Abstract
Purpose
To highlight merits and pitfalls of treatment plan comparison between photon-based conventional radiation therapy (XRT) and particle-based (proton and carbon ions) therapy (PT).
Methods
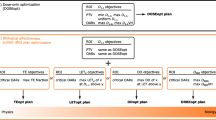
The physical and dosimetric rationales for the use of PT will be explored looking at their advantages and disadvantages with a specific focus on sensitivity to range and biological uncertainties. Next, the analysis will focus on linear energy transfer (LET) and its correlation with relative biological effectiveness (RBE), particularly within the context of plan optimization and evaluation phases in proton therapy. This examination aims to address the impact of the LET/RBE interplay on radiobiological uncertainties.
Results
There is a wide literature of planning studies comparing PT and the most advanced XRT techniques. Purely dosimetric advantages are translated into normal tissue complication probability (NTCP) variation to drive patient optimal allocation, but the impact of range uncertainty needs to be accounted for, together with the consequent need for plan adaptation and organ motion management. RBE modelling and entailed clinical effects are currently central in PT research, while new degrees of freedom are being introduced with LET-based plan optimization and evaluation.
Conclusions
PT shows many dosimetric advantages but emphasizes challenges like range uncertainty. RBE modelling plays a crucial role, and LET-based optimization introduces new possibilities. Addressing uncertainties and embracing innovation are vital for enhancing PT’s efficacy.


Similar content being viewed by others
Data availability
The data supporting the findings of this study can be accessed from the corresponding author upon a reasonable request. However, please note that restrictions may apply due to the potential inclusion of information that could compromise the privacy of participants.
References
Rackwitz T, Debus J. Clinical applications of proton and carbon ion therapy, Seminars in Oncology, vol. 46, no. 3. W.B. Saunders, pp. 226–232, Jun. 2019, https://doi.org/10.1053/j.seminoncol.2019.07.005.
Paganetti H, et al. Roadmap: Proton therapy physics and biology. Phys Med Biol. 2021;66(5). https://doi.org/10.1088/1361-6560/abcd16.
Durante M, Paganetti H. Nuclear physics in particle therapy: a review. Rep Prog Phys. 2016;79(9). https://doi.org/10.1088/0034-4885/79/9/096702.
Paganetti H. Mechanisms and review of clinical evidence of variations in relative Biological effectiveness in Proton Therapy. Int J Radiat Oncol Biol Phys. 2022;112(1):222–36. https://doi.org/10.1016/j.ijrobp.2021.08.015.
Kramer M, Scholz M. Treatment planning for heavy-ion radiotherapy: calculation and optimization of biologically effective dose. Phys Med Biol. 2000;45(11):3319–30. https://doi.org/10.1088/0031-9155/45/11/314.
Inaniwa T, et al. Treatment planning for a scanned carbon beam with a modified microdosimetric kinetic model. Phys Med Biol. 2010;55(22):6721–37. https://doi.org/10.1088/0031-9155/55/22/008.
Tinganelli W, Durante M. Carbon ion radiobiology, vol. 12, no. 10. 2020.
Visser S, et al. Robustness assessment of clinical adaptive proton and photon radiotherapy for oesophageal cancer in the model-based approach. Radiother Oncol. 2022;177:197–204. https://doi.org/10.1016/j.radonc.2022.11.001.
Hansen CR et al. Evaluation of decentralised model-based selection of head and neck cancer patients for a proton treatment study. DAHANCA 35, Radiother. Oncol, no. xxxx, p. 109812, 2023, https://doi.org/10.1016/j.radonc.2023.109812.
Vai A, et al. Proton Radiation Therapy for Nasopharyngeal Cancer patients: dosimetric and NTCP evaluation supporting clinical decision. Cancers (Basel). 2022;14(5):1–12. https://doi.org/10.3390/cancers14051109.
