Abstract
The pandemic of Coronavirus Disease 2019 (COVID-19) caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) is still impacting not only on human health but also all economic activities, especially in those related to tourism. In this study, in order to characterize the presence of SARS-CoV-2 in a hot spring park in Uruguay, swimming pools water, wastewater, and surface water from this area were analyzed by quantitative PCR. Wastewater from Salto city located next to the hydrothermal spring area was also evaluated as well as the presence of Rotavirus (RV). Overall, SARS-CoV-2 was detected in 13% (13/102) of the analyzed samples. Moreover, this virus was not detected in any of the samples from the swimming pools water and was present in 18% (3/17) of wastewater samples from the hotels area showing the same trend between the titer of SARS-CoV-2 and the number of infected people in Salto city. SARS-CoV-2 was also detected in wastewater samples (32% (11/34)) from Salto city, detecting the first positive sample when 105 persons were positive for SARS-CoV-2. Rotavirus was detected only in 10% (2/24) of the wastewater samples analyzed in months when partial lockdown measures were taken, however, this virus was detected in nearly all wastewater samples analyzed when social distancing measures and partial lockdown were relaxed. Wastewater results confirmed the advantages of using the detection and quantification of viruses in this matrix in order to evaluate the presence of these viruses in the population, highlighting the usefulness of this approach to define and apply social distancing. This study suggests that waters from swimming pools are not a source of infection for SARS-CoV-2, although more studies are needed including infectivity assays in order to confirm this statement.
Similar content being viewed by others
Introduction
Since the first detection of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Wuhan, China Zhu et al., 2019) and the posterior declaration of the pandemic by the World Health Organization (WHO) (WHO, 2020), many actions have been taken around the world to contain the virus dissemination.
Diagnosis tools as PCR were described rapidly to diagnose people who were coursing the Coronavirus Disease 2019 (COVID-19) or asymptomatic patients (Chu et al., 2020; CDC, 2020; Corman et al., 2020), in order to identify and isolate infected people and avoid viral dispersion. Other tools as immunology-based techniques were developed with the same purpose (Chau et al., 2020) such as detection of viral antigens or antibodies.
The high number of cases that rapidly started to grow and the extensive population around the world makes that clinical diagnoses were not enough to know the real number of people infected in a region or country. Therefore, a tool already described by WHO to evaluate environmental surveillance of poliovirus in the world (WHO, 2003) became widely used by many scientific groups. Wastewater-Based Epidemiology (WBE) has been used for poliovirus and other enteric viruses in wastewater and other water matrixes in order to evaluate the viral circulation in the population (Fumian et al., 2019; Gharbi-Khelifi et al., 2007; Pina et al., 2001). Rotavirus, one of the most important causal agents of gastroenteritis in children is an example of an enteric virus already evaluated by a WBE strategy (Kumazaki and Usuku, 2015). Unfortunately, in our country, we do not have a surveillance of Rotavirus in order to provide information of its national incidence, however, the presence of this virus in wastewater samples was already described in the region previous to COVID-19 pandemic, evidencing a wide dissemination in this kind of matrix (Victoria et al., 2014; Lizasoain et al., 2018).
WBE has many advantages, a large number of people could be evaluated with only one sample, detection and quantification of viruses could be used to take decisions about social distancing or lockdown, and viral diversity could be evaluated with the same sample (Murakami et al., 2020). The use of this tool already describes the presence of other coronavirus in water matrix samples previous to the COVID-19 pandemic (Bibby & Peccia, 2013), therefore it was rapidly used to evaluate the presence of SARS-CoV-2 in environmental matrices. Some authors even associate (by statistical estimations) the number of people infected with SARS-CoV-2 with the viral load in wastewater (Ahmed et al., 2020).
Social distancing and lockdown measures forced many shops, gyms, swimming pools, and tourist attractions to close their facilities. Swimming pools present special attention since the transmission of SARS-CoV-2 by the fecal–oral route is not ruled out (Chen et al., 2020; Xiao et al., 2020; Dergham et al., 2021). Moreover, the presence of an infected person in a swimming pool can contaminate this water through the nasal secretion with high viral titer (1010 copies/ml in individuals with only runny nose as symptoms [Savela et al, 2022]) which can infect a susceptible person sharing the pool.
