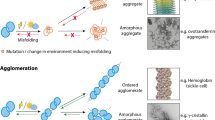
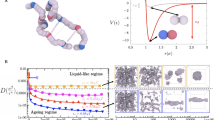
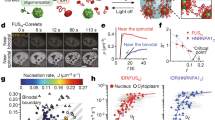
Abstract
Over the past decade, myriads of studies have highlighted the central role of protein condensation in subcellular compartmentalization and spatiotemporal organization of biological processes. Conceptually, protein condensation stands at the highest level in protein structure hierarchy, accounting for the assembly of bodies ranging from thousands to billions of molecules and for densities ranging from dense liquids to solid materials. In size, protein condensates range from nanocondensates of hundreds of nanometers (mesoscopic clusters) to phase-separated micron-sized condensates. In this review, we focus on protein nanocondensation, a process that can occur in subsaturated solutions and can nucleate dense liquid phases, crystals, amorphous aggregates, and fibers. We discuss the nanocondensation of proteins in the light of general physical principles and examine the biophysical properties of several outstanding examples of nanocondensation. We conclude that protein nanocondensation cannot be fully explained by the conceptual framework of micron-scale biomolecular condensation. The evolution of nanocondensates through changes in density and order is currently under intense investigation, and this should lead to the development of a general theoretical framework, capable of encompassing the full range of sizes and densities found in protein condensates.

Similar content being viewed by others
Abbreviations
- ALS:
-
Amyotrophic lateral sclerosis
- BR:
-
Bodies bacterial ribonucleoprotein bodies
- DLS:
-
Dynamic light scattering
- FTD:
-
Frontotemporal dementia
- IR:
-
Insulin receptor
- IDD:
-
Intrinsically disordered domain
- IGF:
-
Insulin-like growth factor
- MRG:
-
Mitochondrial RNA granule
- N-protein:
-
Nucleocapsid protein
- Pol II:
-
RNA polymerase II
- polyQ:
-
Polyglutamine track
- PRM:
-
Proline-rich motif
- RBP:
-
RNA binding protein
- SVs:
-
Synaptic vesicles
- SLS:
-
Static light scattering
- SG:
-
Secretory granule
- SH3:
-
SRC homology 3
- α-Syn:
-
α-Synuclein
- TEM:
-
Transmission electron microscopy.
References
Aarum J, Cabrera CP, Jones TA et al (2020) Enzymatic degradation of RNA causes widespread protein aggregation in cell and tissue lysates. EMBO Rep 21:e49585. https://doi.org/10.15252/embr.201949585
Abyzov A, Blackledge M, Zweckstetter M (2022) Conformational dynamics of intrinsically disordered proteins regulate biomolecular condensate chemistry. Chem Rev 122:6719–6748. https://doi.org/10.1021/acs.chemrev.1c00774
Adamcik J, Mezzenga R (2018) Amyloid polymorphism in the protein folding and aggregation energy landscape. Angew Chem Int Ed 57:8370–8382. https://doi.org/10.1002/anie.201713416
Alberti S, Gladfelter A, Mittag T (2019) Considerations and challenges in studying liquid-liquid phase separation and biomolecular condensates. Cell 176:419–434. https://doi.org/10.1016/j.cell.2018.12.035
Alberti S, Hyman AA (2021) Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat Rev Mol Cell Biol 22:196–213. https://doi.org/10.1038/s41580-020-00326-6
Arakawa T, Timasheff SN (1985) Theory of protein solubility. Methods Enzymol 114:49–77. https://doi.org/10.1016/0076-6879(85)14005-X
