Abstract
Purpose
To present novel approaches in particle therapy that could result in an improvement of patient outcome.
Methods
Technological/planning and biological innovations could bring particle therapy into a new area of precision medicine. However, several hurdles have to be overcome in order to transform these R&D opportunities into clinical advantages. In this contribution, we summarize the potential advantages of novel tumor targeting, through high-LETd boosting strategies with carbon ions, over standard IMPT: LETd-optimization for IMPT plan, IMPTLET, and spot-scanning hadron arc (SHArc) therapy. Two patient cases are presented to showcase the benefit: a pancreatic cancer patient (PATA) and a recurrent glioblastoma patient (PATB).
Results
For both patients, the prescription dose and target/organs at risk (OARs) optimization goals were reached for the three techniques. In standard IMPT, the maximum LETd is placed outside of the target volume and extends into normal tissues. For the gross target volume (GTV), mean LETd values were, on average, around ∼40–60 keV/µm. IMPTLET allowed an increase in the GTV minimum LETd from 38.4 keV/µm to 48.6 keV/µm, and from 55.1 to 87.1 keV/µm, for PATA and PATB, respectively. SHArc led to an enhancement of the maximum LETd in the GTV up to at least 125 keV/µm, while the minimum GTV LETd were 47.2 keV/µm and 46.1 keV/µm, respectively. For PATA, SHArc lowers the maximum LETd in the gastrointestinal tract to 47.5 keV/µm compared to 88.0 keV/µm and 83.0 keV/µm found for the IMPT and IMPTLET plans, respectively.
Conclusions
Many technological and biological innovations could enhance our current clinical approach. Following the current success of the IMPTLET introduction in clinic, SHARc will represent an interesting clinical option in carbon ion therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Radiotherapy can be delivered with photons or charged particles [1, 2]. Particle therapy includes treatment with different types of ions, such as protons, helium or carbon ions having different linear energy transfer (LET). In contrast to conventional photon therapy, all ion beam treatments feature the so-called Bragg peak resulting in highly conformal tumor irradiation and greater normal tissue sparing [1, 2]. Heavier charged particles, such as helium and carbon ions, result in improved tumor targeting capability due to their reduced lateral scattering. Additionally, carbon ions offer biological advantages untapped by lower LET particles. One of the major differences between carbon ions and lighter particles is their larger biological effectiveness in cell killing [2]. This made them a prime candidate for treating tumor radio-resistant to conventional photon radiotherapy such as pancreatic cancer and glioblastoma. This increase in efficiency is due to their higher LET which induces more complex, non-repairable, DNA damage [3].
Pancreatic cancer is currently one of the most lethal tumors, with a five-year overall survival rate at merely 5–10% [4]. Carbon ion radiotherapy, as reported by the Japanese experience, comes as a valuable treatment option for pancreatic cancer and a minimum dose-averaged LET (LETd) of 44 keV/µm in the gross target volume (GTV) has been correlated with an improved local control [5].
Glioblastoma, also defined as a grade IV glioma based on the WHO classification, is the most common malignant primary brain tumor in adults, representing up to 46% of all gliomas and 16% of all primary brain tumors [6]. It is one of the most aggressive tumors and has a poor prognosis, with a median overall survival of about 15–18 months [7]. It is known that glioblastoma is resistant to low LET radiation, in particular due to the presence of hypoxia regions [8] and it has been postulated in several works that high LETd (⪆ 75 keV/µm) carbon ions could overcome hypoxia-induced radio-resistance as shown in vitro and in vivo [9].
For these challenging tumors with poor prognosis, in particular, novel approaches in particle therapy should be clinically introduced aiming to maximize tumor targeting in terms of biological dose and LETd while maintaining a low toxicity profile of the normal tissue. In terms of improving dose calculation accuracy, Monte Carlo (MC) dose engine should be included in clinic for helium and carbon ions [10, 11] following the success of proton MC dose engine. When available, helium could substitute proton beams when a “low-LET” arm, compared to carbon ions, is required [12].
Current clinical planning and delivery methods for intensity modulated particle therapy (IMPT) using carbon ions for patient treatment do not yield enough high LETd in the tumor target [13, 14] to efficiently eradicate very radio-resistant tumor micro-environment (TME) such as in hypoxic niches characteristic of pancreatic cancer and glioblastoma. More specifically, values in patient treatment expressed as voxel-based LETd typically reach values up to 30–50 keV/µm, which can be considered low to mid-range values, with minimum, maximumFootnote 1 and mean LETd strongly varying with tumor volume and beam arrangements [13, 15]. The clinical standard for IMPT entails delivering static beams at predetermined angles and selecting spots that overlap, effectively blending the dose deposition in the entrance channel (low-LET) and Bragg peak (high-LET) to achieve the planned target biological dose distribution. Novel planning approaches will be presented in this short communication aiming to overcome this limitation by treating tumor targets with high LETd carbon ion beams.