In Uruguay, the first case of COVID-19 was confirmed in March 18th, 2020, from that date until November 7th, 2020, daily new COVID-19 cases per million people were below 15. The first peak of detection was in January 15th, 2021 with 275 cases. The second and third peaks were in April 12th and May 27th, 2021 with 1134 and 1102 daily new cases per million people, respectively.
In this work, different water matrices from a geothermal hot spring area were studied in order to evaluate the presence of SARS-CoV-2 to know the epidemiology of these viruses in tourists that visit the hydrothermal complex and in the population that resides in the same area. Nonpharmaceutical interventions such as social distancing and lockdown likely affect not only the epidemiology of SARS-CoV-2, but also the epidemiology of other viruses. In this sense, an enteric virus like Rotavirus was also evaluated.
Materials and Methods
Sampling Sites
Grab samples were collected in a period of one year from August 2020 to July 2021, the first 6 months of samples were taken every 15 days, while in the last period (January–July 2021), samples were taken monthly (with exception of February when samples could not be collected).
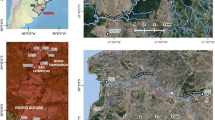
Wastewater, swimming pools water (regular swimming pool with hot water from the hot spring with 1.5 ppm chlorination) from the Daymán Hot Spring Park, and surface water from a stream close to the park were evaluated. Wastewater samples were obtained in two sites from Salto city (Obras Sanitarias del Estado—OSE) and two sites of the hydrothermal spring. In Salto city one wastewater sampling site collect wastewater from the Northern region of the city, while the other site collects wastewater from the southern region. In the hydrothermal springs also two sampling sites were defined, one collects wastewater from the hydrothermal park, and the other collects wastewater from hotels located next to the hot spring park. Doña Jacinta stream receives wastewater from a neighborhood that surrounds the hot spring park. All grab samples were collected at 8:00 A.M. for Salto samples and 10:00 A.M. for Dayman samples. 34 wastewater samples were from Salto city, 34 samples from Dayman wastewater, 17 samples from swimming pools (one composite sample from two swimming pools), and 17 samples from Doña Jacinta stream. Swimming pool samples were always collected on Monday, after the weekend where the hot spring park is crowed. Sampling sites are shown in Fig. 1.
Viral Concentration
In the case of wastewater a volume of 500 ml was collected, while 10 L of swimming pool water and surface water of Doña Jacinta stream were collected. Swimming pools waters were sampled in two sites of collection and a composite sample was analyzed. In all cases, samples were refrigerated and transported to the laboratory to be analyzed immediately.
As a control of the viral concentration process, 100 μL and 50 μL of PP7 bacteriophage (1.0 × 106 genomic copies/μL) were added to 10 L of surface water and 500 ml of wastewater samples, respectively. Wastewater samples were concentrated with PEG precipitation method, according to the protocol described by Lewis and Metcalf (1988) with modifications (Salvo et al., 2021). Briefly, weights of centrifugation pots were registered, 500 ml of sample was distributed in two bottles (250 ml each) and centrifuged at 4750 xg for 20 min at 4 °C. Supernatant (S1) was maintained at 4 °C to be used later. Pots were weighted again and weight of each pellet was calculated. Sediment was mixed with 3 volumes respect to pellet volume with 3% Beef extract (Oxoid, Hampshire, England), 2 M NaNO3 eluant (pH 5.5). Volumes of the same sample were mixed in one pot, pH was adjusted to 5.5 and sample was stirred for 1 h at 4 °C. Solids were then removed by centrifugation at 10000 xg for 20 min and the eluted was mixed with the first supernatant obtained (S1) and adjusted to pH 6.5–7.2. PEG 6000 (Sasol. Hamburg, Germany) was added to a final concentration of 10% (w/v) and NaCl to 2% (w/v). The resulting suspension was stirred for 6 h at 4 °C and centrifuged at 10,000 xg for 25 min. The supernatant was discarded and the pellet was resuspended in 5 ml of PBS buffer (Dako Inc., California, USA) (pH 7.2), adjusted to pH 8.0, incubated for 1 h with occasional vortex, and centrifuged at 10,000 xg for 20 min. Supernatant was stored for nucleic acid extraction.