Tambas M et al. November., First experience with model-based selection of head and neck cancer patients for proton therapy, Radiother. Oncol, vol. 151, no. 2017, pp. 206–213, 2020, https://doi.org/10.1016/j.radonc.2020.07.056.
Mirandola A, et al. A patient selection Approach based on NTCP Models and DVH parameters for definitive Proton Therapy in locally Advanced Sinonasal Cancer patients. Cancers (Basel). 2022;14(11). https://doi.org/10.3390/cancers14112678.
Stick LB, et al. Radiation-Induced toxicity risks in Photon Versus Proton Therapy for Synchronous bilateral breast Cancer. Int J Part Ther. 2022;8(4):1–13. https://doi.org/10.14338/IJPT-21-00023.1.
Scorsetti M, et al. Intensity modulated proton therapy compared to volumetric modulated arc therapy in the irradiation of young female patients with hodgkin’s lymphoma. Assessment of risk of toxicity and secondary cancer induction. Radiat Oncol. 2020;15(1):1–12. https://doi.org/10.1186/s13014-020-1462-2.
Boersma LJ, et al. Model-based selection for Proton Therapy in breast Cancer: development of the National Indication Protocol for Proton Therapy and First Clinical experiences. Clin Oncol. 2022;34(4):247–57. https://doi.org/10.1016/j.clon.2021.12.007.
Berrington de González A, et al. The Pediatric Proton and Photon Therapy Comparison Cohort: Study Design for a Multicenter Retrospective Cohort to investigate subsequent cancers after Pediatric Radiation Therapy. Adv Radiat Oncol. 2023;8(6). https://doi.org/10.1016/j.adro.2023.101273.
Hamming-Vrieze O, et al. Impact of setup and range uncertainties on TCP and NTCP following VMAT or IMPT of oropharyngeal cancer patients. Phys Med Biol. 2019;64(9). https://doi.org/10.1088/1361-6560/ab1459.
Paganetti H, Botas P, Sharp GC, Winey B. Adaptive proton therapy. Phys Med Biol. 2021;66(22). https://doi.org/10.1088/1361-6560/ac344f.
Fossati P, et al. Dose prescription in carbon ion radiotherapy: a planning study to compare NIRS and LEM approaches with a clinically-oriented strategy. Phys Med Biol. 2012;57(22):7543–54. https://doi.org/10.1088/0031-9155/57/22/7543.
Paganetti H, et al. Report of the AAPM TG-256 on the relative biological effectiveness of proton beams in radiation therapy. Med Phys. 2019;46(3):e53–78. https://doi.org/10.1002/mp.13390.
Molinelli S et al. RBE-weighted dose in carbon ion therapy for ACC patients: Impact of the RBE model translation on treatment outcomes, Radiother. Oncol, vol. 141, no. xxxx, pp. 227–233, 2019, https://doi.org/10.1016/j.radonc.2019.08.022.
Molinelli S, et al. How LEM-based RBE and dose-averaged LET affected clinical outcomes of sacral chordoma patients treated with carbon ion radiotherapy: RBE and LET in carbon ion therapy for sacral chordoma. Radiother Oncol. 2021;163:209–14. https://doi.org/10.1016/j.radonc.2021.08.024.
Molinelli S, et al. Dose prescription in carbon ion radiotherapy: how to compare two different RBE-weighted dose calculation systems. Radiother Oncol. 2016;120(2):307–12. https://doi.org/10.1016/j.radonc.2016.05.031.
Dale JE, et al. Optic nerve constraints for carbon ion RT at CNAO – reporting and relating outcome to European and Japanese RBE. Radiother Oncol. 2019;140:175–81. https://doi.org/10.1016/j.radonc.2019.06.028.
Dale JE et al. Brainstem NTCP and Dose Constraints for Carbon Ion RT—Application and Translation From Japanese to European RBE-Weighted Dose, Front. Oncol, vol. 10, no. November, pp. 1–11, 2020, https://doi.org/10.3389/fonc.2020.531344.