2 Methods
Two anonymized patients: a pancreatic cancer patient (PATA) and a recurrent glioblastoma (PATB) were taken as proof of principle for comparing different planning strategies. Following clinical standard, the RBE-weighted dose was planned at 48 Gy (RBE) in 12 fractions and at 51 Gy (RBE) in 17 fractions, for PATA and PATB, respectively, using RaySearch RayStation treatment planning system (version 2024 A). For PATA and PATB, the local effect model version I [16] and the modified microdosimetric kinetic model [17] have been used, respectively.
For both patients, the IMPT LETd-optimized plans (IMPTLET) used the same beam arrangement as the clinical standard IMPT, but adding a LETd optimization boosting objective to the GTV to reach 50 and 90 keV/µm in the GTV, for PATA and PATB, respectively. Moreover, the beam energy ranged between 221.05 MeV/u and 334.94 MeV/u for PATA, and 118.52 MeV/u and 267.2 MeV/u for PATB.
In the case of the SHArc plans, different beam configurations were used for each patient. For PATA, the plan was optimized using 90 beams (with 4° gantry angle spacing) over a 360° arc. Moreover, each beam was planned for only 3 energies separated by 3 mm depth. For PATB, 20 beams (with 18° gantry angle spacing) over a 360° arc were used instead. Additionally, each beam was planned with 5 energies (while maintaining the same 3 mm depth separation and configuration at the center of the target). In the SHArc plans, the energies range for PATA and PATB were 197.58 MeV/u − 309.52 MeV/u and 156.85 MeV/u − 283.76 MeV/u, respectively.
3 Results
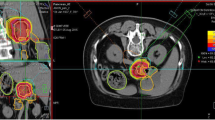
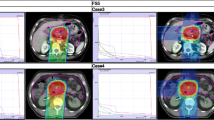
Evaluated plans for pancreatic adenocarcinoma (A) and for glioblastoma (B) patient cases are displayed in Fig. 1. The prescription dose and target optimization goals were achieved as shown in biological dose maps and the dose volume histograms (DVHs) outlined in panels (D). LETd maps, LETd profiles and LETd-volume histograms (LVHs) demonstrate that the maximum LETd for IMPT plans is placed outside of the target volume and extends into normal tissues. For the GTV, LETd values were around ∼40–65 keV/µm. In order to increase LETd in the target, LETd optimization feature was also investigated in Fig. 1 (IMPTLET). As observed from the LETd distribution, the inclusion of LETd-boosting allows LETd-escalation at the GTV, while having a minimum impact on the target dose coverage (see DVH for the clinical target volume, CTV). IMPTLET allowed an increase in the GTV minimum LETd from 55.1 keV/µm to 87.1 keV/µm, and from 38.4 to 48.6 keV/µm, for the glioblastoma and pancreatic adenocarcinoma cases, respectively. However, LETd optimization comes at the expense of an increase in the beam’s entrance biological dose. For the glioblastoma patient, for example, there could be an increase of up to 30% in the biological dose delivered to the skin directly exposed to the entrance channel. Additionally, for IMPTLET plans, normal tissue DVH highlights an increase of the volume in the middle range doses (20–30 Gy (RBE)) compared to standard IMPT.
Simultaneously, the LETd escalation is still constrained by the chosen beam arrangement. In scenarios in which two posterior beams are used, as seen in pancreatic cases currently treated at HIT, although IMPTLET raises LETd in the GTV, the high-LETd region is still focused at the distal edge of the tumour volume.
For both glioblastoma and pancreatic cases shown in Fig. 1, SHArc led to an enhancement of the maximum LETd in the GTV up to at least 125 keV/µm, while the minimum GTV LETd values were 46.1 keV/µm and 47.2 keV/µm, respectively. The plotted LETd profiles further demonstrate that the maximum LETd for SHArc plans is concentrated in the central region of the tumour.
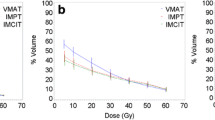
Maps of biological dose (DRBE) and LETd for IMPT, IMPTLET and SHArc plans for (A) pancreatic adenocarcinoma and (B) glioblastoma patient cases using carbon ions. For visualisation, the LETd values are displayed with a 5 and 20 Gy low-dose threshold, for glioblastoma and pancreatic cases, respectively. The white lines in the LETd map designate the LETd profiles presented in panel (C). (C) Profiles for LETd are presented highlighting the LETd range within the GTV for both cases. (D) DRBE- and LETd-volume histograms are shown for relevant organs of interest, for the IMPT (full line), IMPTLET (dashed line) and SHArc plans (dotted line). For the DVH plots (top row), the CTV is shown (orange), as well as either the body excluding the PTV or the brain excluding PTV (pink), for the pancreatic and the head case, respectively. In the LVH plots (bottom row), the GTV is shown for both cases (green), as well as the main OAR for each case (red): the gastrointestinal tract for the pancreatic adenocarcinoma and the ventricles of the brain for the case of the glioblastoma. For the pancreatic case, two lines are mostly indistinguishable for the OAR shown in both the DVH and the LVH, due to the nearly overlap between IMPT and IMPTLET volume histograms (i.e., full and dashed lines).