Surface and swimming pool waters were concentrated by using the skimmed milk flocculation, according to the protocol described by Calgua et al. (2008). Briefly, a pre-flocculated 1% (w/v) skimmed milk solution (PSM) was prepared, the sample was carefully acidified to pH 3.5 by adding HCl 1 N and the PSM was added. Samples were stirred for 8 h at room temperature and flocs were allowed to form sediment by gravity for another 8 h. The supernatant was carefully removed and a final volume of about 500 ml containing the sediment was transferred to a centrifuge tube and centrifuged at 7000 xg for 30 min at 12 ºC. The supernatant was carefully removed and the pellet dissolved in 10 ml of phosphate buffer (1:2, v/v of Na2HPO4 0.2 M and NaH2PO4 0.2 M) at pH 7.5. The viral concentrate was stored at − 80 ºC.
Viral Detection
Nucleic acid extraction was performed with QIAmp Viral RNA mini kit according to the manufacturer’s instructions (QIAGEN, Germany). Detection of SARS-CoV-2 was performed using a one-step multiplex qPCR kit developed by the University of the Republic (UdelaR), ATGen and Institute Pasteur of Montevideo based on the protocol described by Chu et al. (2020). The same reaction allows the detection of SARS-CoV-2 (N gene) and Ribonuclease P (RNase P), used as an endogenous control. The SARS-CoV-2 amplicon inserted into the TOPO™ TA cloning vector provided by the kit was quantified and serially diluted in our laboratory for the construction of the standard curve in order to quantify SARS-CoV-2 in the analyzed samples. Standard curve for SARS-CoV-2 has a range of four logs (100–103 genome copies/reaction). The cycling conditions were as follows: reverse transcription for 5 min at 50 °C, initial denaturation for 20 s at 95 °C followed by 40 cycles for 15 s at 95 °C and 60 °C for 30 s.
For the detection of Rotavirus, a first reverse transcription reaction was performed with random primers pd(N)6 (Macrogen, South Korea) and Revert Aid reverse transcriptase (Revert Aid RT Thermo Scientific™, Carlsbad, CA, USA) according to the manufacturer’s instructions. After the synthesis of the cDNA a Real-Time PCR reaction with TaqMan® technology was carried out. SensiMix™ II Probe kit (BIOLINE®, England) and Rotor Gene Q thermocycler (Qiagen®, Germany) were used following the manufacturer’s instructions. A set of RVA-specific primers that amplify NSP3 gene and one probe with FAM fluorophore were used in the reaction. A standard curve was generated by using tenfold dilutions (107–100 genome copies reaction−1) of a plasmid containing an amplicon of 86 bp (Zeng et al., 2008).
The cycling conditions were as follows: initial denaturation at 95 °C for 10 min followed by 45 cycles at 95 °C for 10 s and extension at 60 °C for 1 min.
Positive and negative controls were included in each step of the viral detection and samples that were negative for SARS-CoV-2 and RV were diluted 1/10 to evaluated possible inhibition of the enzymatic reactions.
Physicochemical Parameters Determinations
In order to compare the presence of the analyzed viruses with physicochemical parameters, temperature, dissolved oxygen (DO), redox potential (ORP), and free ammonia were measured in situ in swimming pools water and Doña Jacinta stream. Temperature and ORP were obtained using a multiparametric probe Sense ION HACH (MM150), ammonium and dissolved oxygen were determined with sensors IntelliCAL, HACH 30HQd, (IntelliCAL Nitrate ISENO3181, and LDO101, respectively). Calibration buffers were purchased from HACH.