Deng W, et al. A critical review of LET-based intensity- modulated proton therapy plan evaluation and optimization for head and neck cancer management. Int J Part Ther. 2021;8(1):36–49. https://doi.org/10.14338/IJPT-20-00049.1.
Hahn C, et al. Comparing biological effectiveness guided plan optimization strategies for cranial proton therapy: potential and challenges. Radiat Oncol. 2022;17(1):1–13. https://doi.org/10.1186/s13014-022-02143-x.
Magro G, et al. Dosimetric validation of a GPU-based dose engine for a fast in silico patient-specific quality assurance program in light ion beam therapy. Med Phys. 2022;49(12):7802–14. https://doi.org/10.1002/mp.16002.
Kalholm F, Grzanka L, Traneus E, Bassler N. A systematic review on the usage of averaged LET in radiation biology for particle therapy. Radiother Oncol. 2021;161:211–21. https://doi.org/10.1016/j.radonc.2021.04.007.
Unkelbach J, et al. Robust radiotherapy planning. Phys Med Biol. 2018;63(22). https://doi.org/10.1088/1361-6560/aae659.
Molinelli S et al. November., The role of multiple anatomical scenarios in plan optimization for carbon ion radiotherapy of pancreatic cancer: Inter-fraction robustness in CIRT for pancreatic cancer, Radiother. Oncol, vol. 176, no. 2019, pp. 1–8, 2022, https://doi.org/10.1016/j.radonc.2022.09.005.
Li H, Durante M, Jäkel O, Kong L, Lu L. Editorial: image-guided particle therapy. Front Oncol. 2023;13:10–2. https://doi.org/10.3389/fonc.2023.1175511.
Mori S, Knopf A, Umegaki K. Motion management in particle therapy. Med Phys. Nov. 2018;45(11):e994–1010. https://doi.org/10.1002/mp.12679.
Hagiwara Y, et al. Efficacy and feasibility of re-irradiation using carbon ions for pancreatic cancer that recurs after carbon-ion radiotherapy. Clin Transl Radiat Oncol. 2021;26:24–9. https://doi.org/10.1016/j.ctro.2020.10.007.
Matsumoto S, et al. Unresectable chondrosarcomas treated with carbon ion radiotherapy: relationship between dose-averaged linear energy transfer and local recurrence. Anticancer Res. 2020;40(11):6429–35. https://doi.org/10.21873/anticanres.14664.
Nenoff L, et al. Experimental validation of daily adaptive proton therapy. Phys Med Biol. 2021;66(20). https://doi.org/10.1088/1361-6560/ac2b84.
Placidi L, et al. Effect of anatomic changes on Pencil Beam scanned Proton Dose distributions for cranial and extracranial tumors. Int J Radiat Oncol Biol Phys. 2017;97(3):616–23. https://doi.org/10.1016/j.ijrobp.2016.11.013.
Giantsoudi D, Adams J, MacDonald SM, Paganetti H. Proton Treatment Techniques for Posterior Fossa Tumors: consequences for Linear Energy transfer and dose-volume parameters for the Brainstem and organs at Risk. Int J Radiat Oncol Biol Phys. 2017;97(2):401–10. https://doi.org/10.1016/j.ijrobp.2016.09.042.
MacDonald SM, Laack NN, Terezakis S. Humbling advances in Technology: Protons, Brainstem Necrosis, and the self-driving Car. Int J Radiat Oncol Biol Phys. 2017;97(2):216–9. https://doi.org/10.1016/j.ijrobp.2016.08.001.
Niemierko A, et al. Brain necrosis in adult patients after Proton Therapy: is there evidence for dependency on Linear Energy transfer? Int J Radiat Oncol Biol Phys. 2021;109(1):109–19. https://doi.org/10.1016/j.ijrobp.2020.08.058.