This comes at the cost of a low dose-bath as shown in the normal tissue DVHs. In terms of LETd in the OARs, SHArc could be particularly beneficial for pancreatic adenocarcinoma. In this case, the clinical beam arrangement results in higher LETd values towards the beam’s distal edge, thus directly leading to high LETd in the gastrointestinal tract, which is posterior to the tumour. With SHArc shifting the high LETd towards the tumour’s centre, there is a direct reduction of the near-maximum LETd in the gastrointestinal tract. Specifically, SHArc lowers the maximum LETd in the gastrointestinal tract to 47.5 keV/µm compared to 88.0 keV/µm and 83.0 keV/µm found for the IMPT and IMPTLET plans, respectively. For the glioblastoma case, in which opposite fields are considered as the standard, the reduction of the near maximum LETd in the surrounding OAR, i.e., the brain ventricles, is less pronounced. Nonetheless, there is still an observed decrease in the LETd5%, from 88.4 keV/µm to 79.7 keV/µm.
4 Discussion
Pancreatic cancer and glioblastoma TME are often hypoxic, which in part explains the poor clinical outcome of these indications. Carbon ions are often used to treat these indications due to their higher LET compared to proton and helium ions. Clinical IMPT, as shown in Fig. 1, resulted in low to mid-range LETd values in the target which only in part overcomes tumor radio-resistance. Therefore, to most effectively use carbon ions, LETd boosting strategies in the target may be necessary to overcome TME related radio-resistance while preserving healthy tissues. Novel approaches are emerging, such as heavy ion LETd-optimization for IMPT plan (IMPTLET), heavy ion arc therapy (SHARc), or multi-ion therapy (MIT) strategies combining lower and higher LET particles [18, 19]. For instance, MIT was proposed by Inaniwa et al., combining neon and helium ions to optimize for higher LETd in the GTV [19]. Unfortunately, access to ion beams like neon is highly limited due to the high cost of the technology and lack of industry support. IMPTLET allows the combination of biological dose- and LETd-based optimization in treatment planning generation. As shown in Fig. 1, it allows efficient boost of LETd in the target at the cost of higher middle dose in the OARs. More recently, interest is rising for more unconventional ion beam delivery techniques, such as SHArc [13, 14, 18] which offers potential treatment benefits such as increased normal tissue sparing from middle-high doses, enhanced target LETd, and potential reduction in high-LETd components in organs at risk as shown in Fig. 1. However, SHArc results in a low dose bath and theoretically in a less robust plan (both in terms of dose and LETd). The choice between SHArc and IMPTLET should ideally align with the individual needs of each patient, as its effectiveness is contingent on various factors including tumor characteristics, patient anatomy, and treatment facility capabilities. Including LETd consideration in planning optimization and evolution is the way to follow towards a modernization of particle therapy. Additionally, the clinical impact of LETd uniformity in the target should be evaluated especially in scenarios where reliable imaging of the tumor hypoxic regions is lacking.
Notes
In the text, we consider LETd98 (LETd at 98% of the volume) and LETd2 (LETd at 2% of the volume) as minimum and maximum, respectively.
References
Schaub L, Harrabi SB, Debus J. Particle therapy in the future of precision therapy. Br J Radiol. 2020;93(1114):20200183. https://doi.org/10.1259/bjr.20200183.
Durante M, Debus J, Loeffler JS. Physics and biomedical challenges of cancer therapy with accelerated heavy ions. Nat Rev Phys. 2021;3:777–90. https://doi.org/10.1038/s42254-021-00368-5.
Joiner M, van der Kogel A, Francis Group. (2018) Basic Clinical Radiobiology, 5th ed.; CRC Press/Taylor & : Boca Raton, FL, USA, 2018; ISBN 978-1-4441-7963-7. https://doi.org/10.1201/9780429490606.
Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic Cancer: global trends, etiology and risk factors. World J Oncol. 2019;10(1):10–27. https://doi.org/10.14740/wjon1166.