Attri AK, Fernández C, Minton AP (2010) pH-dependent self-association of zinc-free insulin characterized by concentration-gradient static light scattering. Biophys Chem 148:28–33. https://doi.org/10.1016/j.bpc.2010.02.002
Azaldegui CA, Vecchiarelli AG, Biteen JS (2021) The emergence of phase separation as an organizing principle in bacteria. Biophys J 120:1123–1138. https://doi.org/10.1016/j.bpj.2020.09.023
Banani SF, Lee HO, Hyman AA, Rosen MK (2017) Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 18:285–298. https://doi.org/10.1038/nrm.2017.7
Banani SF, Rice AM, Peeples WB et al (2016) Compositional control of phase-separated cellular bodies. Cell 166:651–663. https://doi.org/10.1016/j.cell.2016.06.010
Banjade S, Rosen MK (2014) Phase transitions of multivalent proteins can promote clustering of membrane receptors. eLife 3:e04123. https://doi.org/10.7554/eLife.04123
Barrera-Vilarmau S, Teixeira JMC, Fuxreiter M (2022) Protein interactions: anything new? Essays Biochem 66:821–830. https://doi.org/10.1042/EBC20220044
Bearrows SC, Bauchle CJ, Becker M et al (2019) Chromogranin B regulates early-stage insulin granule trafficking from the Golgi in pancreatic islet β-cells. J Cell Sci 132:jcs231373. https://doi.org/10.1242/jcs.231373
Berland CR, Thurston GM, Kondo M et al (1992) Solid-liquid phase boundaries of lens protein solutions. Proc Natl Acad Sci U S A 89:1214–1218. https://doi.org/10.1073/pnas.89.4.1214
Beutel O, Maraspini R, Pombo-Garcia K et al (2019) Phase separation of zonula occludens proteins drives formation of tight junctions. Cell 179:923–936. https://doi.org/10.1016/j.cell.2019.10.011
Babinchak WM, Haider R, Dumm BK et al (2019) The role of liquid–liquid phase separation in aggregation of the TDP-43 low-complexity domain. J Biol Chem 294:6306–6317. https://doi.org/10.1074/jbc.RA118.007222
Boeynaems S, Alberti S, Fawzi NL et al (2018) Protein phase separation: a new phase in cell biology. Trends Cell Biol 28:420–435. https://doi.org/10.1016/j.tcb.2018.02.004
Boisvert F-M, van Koningsbruggen S, Navascués J, Lamond AI (2007) The multifunctional nucleolus. Nat Rev Mol Cell Biol 8:574–585. https://doi.org/10.1038/nrm2184
Botterbusch S, Baumgart T (2021) Interactions between phase-separated liquids and membrane surfaces. Appl Sci 11:1288. https://doi.org/10.3390/app11031288
Brady JP, Farber PJ, Sekhar A et al (2017) Structural and hydrodynamic properties of an intrinsically disordered region of a germ cell-specific protein on phase separation. Proc Natl Acad Sci U S A 114:E8194–E8203. https://doi.org/10.1073/pnas.1706197114
Brangwynne CP, Eckmann CR, Courson DS et al (2009) Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324:1729–1732. https://doi.org/10.1126/science.1172046
Broide ML, Berland CR, Pande J et al (1991) Binary-liquid phase separation of lens protein solutions. Proc Natl Acad Sci U S A 88:5660–5664. https://doi.org/10.1073/pnas.88.13.5660
Bunnell SC, Singer AL, Hong DI et al (2006) Persistence of cooperatively stabilized signaling clusters drives T-cell activation. Mol Cell Biol 26:7155–7166. https://doi.org/10.1128/MCB.00507-06
Byington MC, Safari MS, Lubchenko V et al (2018) Weakly-bound dimers that underlie the crystal nucleation precursors in lysozyme solutions. bioRxiv:275222. https://doi.org/10.1101/275222