Bauer J, Bahn E, Harrabi S, Herfarth K, Debus J, Alber M. How can scanned proton beam treatment planning for low-grade glioma cope with increased distal RBE and locally increased radiosensitivity for late MR-detected brain lesions? Med Phys. 2021;48(4):1497–507. https://doi.org/10.1002/mp.14739.
Underwood TSA, McNamara AL, Appelt A, Haviland JS, Sørensen BS, Troost EGC. A systematic review of clinical studies on variable proton relative biological effectiveness (RBE). Radiother Oncol. 2022;175:79–92. https://doi.org/10.1016/j.radonc.2022.08.014.
Traneus E, Ödén J. Introducing Proton Track-End objectives in Intensity Modulated Proton Therapy Optimization to Reduce Linear Energy Transfer and relative Biological effectiveness in critical structures. Int J Radiat Oncol Biol Phys. 2019;103(3):747–57. https://doi.org/10.1016/j.ijrobp.2018.10.031.
Choi K, et al. Rectum dose constraints for carbon ion therapy: relative biological effectiveness model dependence in relation to clinical outcomes. Cancers (Basel). 2020;12(1). https://doi.org/10.3390/cancers12010046.
Held T, et al. Ways to unravel the clinical potential of carbon ions for head and neck cancer reirradiation: dosimetric comparison and local failure pattern analysis as part of the prospective randomized CARE trial. Radiat Oncol. 2022;17(1):1–12. https://doi.org/10.1186/s13014-022-02093-4.
Mein S et al. Assessment of RBE-Weighted Dose Models for Carbon Ion Therapy Toward Modernization of Clinical Practice at HIT: In Vitro, in Vivo, and in Patients, Int. J. Radiat. Oncol. Biol. Phys, vol. 108, no. 3, pp. 779–791, 2020, https://doi.org/10.1016/j.ijrobp.2020.05.041.
Liermann J, Naumann P, Weykamp F, Hoegen P, Debus J, Herfarth K. Effectiveness of Carbon Ion Radiation in Locally Advanced Pancreatic Cancer, Front. Oncol, vol. 11, no. July, pp. 1–9, 2021, https://doi.org/10.3389/fonc.2021.708884.
Bassler N, Jäkel O, Søndergaard CS, Petersen JB. Dose-and LET-painting with particle therapy. Acta Oncol (Madr). 2010;49(7):1170–6. https://doi.org/10.3109/0284186X.2010.510640.
Inaniwa T, Kanematsu N, Noda K, Kamada T. Treatment planning of intensity modulated composite particle therapy with dose and linear energy transfer optimization. Phys Med Biol. 2017. https://doi.org/10.1088/1361-6560/aa68d7.
Mein S, et al. Spot-scanning Hadron Arc (SHArc) Therapy: a Study with Light and Heavy ions. Adv Radiat Oncol. 2021;6(3):100661. https://doi.org/10.1016/j.adro.2021.100661.
Fredriksson A, Glimelius L, Bokrantz R. The LET trilemma: conflicts between robust target coverage, uniform dose, and dose-averaged LET in carbon therapy. Med Phys. 2023;50(12):7338–48. https://doi.org/10.1002/mp.16771.
Acknowledgements
This work has been partly founded by the European Union’s Horizon 2020 research and innovation program under grant agreement No. 101008548 (HITRIplus).
Funding
The authors declare that no funds, grants, or any other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SM, GM and AV. The first draft of the manuscript was written by SM and all authors commented on previous versions of the manuscript. Further revisions have been managed by GM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no competing interests to disclose.
Ethical approval
No ethical approval was required for this article.
Consent to participate
No consent to participate was required for this article.
Consent to publish
The authors confirm that human participants provided informed consent for publication of the images in Figs. 1 and 2.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Molinelli, S., Mirandola, A., Magro, G. et al. Treatment Planning: comparing techniques and standards. Health Technol. (2024). https://doi.org/10.1007/s12553-024-00845-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12553-024-00845-8