Hagiwara Y, Bhattacharyya T, Matsufuji N, Isozaki Y, Takiyama H, Nemoto K, Tsuji H, Yamada S. Influence of dose-averaged linear energy transfer on tumour control after carbon-ion radiation therapy for pancreatic cancer. Clin Transl Radiat Oncol. 2019;21:19–24. https://doi.org/10.1016/j.ctro.2019.11.002.
Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. (2015) CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-Oncology. 2015;17(4):iv1–iv62. https://doi.org/10.1093/neuonc/nov189.
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. https://doi.org/10.1016/S1470-2045(09)70025-7.
Gérard M, Corroyer-Dulmont A, Lesueur P, Collet S, Chérel M, Bourgeois M, Stefan D, Limkin EJ, Perrio C, Guillamo JS, et al. Hypoxia Imaging and Adaptive Radiotherapy: a state-of-the-art Approach in the management of Glioma. Front Med. 2019;6:117. https://doi.org/10.3389/fmed.2019.00117.
Valable S, Gérault AN, Lambert G, Leblond MM, Anfray C, Toutain J, Bordji K, Petit E, Bernaudin M, Pérès EA. (2020) Impact of Hypoxia on Carbon Ion Therapy in Glioblastoma Cells: Modulation by LET and Hypoxia-Dependent Genes. Cancers (Basel). 12(8): 2019. https://doi.org/10.3390/cancers12082019.
Lysakovski P, et al. Development and benchmarking of the first fast Monte Carlo engine for Helium ion beam dose calculation: MonteRay. Med Phys. 2023;50:2510–24. https://doi.org/10.1002/mp.16178.
Lysakovski P, et al. Development and validation of MonteRay, a fast Monte Carlo dose engine for carbon ion beam radiotherapy. Med Phys. 2023. https://doi.org/10.1002/mp.16754.
Tessonnier T, et al. Commissioning of Helium Ion Therapy and the first patient treatment with active Beam Delivery. Int J Radiat Oncol Biol Phys. 2023;116(4):935–48. https://doi.org/10.1016/j.ijrobp.2023.01.015.
Mein S, Tessonnier T, Kopp B, Harrabi S, Abdollahi A, Debus J, Haberer T, Mairani A. Spot-scanning Hadron Arc (SHArc) Therapy: a Study with Light and Heavy ions. Adv Radiation Oncol. 2021;6(3):2452–1094. https://doi.org/10.1016/j.adro.2021.100661.
Mein S, Tessonnier T, Kopp B, Schömers C, Harrabi S, Abdollahi A, Debus J, Haberer T, Mairani A. Biological Dose optimization for particle Arc Therapy using Helium and Carbon ions. Int J Radiat Oncol Biol Phys. 2022;114(2):334–48. https://doi.org/10.1016/j.ijrobp.2022.04.025.
Bassler N, Jäkel O, Søndergaard CS, Petersen JB. Dose-and LET-painting with particle therapy. Acta Oncol (Madr). 2010;49(7):1170–6. https://doi.org/10.3109/0284186X.2010.510640.
Baltazar F, Tessonnier T, Haberer T, Debus J, Herfarth K, Tawk B, Knoll M, Abdollahi A, Liermann J, Mairani A. Carbon-ion radiotherapy (CIRT) as treatment of pancreatic cancer at HIT: initial radiation plan analysis of the prospective phase II PACK-study. Radiother Oncol. 2023;188. https://doi.org/10.1016/j.radonc.2023.109872.
Kopp B, Mein S, Tessonnier T, Besuglow J, Harrabi S, Heim E, Abdollahi A, Haberer T, Debus J, Mairani A. Rapid effective dose calculation for raster-scanning 4He ion therapy with the modified microdosimetric kinetic model (mMKM). Phys Med. 2021;81:273–84. https://doi.org/10.1016/j.ejmp.2020.11.028.
Mein S, Kopp B, Tessonnier T, Liermann J, Abdollahi A, Debus J, Haberer T, Mairani A. Spot-scanning hadron arc (SHArc) therapy: a proof of concept using single- and multi-ion strategies with Helium, carbon, oxygen, and neon ions. Med Phys. 2022;49:6082–97. https://doi.org/10.1002/mp.15800.
Inaniwa T, Kanematsu N, Noda K, Kamada T. Treatment planning of intensity modulated composite particle therapy with dose and linear energy transfer optimization. Phys Med Biol. 2017;62:5180. https://doi.org/10.1088/1361?6560/aa68d7.
Acknowledgements
This contribution has been written on behalf of the Heidelberg Biophysics in Particle Therapy (BioPT) group.
Funding
No funding to report for this work.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
No conflict of interest to declare.
Ethical approval
No ethical approval needed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mairani, A. Innovative approaches to enhance high-LETd tumor targeting in carbon ion radiotherapy. Health Technol. (2024). https://doi.org/10.1007/s12553-024-00842-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12553-024-00842-x