In addition, to characterize Daymán geothermal water, a composed sample was collected at a flow rate of 70–80 m3/min of the geothermal borehole. The composed sample was obtained collecting 10 subsamples every 10 min and mixing. Hydrochemical characterization of the composed sample by majority ions analyzed provides a better awareness of the chemical baseline of this geothermal groundwater source. Temperature, Electrical Conductivity (EC), ORP, and pH were measured in situ. Sample was filtered through a 0.45 μm membrane (mixed cellulose esters-Merck Millipore), and major ions were analyzed in Ion Chromatograph DIONEX THERMO® with cation and anion standards and, respectively, solvents from THERMO®. MilliQ water was obtained from Arium Mini, SARTORIUS®.
Ion Chromatography, Alkalinity, HCO3−, and Hardness were analyzed in the Water and Soils Laboratory from Water Department, Litoral North Regional Centre of the University of the Republic in Uruguay, according to the methodology established by Standard Methods (APHA, 2017).
Piper Diagram (Piper, 1944) and Schöeller-Berkaloff Diagram from EASY CHEM 5.0 (2012) software were performed to characterize the groundwater.
Results and Discussion
In this study, the contamination of different water matrices was assessed in order to determine their contamination with SARS-CoV-2 and Rotavirus comparing also the dynamic of SARS-CoV-2 in wastewater with the infected population in the same area.
PP7 was added as a control of the viral concentration process and was detected in all the analyzed samples (100% recovery success), with means of viral recovery of 26% for wastewater, 7% for swimming pools water, and 2% for Doña Jacinta stream. Differences in recovery percentage between wastewater and surface water can be explained by the fact that different methods were used for viral concentration and different volumes were analyzed causing different dilution factor for PP7.
Human RNase P amplified in 66 out of 67 wastewater samples, 15 out of 17 swimming pool water, and 5 out of 17 surface water of Doña Jacinta stream. RNase P according to the Center for Disease Control and Prevention protocol (CDC, 2019) serves as an endogenous control in clinical samples, and no results can be emitted if negative samples for SARS-CoV-2 are also negative for RNAse P. In water matrices such as wastewater, RNAse P have also been used as an endogenous control (Peccia et al., 2020; Ahmed et al., 2021). Our results confirm the advantages of use RNAse P as an extra control, where 99% of the analyzed wastewater samples were positive for RNAse P. Only one sample (1/67) was negative and corresponds to a wastewater sample from the hot spring park, in this sample the presence of chemical products could be more concentrated than the other wastewater samples evaluated and degradation of RNA/DNA molecules are more likely to occur. Moreover, this sample was positive for Rotavirus, thus this result is supported by the fact that RNAse P is more vulnerable to degradation than viral genes as suggested by previous results (Ahmed et. al., 2021). For swimming pools and Doña Jacinta stream, the percentage of positive results for RNAse P were 88% and 29%, respectively. This is consistent with the matrix analyzed, as a mayor dilution factor is present in the stream samples, suggesting that this endogenous control could be used when wastewater matrices are analyzed for viral presence. Negative and positive viral controls presented correct results for all the analyzed samples for PP7, RNase P, Rotavirus, and SARS-CoV-2.
SARS-CoV-2 and Rotavirus were not detected in swimming pools water and surface water of the Doña Jacinta stream. The absence of viruses in the volume analyzed of swimming pools water is in concordance with evidence that swimming pools would not be a route of transmission for SARS-CoV-2 (CDC, 2020; Romano Spica et al., 2020; Brown et al., 2021; Sobsey, 2022).
In wastewater, Rotavirus was detected in samples collected in the hot spring (7/17), in wastewater from hotels next to the hot spring (6/17) and from Salto city (22/34). The frequency of detection and quantification for this virus in wastewater samples is shown in Fig. 2.
From August to October in 2020, the frequency of Rotavirus in wastewater was around 10%, which is a low frequency compared with the previous results on the same area, and in other region of the country during the pre-pandemic period (Victoria et al., 2014; Lizasoain et al., 2018). In this period with a low frequency of Rotavirus, social distancing and partial lockdown measures were applied by government authorities. When these measures were relaxed (November 2020–March 2021), the frequency of Rotavirus was higher (roughly 90%). This phenomenon was already described by other authors in different regions of the world, mainly for enteric viruses (Wang et al., 2021; Chan, 2022; Shen et al. 2022).
