAO, Rome, pp 269–347
FAO (2012) FAO yearbook. Fishery and aquaculture statistics. 2010. FAO, Rome, p 78
Okutani T (1983) Todarodes pacificus. In: Boyle PR (ed) Cephalopod life cycle. Academic, London, pp 201–214
Murata M (1990) Oceanic resources of squids. Mar Behav Physiol 18:19–71
Bower JR, Sakurai Y (1996) Laboratory observations on Todarodes pacificus (Cephalopoda: Ommastrephidae) egg masses. Am Malacol Bull 13:65–71
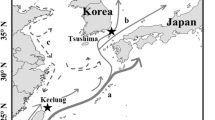
Sakurai Y, Kiyofuji H, Saitoh S, Goto T, Hiyama Y (2000) Changes in inferred spawning areas of Todarodes pacificus (Cephalopoda: Ommastrephidae) due to changing environmental conditions. ICES J Mar Sci 57:24–30. doi:10.1006/jmsc.2000.0667
Yamamoto J, Shimura T, Uji R, Masuda S, Watanabe S, Sakurai Y (2007) Vertical distribution of Todarodes pacificus (Cephalopoda: Ommastrephidae) paralarvae near the Oki Islands, southwestern Sea of Japan. Mar Biol 153:7–13. doi:10.1007/s00227-007-0775-0
Sakurai Y, Bower JR, Nakamura Y, Yamamoto S, Watanabe K (1996) Effect of temperature on development and survival of Todarodes pacificus embryos and paralarvae. Am Malacol Bull 13:89–95
Yamamoto J, Miyanaga S, Fukui S, Sakurai Y (2012) Effect of temperature on swimming behavior of paralarvae of the Japanese common squid Todarodes pacificus. Bull Jpn Soc Fish Oceanogr 76:18–23 (in Japanese with English abstract)
Bower JR, Nakamura Y, Mori K, Yamamoto J, Isoda Y, Sakurai Y (1999) Distribution of Todarodes pacificus (Cephalopoda: Ommastrephidae) paralarvae near the Kuroshio off southern Kyushu, Japan. Mar Biol 135:99–106. doi:10.1007/s002270050606
Sakurai Y, Young RE, Hirota J, Mangold K, Vecchione M, Clarke MR, Bower J (1995) Artificial fertilization and development through hatching in the oceanic squids Ommastrephes bartramii and Sthenoteuthis oualaniensis (Cephalopoda: Ommastrephidae). The Veliger 38:185–191
Villanueva R, Quintana D, Petroni G, Bozzano A (2011) Factors influencing the embryonic development and hatchling size of the oceanic squid Illex coindetii following in vitro fertilization. J Exp Mar Biol Ecol 407:54–62. doi:10.1016/j.jembe.2011.07.012
Staaf DJ, Camarillo-Coop S, Haddock SHD, Nyack AC, Payne J, Salinas-Zavala CA, Seibel BA, Trueblood L, Widmer C, Gilly WF (2008) Natural egg mass deposition by the Humboldt squid (Dosidicus gigas) in the Gulf of California and characteristics of hatchlings and paralarvae. J Mar Biol Assoc UK 88:759–770. doi:10.1017/S0025315408001422
Watanabe K, Sakurai Y, Segawa S, Okutani T (1996) Development of the ommastrephid squid Todarodes pacificus, from fertilized egg to rhynchoteuthion paralarva. Am Malacol Bull 13:73–88
Sakai M, Brunetti N, Ivanovic M, Elena B, Nakamura K (2004) Interpretation of statolith microstructure in reared hatchling paralarvae of the squid Illex argentinus. Mar Freshw Res 55:403–413. doi:10.1071/MF03148
Houde ED (1987) Fish early life dynamics and recruitment variability. Am Fish Soc Symp 2:17–29
Houde ED (1996) Evaluating stage-specific survival during the early life of fish. In: Watanabe Y, Yamashita Y, Oozeki Y (eds) Survival strategies in early life stages of marine resources. Balkema, Brookfield, Yokohama, pp 51–66
Bakun A, Csirke J (1998) Environmental processes and recruitment variability. In: Rodhouse PG, Dawe EG, O’Dor RK (eds) FAO fisheries technical paper, 376th edn. FAO, Rome, pp 105–124
Sakurai Y, Kiyofuji H, Saitoh S, Yamamoto J, Goto T, Mori K, Kinoshita T (2002) Stock fluctuations of the Japanese common squid, Todarodes pacificus, related to recent climate changes. Fish Sci 68:226–229
Acknowledgments
We thank Akebono-Suisan, Hakodate, Japan for providing the live squid, and Dr. John R. Bower, Hokkaido University for his comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoo, HK., Yamamoto, J., Saito, T. et al. Laboratory observations on the vertical swimming behavior of Japanese common squid Todarodes pacificus paralarvae as they ascend into warm surface waters. Fish Sci 80, 925–932 (2014). https://doi.org/10.1007/s12562-014-0767-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12562-014-0767-1