Cardinaux F, Gibaud T, Stradner A, Schurtenberger P (2007) Interplay between spinodal decomposition and glass formation in proteins exhibiting short-range attractions. Phys Rev Lett 99:118301. https://doi.org/10.1103/PhysRevLett.99.118301
Case LB, Ditlev JA, Rosen MK (2019) Regulation of transmembrane signaling by phase separation. Phys Rev Lett 48:465–494. https://doi.org/10.1146/annurev-biophys-052118-115534
Chan HY, Lubchenko V (2019) A mechanism for reversible mesoscopic aggregation in liquid solutions. Nat Commun 10:1–11. https://doi.org/10.1038/s41467-019-10270-5
Chen X, Wu X, Wu H, Zhang M (2020) Phase separation at the synapse. Nat Neurosci 23:301–310. https://doi.org/10.1038/s41593-019-0579-9
Cho W-K, Spille J-H, Hecht M et al (2018) Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. Science 361:412–415. https://doi.org/10.1126/science.aar4199
Choi J-M, Holehouse AS, Pappu RV (2020) Physical principles underlying the complex biology of intracellular phase transitions. Annu Rev Biophys 49:107–133. https://doi.org/10.1146/annurev-biophys-121219-081629
Chong PA, Forman-Kay JD (2016) Liquid–liquid phase separation in cellular signaling systems. Curr Opin Struct Biol 41:180–186. https://doi.org/10.1016/j.sbi.2016.08.001
Correll CC, Bartek J, Dundr M (2019) The nucleolus: a multiphase condensate balancing ribosome synthesis and translational capacity in health, aging and ribosomopathies. Cells 8:869. https://www.mdpi.com/2073-4409/8/8/869
Curtis RA, Newman J, Blanch HW, Prausnitz JM (2001) McMillan–Mayer solution thermodynamics for a protein in a mixed solvent. Fluid Phase Equilib 192:131–153. https://doi.org/10.1016/S0378-3812(01)00635-5
Dall’Agnese A, Platt JM, Zheng MM et al (2022) The dynamic clustering of insulin receptor underlies its signaling and is disrupted in insulin resistance. Nat Commun 13:7522. https://doi.org/10.1038/s41467-022-35176-7
Davis RB, Moosa MM, Banerjee PR (2022) Ectopic biomolecular phase transitions: fusion proteins in cancer pathologies. Trends Cell Biol 32:681–695. https://doi.org/10.1016/j.tcb.2022.03.005
Dignon GL, Best RB, Mittal J (2020) Biomolecular phase separation: from molecular driving forces to macroscopic properties. Annu Rev Phys Chem 71:53–75. https://doi.org/10.1146/annurev-physchem-071819-113553
Ditlev JA (2021) Membrane-associated phase separation: organization and function emerge from a two-dimensional milieu. J Mol Cell Biol 13:319–324. https://doi.org/10.1093/jmcb/mjab010
Dodson G, Steiner D (1998) The role of assembly in insulin’s biosynthesis. Curr Opin Struc Biol 8:189–194. https://doi.org/10.1016/S0959-440X(98)80037-7
Du M, Chen ZJ (2018) DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science 361:704–709. https://doi.org/10.1126/science.aat1022
Dumetz AC, Chockla AM, Kaler EW, Lenhoff AM (2008) Protein phase behavior in aqueous solutions: crystallization, liquid-liquid phase separation, gels, and aggregates. Biophys J 94:570–583. https://doi.org/10.1529/biophysj.107.116152
Etibor TA, Yamauchi Y, Amorim MJ (2021) Liquid biomolecular condensates and viral lifecycles: Review and perspectives. Viruses 13:366. https://www.mdpi.com/1999-4915/13/3/366
Feng Z, Jia B, Zhang M (2021) Liquid--liquid phase separation in biology: specific stoichiometric molecular interactions vs promiscuous interactions mediated by disordered sequences. Biochemistry 60:2397–2406. https://doi.org/10.1021/acs.biochem.1c00376