SARS-CoV-2 in the hot spring area was detected in three wastewater samples from hotels next to hot spring corresponding to samples collected in January, May, and June of 2021. In Fig. 3, the number of active cases of SARS-CoV-2 in Salto and the concentration of SARS-CoV-2 observed in wastewater of the area of hydrothermal spring are shown.
The first positive sample for SARS-CoV-2 in wastewater from the hot spring area was observed when the number of active cases exceed 100 which is in accordance with the moment when social distancing and partial lockdown measures started to be relaxed. Moreover, the peak of detection of SARS-CoV-2 in wastewater was observed previous to the peak of active cases in the Salto population which is consistent with the previous studies, suggesting the use of wastewater as an early warning of the number of SARS-CoV-2 infections in the population (Robotto et al., 2022; Prado et al., 2021).
In Salto city, SARS-CoV-2 was detected in 11 out of 34 wastewater samples analyzed, 5 corresponding to the Northern sampling site and 6 to the Southern sampling site (Fig. 4).
In these samples, the association between the number of active cases and genome copies/L detected is more evident. Concentration of SARS-CoV-2 in the Northern and Southern pump stations of the city was clearly accompanied by the number of active cases in the city. These results confirm that, as it was already described by various authors around the world, the use of WBE is a powerful tool to describe the viral dissemination in the population based on the analysis of wastewater samples not only for enteric viruses but also for SARS-CoV-2 (Randazzo et al., 2020; Peccia et al., 2020; Xagoraraki and O’Brien, 2019). The first detection of SARS-CoV-2 in wastewater from Salto city was observed when 105 active cases were reported. Taking in consideration the difference between populations, concentration methods, environmental, and other factors, the number of infected people and the presence of a positive wastewater sample for SARS-CoV-2 are consistent with the results from Ahmed et al. (2020) where they detect SARS-CoV-2 in wastewater samples when the number of active cases was higher than 171 active cases.
Differences observed in the dynamic of SARS-CoV-2 in wastewater between both sites (hot spring park and Salto city) respect to SARS-CoV-2 active cases in Salto could be explained since that hot spring receives tourists coming from different places of the country.
The absence of virus in swimming pools and Doña Jacinta stream water did not allow a possible association between the physicochemical parameters evaluated in these matrices and the presence of virus. In any case, the results obtained for these parameters are shown in Fig. 5.
Regarding the hydrochemical characterization of the Daymán groundwater, this is a Hyperthermal groundwater, in situ physicochemical parameters were as follows: Temperature 43.2 °C, pH 7.32, ORP 190.3 mV, EC 754 µS/cm, and alkalinity 330.0 mgCaCO3/ml (see Table A from supplementary materials). Results from ion chromatography of majority ions analyzed by Piper Diagram indicated that this geothermal source is a Sodium bicarbonate type (See Figure A and EasyChem files from supplementary materials).
Daymán water showed no detected values of NH4+. However, free ammonia was detected in swimming pools water (Fig. 5). This could be related to the possible presence of the decomposition of human urine products.
Conclusions
The absence of SARS-CoV-2 in swimming pools water in this one-year surveillance suggests that waters from swimming pools would not be a source of transmission for this virus although infectivity assays are needed in order to confirm this statement. The concentration of SARS-CoV-2 in wastewater follows the tendency of the number of infected people in the area of this study, supporting the advantage of this approach in order to determine the viral dispersion in the population and also serves as an early warning. Changes in frequencies of Rotavirus in wastewater samples evidence that the effects of social distancing and partial lockdown measures applied to the population in order to avoid SARS-CoV-2 transmission dispersion also affect the dissemination and transmission for other pathogens like enteric viruses.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ahmed, W., Angel, N., Edson, J., Bibby, K., Bivins, A., O’Brien, J. W., Choi, P. M., Kitajima, M., Simpson, S. L., Li, J., Tscharke, B., Verhagen, R., Smith, W. J. M., Zaugg, J., Dierens, L., Hugenholtz, P., Thomas, K. V., & Mueller, J. F. (2020). First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: A proof of concept for the wastewater surveillance of COVID-19 in the community. Science of the Total Environment, 728, 138764.
Ahmed, F., Islam, M. A., Kumar, M., Hossain, M., Bhattacharya, P., Islam, M. T., Hossen, F., Hossain, M. S., Islam, M. S., Uddin, M. M., Islam, M. N., Bahadur, N. M., Didar-Ul-Alam, M., Reza, H. M., & Jakariya, M. (2021). First detection of SARS-CoV-2 genetic material in the vicinity of COVID-19 isolation Centre in Bangladesh: Variation along the sewer network. Science of the Total Environment, 776, 145724.
American Public Health Association (APHA). (2017). Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association.
Bibby, K., & Peccia, J. (2013). Identification of viral pathogen diversity in sewage sludge by metagenome analysis. Environmental Science and Technology, 47, 1945–1951.
Brown, J. C., Moshe, M., Blackwell, A., & Barclay, W. S. (2021). Inactivation of SARS-CoV-2 in chlorinated swimming pool water. Water Research, 205, 117718.
Calgua, B., Mengewein, A., Grunert, A., Bofill-Mas, S., Clemente-Casares, P., Hundesa, A., Wyn-Jones, A. P., López-Pila, J. M., & Girones, R. (2008). Development and application of a one-step low cost procedure to concentrate viruses from seawater samples. Journal of Virological Methods, 153, 79–83.
Center for Disease Control and prevention (CDC). (2019). Real-time rRT-PCR panel primers and probes [WWW document]. Coronavirus disease 2019 (COVID-19). Retrieved Nov 15, 2021 from https://www.fda.gov/media/134922/download
Centers for Disease Control and Prevention (CDC). 2020. Research use only 2019-novel coronavirus (2019-nCoV) real-time RT-PCR primer and probe information.
Chan, M. C. (2022). Return of norovirus and rotavirus activity in winter 2020–21 in city with strict COVID-19 control strategy. China. Emerg Infect Dis., 28(3), 713–716.
Chau, C. H., Strope, J. D., & Figg, W. D. (2020). COVID-19 clinical diagnostics and testing technology. Pharmacotherapy, 40(8), 857–868.
Chen, Y., Chen, L., Deng, Q., Zhang, G., Wu, K., Ni, L., Yang, Y., Liu, B., Wang, W., Wei, C., Yang, J., Ye, G., & Cheng, Z. (2020). The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. Journal of Medical Virology, 92(7), 833–840.
Chu, D. K. W., Pan, Y., Cheng, S. M. S., Hui, K. P. Y., Krishnan, P., Liu, Y., Ng, D. Y. M., Wan, C. K. C., Yang, P., Wang, Q., Peiris, M., & Poon, L. L. M. (2020). Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clinical Chemistry, 66(4), 549–555.
Corman, V. M., Landt, O., Kaiser, M., Molenkamp, R., Meijer, A., Chu, D. K., Bleicker, T., Brünink, S., Schneider, J., Schmidt, M. L., Mulders, D. G., Haagmans, B. L., van der Veer, B., van den Brink, S., Wijsman, L., Goderski, G., Romette, J. L., Ellis, J., Zambon, M., … Drosten, C. (2020). Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance Weekly, 25(3), 2000045.
Dergham, J., Delerce, J., Bedotto, M., La Scola, B., & Moal, V. (2021). Isolation of viable SARS-CoV-2 virus from feces of an immunocompromised patient suggesting a possible fecal mode of transmission. Journal of Clinical Medicine, 10(12), 2696. https://doi.org/10.3390/jcm10122696.PMID:34207314;PMCID:PMC8235306
EASY CHEM 5.0 (2012). Software. Designed by Grupo de Hidrología Subterránea—Departamento de Ingeniería del Terreno de la UPC. Retrieved , 05/04/2021 from https://h2ogeo.upc.edu/es/investigacion-hidrologia-subterrania/software/42-easy-quim.
Fumian, T. M., Fioretti, J. M., Lun, J. H., Dos Santos, I. A. L., White, P. A., & Miagostovich, M. P. (2019). Detection of norovirus epidemic genotypes in raw sewage using next generation sequencing. Environment International, 123, 282–291.