Flory PJ (1942) Thermodynamics of high polymer solutions. J Chem Phys 10:51–61. https://doi.org/10.1063/1.1723621
Folkmann AW, Putnam A, Lee CF, Seydoux G (2021) Regulation of biomolecular condensates by interfacial protein clusters. Science 373:1218–1224. https://doi.org/10.1126/science.abg7071
Fox AH, Nakagawa S, Hirose T, Bond CS (2018) Paraspeckles: where long noncoding RNA meets phase separation. Trends Biochem Sci 43:124–135. https://doi.org/10.1016/j.tibs.2017.12.001
Frey S, Görlich D (2007) A saturated FG-repeat hydrogel can reproduce the permeability properties of nuclear pore complexes. Cell 130:512–523. https://doi.org/10.1016/j.cell.2007.06.024
Galkin O, Pan W, Filobelo L et al (2007) Two-step mechanism of homogeneous nucleation of sickle cell hemoglobin polymers. Biophys J 93:902–913. https://doi.org/10.1529/biophysj.106.103705
Gao XK, Rao XS, Cong XX et al (2022) Phase separation of insulin receptor substrate 1 drives the formation of insulin/IGF-1 signalosomes. Cell Discov 8:1–19. https://doi.org/10.1038/s41421-022-00426-x
Georgalis Y, Umbach P, Saenger W et al (1999) Ordering of fractal clusters in crystallizing lysozyme solutions. J Am Chem Soc 121:1627–1635. https://doi.org/10.1021/ja982407y
Giannattasio G, Zanini A, Meldolesi J (1975) Molecular organization of rat prolactin granules: in vitro stability of intact and “ membraneless” granules. J Cell Biol 64:246–251. https://doi.org/10.1083/jcb.64.1.246
Gibson BA, Doolittle LK, Schneider MWG et al (2019) Organization of chromatin by intrinsic and regulated phase separation. Cell 179:470–484.e21. https://doi.org/10.1016/j.cell.2019.08.037
Gliko O, Neumaier N, Fischer M et al (2005) Dense liquid droplets as a step source for the crystallization of lumazine synthase. J Cryst Growth 275:e1409–e1416. https://doi.org/10.1016/j.jcrysgro.2004.11.291
Gliko O, Pan W, Katsonis P et al (2007) Metastable liquid clusters in super-and undersaturated protein solutions. J Phys Chem B 111:3106–3114. https://doi.org/10.1021/jp068827o
Goetz SK, Mahamid J (2020) Visualizing molecular architectures of cellular condensates: hints of complex coacervation scenarios. Dev Cell 55:97–107. https://doi.org/10.1016/j.devcel.2020.09.003
Grouazel S, Bonnete F, Astier J-P et al (2006) Exploring bovine pancreatic trypsin inhibitor phase transitions. J Phys Chem B 110:19664–19670. https://doi.org/10.1021/jp0627123
Haas C, Drenth J (1999) Understanding protein crystallization on the basis of the phase diagram. J Cryst Growth 196:388–394. https://doi.org/10.1016/S0022-0248(98)00831-8
Hardenberg M, Horvath A, Ambrus V et al (2020) Widespread occurrence of the droplet state of proteins in the human proteome. Proc Natl Acad Sci U S A 117:33254–33262. https://doi.org/10.1073/pnas.2007670117
Holehouse AS (2018) Pappu RV (2018a) Collapse transitions of proteins and the interplay among backbone, sidechain, and solvent interactions. Annu Rev Biophys 47:19–39. https://doi.org/10.1146/annurev-biophys-070317-032838
Holehouse AS, Pappu RV (2018) Functional implications of intracellular phase transitions. Biochemistry 57:2415–2423. https://doi.org/10.1021/acs.biochem.7b01136
Hondele M, Heinrich S, De Los RP, Weis K (2020) Membraneless organelles: phasing out of equilibrium. Emerging Topics in Life Sciences 4:343–354. https://doi.org/10.1042/ETLS20190190
Horvath A, Miskei M, Ambrus V et al (2020) Sequence-based prediction of protein binding mode landscapes. PLoS Comput Biol 16:e1007864. https://doi.org/10.1371/journal.pcbi.1007864
Houben L, Weissman H, Wolf SG, Rybtchinski B (2020) A mechanism of ferritin crystallization revealed by cryo-STEM tomography. Nature 579:540–543. https://doi.org/10.1038/s41586-020-2104-4
Huggins ML (1941) Solutions of long chain compounds. J Chem Phys 9:440. https://doi.org/10.1063/1.1750930
Hyman AA, Weber CA, Jülicher F (2014) Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol 30:39–58. https://doi.org/10.1146/annurev-cellbio-100913-013325
Iserman C, Roden CA, Boerneke MA et al (2020) Genomic RNA elements drive phase separation of the SARS-CoV-2 nucleocapsid. Mol Cell 80:1078–1091. https://doi.org/10.1016/j.molcel.2020.11.041
Jonassen I, Havelund S, Hoeg-Jensen T et al (2012) Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res 29:2104–2114. https://doi.org/10.1007/s11095-012-0739-z
Kaissaratos M, Filobelo L, Vekilov PG (2021) Two-step crystal nucleation is selected because of a lower surface free energy barrier. Cryst Growth Des 21:5394–5402. https://doi.org/10.1021/acs.cgd.1c00662
Kar M, Dar F, Welsh TJ et al (2022) Phase-separating RNA-binding proteins form heterogeneous distributions of clusters in subsaturated solutions. Proc Natl Acad Sci U S A 119:e2202222119. https://doi.org/10.1073/pnas.2202222119
Karmakar S, Ghosh T, Sankhla A et al (2022) Insulin biomolecular condensate formed in ionic microenvironment modulates the structural properties of pristine and magnetic cellulosic nanomaterials. J Mol Liq 363:119580. https://doi.org/10.1016/j.molliq.2022.119580
Kashchiev D, Vekilov PG, Kolomeisky AB (2005) Kinetics of two-step nucleation of crystals. J Chem Phys 122:244706. https://doi.org/10.1063/1.1943389
Keber FC, Nguyen T, Brangwynne CP, Wühr M (2021) Evidence for widespread cytoplasmic structuring into mesoscopic condensates. bioRxiv 17:473234. https://doi.org/10.1101/2021.12.17.473234
Kelley FM, Favetta B, Regy RM et al (2021) Amphiphilic proteins coassemble into multiphasic condensates and act as biomolecular surfactants. Proc Natl Acad Sci U S A 118:e2109967118. https://doi.org/10.1073/pnas.2109967118
Kienzle C, von Blume J (2014) Secretory cargo sorting at the trans-Golgi network. Trends Cell Biol 24:584–593. https://doi.org/10.1016/j.tcb.2014.04.007
Klaips CL, Jayaraj GG, Hartl FU (2018) Pathways of cellular proteostasis in aging and disease. J Cell Biol 217:51–63. https://doi.org/10.1083/jcb.201709072
Knee KM, Mukerji I (2009) Real time monitoring of sickle cell hemoglobin fiber formation by UV resonance Raman spectroscopy. Biochemistry 48:9903–9911. https://doi.org/10.1021/bi901352m
Kundra R, Ciryam P, Morimoto RI et al (2017) Protein homeostasis of a metastable subproteome associated with Alzheimer’s disease. Proc Natl Acad Sci U S A 114:E5703–E5711. https://doi.org/10.1073/pnas.1618417114
Lagier-Tourenne C, Polymenidou M, Cleveland DW (2010) TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration. Hum Mol Genet 19:R46–R64. https://doi.org/10.1093/hmg/ddq137
Landreh M, Alvelius G, Willander H et al (2012) Insulin solubility transitions by pH-dependent interactions with proinsulin C-peptide. FEBS J 279:4589–4597. https://doi.org/10.1111/febs.12045