Gharbi-Khelifi, H., Sdiri, K., Ferre, V., Harrath, R., Berthome, M., Billaudel, S., & Aouni, M. (2007). A 1-year study of the epidemiology of hepatitis A virus in Tunisia. Clinical Microbiology & Infection, 13(1), 25–32.
Kumazaki, M., & Usuku, S. (2015). Nucleotide correlations between rotavirus C isolates in clinical samples from outbreaks and in sewage samples. Food Environ Virol., 7(3), 269–275.
Lewis, G. D., & Metcalf, T. G. (1988). Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Applied and Environment Microbiology, 54(8), 1983–1988.
Lizasoain, A., Tort, L. F. L., García, M., Gillman, L., Alberti, A., Leite, J. P. G., Miagostovich, M. P., Pou, S. A., Cagiao, A., Razsap, A., Huertas, J., Berois, M., Victoria, M., & Colina, R. (2018). Human enteric viruses in a wastewater treatment plant: Evaluation of activated sludge combined with UV disinfection process reveals different removal performances for viruses with different features. Letters in Applied Microbiology, 66(3), 215–221.
Murakami, M., Hata, A., Honda, R., & Watanabe, T. (2020). Letter to the editor: Wastewater-based epidemiology can overcome representativeness and stigma issues related to COVID-19. Environmental Science & Technology, 54(9), 5311.
Peccia, J., Zulli, A., Brackney, D. E., Grubaugh, N. D., Kaplan, E. H., Casanovas-Massana, A., Ko, A. I., Malik, A. A., Wang, D., Wang, M., Warren, J. L., Weinberger, D. M., Arnold, W., & Omer, S. B. (2020). Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nature Biotechnology, 38(10), 1164–1167.
Pina, S., Buti, M., Jardí, R., Clemente-Casares, P., Jofre, J., & Girones, R. (2001). Genetic analysis of hepatitis A virus strains recovered from the environment and from patients with acute hepatitis. Journal of General Virology, 82(Pt 12), 2955–2963.
Piper, A. (1944). A graphic procedure in the geochemical interpretation of water-analyses. Transactions, American Geophysical Union., 25(6), 914–928.
Prado, T., Fumian, T. M., Mannarino, C. F., Resende, P. C., Motta, F. C., Eppinghaus, A. L. F., do Vale, V. H. C., Braz, R. M. S., de Andrade, J. D. S. R., Maranhão, A. G., & Miagostovich, M. P. (2021). Wastewater-based epidemiology as a useful tool to track SARS-CoV-2 and support public health policies at municipal level in Brazil. Water Research., 191, 116810.
Randazzo, W., Truchado, P., Cuevas-Ferrando, E., Simón, P., Allende, A., & Sánchez, G. (2020). SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Research, 181, 115942.
Robotto, A., Lembo, D., Quaglino, P., Brizio, E., Polato, D., Civra, A., Cusato, J., & Di Perri, G. (2022). Wastewater-based SARS-CoV-2 environmental monitoring for Piedmont, Italy. Environmental Research, 203, 111901. https://doi.org/10.1016/j.envres.2021.111901
Romano Spica, V., Gallè, F., Baldelli, G., Valeriani, F., Di Rosa, E., Liguori, G., Brandi, G., GSMS-SItI, Working Group on Movement Sciences for Health, Italian Society of Hygiene Preventive Medicine and Public Health. (2020). Swimming pool safety and prevention at the time of Covid-19: a consensus document from GSMS-SItI. Annali Di Igiene, 32(5), 439–448.
Salvo, M., Moller, A., Alvareda, E., Gamazo, P., Colina, R., & Victoria, M. (2021). Evaluation of low-cost viral concentration methods in wastewaters: Implications for SARS-CoV-2 pandemic surveillances. Journal of Virological Methods, 297, 114249.
Savela, E. S., Viloria Winnett, A., Romano, A. E., Porter, M. K., Shelby, N., Akana, R., Ji, J., Cooper, M. M., Schlenker, N. W., Reyes, J. A., Carter, A. M., Barlow, J. T., Tognazzini, C., Feaster, M., Goh, Y. Y., & Ismagilov, R. F. (2022). Quantitative SARS-CoV-2 viral-load curves in paired saliva samples and nasal swabs inform appropriate respiratory sampling site and analytical test sensitivity required for earliest viral detection. Journal of Clinical Microbiology, 60(2), e0178521. https://doi.org/10.1128/JCM.01785-21
Shen, L., Sun, M., Song, S., Hu, Q., Wang, N., Ou, G., Guo, Z., Du, J., Shao, Z., Bai, Y., & Liu, K. (2022). The impact of anti-COVID-19 nonpharmaceutical interventions on hand, foot, and mouth disease-A spatiotemporal perspective in Xi’an, northwestern China. Journal of Medical Virology, 94(7), 3121–3132.
Sobsey, M. D. (2022). Absence of virological and epidemiological evidence that SARS-CoV-2 poses COVID-19 risks from environmental fecal waste, wastewater and water exposures. Journal of Water and Health, 20(1), 126–138.
Victoria, M., Tort, L. F., García, M., Lizasoain, A., Maya, L., Leite, J. P., Miagostovich, M. P., Cristina, J., & Colina, R. (2014). Assessment of gastroenteric viruses from wastewater directly discharged into Uruguay River. Uruguay. Food Environ Virol., 6(2), 116–124.
Wang, L. P., Han, J. Y., Zhou, S. X., Yu, L. J., Lu, Q. B., Zhang, X. A., Zhang, H. Y., Ren, X., Zhang, C. H., Wang, Y. F., Lin, S. H., Xu, Q., Jiang, B. G., Lv, C. L., Chen, J. J., Li, C. J., Li, Z. J., Yang, Y., Liu, W., et al. (2021). The changing pattern of enteric pathogen infections in China during the COVID-19 pandemic: a nation-wide observational study. Lancet Reg Health West Pac., 16, 100268.
World Health Organization. (2003). Guidelines for environmental surveillance of poliovirus circulation.
World Health Organization. (2020). WHO Director-General’s opening remarks at the media briefing on COVID-19–11 March 2020.
Xagoraraki, I., & O’Brien, E. (2019). Wastewater-based epidemiology for early detection of viral outbreaks. Women in water quality: Investigations by prominent female engineers, 2019, 75–97.
Xiao, F., Sun, J., Xu, Y., Li, F., Huang, X., Li, H., Zhao, J., Huang, J., & Zhao, J. (2020). Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerging Infectious Diseases, 26(8), 1920–1922. https://doi.org/10.3201/eid2608.200681
Zeng, S. Q., Halkosalo, A., Salminen, M., Szakal, E. D., Puustinen, L., & Vesikari, T. (2008). One-step quantitative RT-PCR for the detection of rotavirus in acute gastroenteritis. Journal of Virological Methods, 153(2), 238–240.
Zhu, N., Zhang, D., Wang, W., Li, X., Yang, B., Song, J., Zhao, X., Huang, B., Shi, W., Lu, R., Niu, P., Zhan, F., Ma, X., Wang, D., Xu, W., Wu, G., Gao, G. F., Tan, W., China Novel Coronavirus Investigating and Research Team. (2020). A novel coronavirus from patients with pneumonia in China, 2019. New England Journal of Medicine, 382(8), 727–733.
Acknowledgements
We thank Bsc. student in Water Sciences Rafael Banega and the geologist engineer Armando Borrero for giving support in collecting samples and the clinical laboratory graduate Delia Machado from INIA Salto Grande for given support in Ion chromatography analysis. We also want to thank to CSEAM, UdelaR (Project 62) for financial support and to the Cooperation Agreement between Universidad de la República, Ministerio de Salud Pública, Intendencia de Salto, Centro Comercial e Industrial de Salto, and the Fundación Desarrollo Regional de Salto Grande.
Funding
This study was supported by CSEAM, UdelaR, Grant No. 62.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Salvo, M., Azambuya, J., Baccardatz, N. et al. One-Year Surveillance of SARS-CoV-2 and Rotavirus in Water Matrices from a Hot Spring Area. Food Environ Virol 14, 401–409 (2022). https://doi.org/10.1007/s12560-022-09537-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12560-022-09537-w