Latonen L (2019) Phase-to-phase with nucleoli--stress responses, protein aggregation and novel roles of RNA. Front Cell Neurosci 13:151. https://doi.org/10.3389/fncel.2019.00151
Lawrence MC, Colman PM (1993) Shape complementarity at protein/protein interfaces. J Mol Biol 234:946–950. https://doi.org/10.1006/jmbi.1993.1648
Lerman S, Zigman S, Forbes WF (1966) Properties of a cryoprotein in the ocular lens. Biochem Biophys Res Commun 22:57–61. https://doi.org/10.1016/0006-291X(66)90602-4
Levine AJ (2019) Targeting therapies for the p53 protein in cancer treatments. Annu Rev Cancer Biol 3:21–34. https://doi.org/10.1146/annurev-cancerbio-030518-055455
Li L, Srivastava S, Andreev M et al (2018) Phase behavior and salt partitioning in polyelectrolyte complex coacervates. Macromolecules 51:2988–2995. https://doi.org/10.1021/acs.macromol.8b00238
Li P, Banjade S, Cheng H-C et al (2012a) Phase transitions in the assembly of multivalent signalling proteins. Nature 483:336–340. https://doi.org/10.1038/nature10879
Li Y, Lubchenko V, Vekilov PG (2011) The use of dynamic light scattering and Brownian microscopy to characterize protein aggregation. Rev Sci Instrum 82:53106. https://doi.org/10.1063/1.3592581
Li Y, Lubchenko V, Vorontsova MA et al (2012b) Ostwald-like ripening of the anomalous mesoscopic clusters in protein solutions. J Phys Chem B 116:10657–10664. https://doi.org/10.1021/jp303316s
Lin C-W, Nocka LM, Stinger BL et al (2022) A two-component protein condensate of the EGFR cytoplasmic tail and Grb2 regulates Ras activation by SOS at the membrane. Proc Natl Acad Sci U S A 119:e2122531119. https://doi.org/10.1073/pnas.2122531119
Lyon AS, Peeples WB, Rosen MK (2021) A framework for understanding the functions of biomolecular condensates across scales. Nat Rev Mol Cell Biol 22:215–235. https://doi.org/10.1038/s41580-020-00303-z
Machyna M, Heyn P, Neugebauer KM (2013) Cajal bodies: where form meets function. WIREs RNA 4:17–34. https://doi.org/10.1002/wrna.1139
Maes D, Vorontsova MA, Potenza M et al (2015) Do protein crystals nucleate within dense liquid clusters? Acta Crystallogr Sect F 71:815–822. https://doi.org/10.1107/S2053230X15008997
Maji SK, Perrin MH, Sawaya MR et al (2009) Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science 325:328–332. https://doi.org/10.1126/science.1173155
Malay AD, Suzuki T, Katashima T et al (2020) Spider silk self-assembly via modular liquid-liquid phase separation and nanofibrillation. Sci Adv 6:eabb6030. https://doi.org/10.1126/sciadv.abb6030
Manoharan VN (2015) Colloidal matter: Packing, geometry, and entropy. Science 349:1253751. https://doi.org/10.1126/science.1253751
Martin EW, Harmon TS, Hopkins JB et al (2021) A multi-step nucleation process determines the kinetics of prion-like domain phase separation. Nat Commun 12:4513. https://doi.org/10.1038/s41467-021-24727-z
Martin EW, Mittag T (2018) Relationship of sequence and phase separation in protein low-complexity regions. Biochemistry 57:2478–2487. https://doi.org/10.1021/acs.biochem.8b00008
Mathieu C, Pappu RV, Taylor JP (2020) Beyond aggregation: pathological phase transitions in neurodegenerative disease. Science 370:56–60. https://doi.org/10.1126/science.abb8032
Matthews BW (1968) Solvent content of protein crystals. J Mol Biol 33:491–497. https://doi.org/10.1016/0022-2836(68)90205-2
McAlary L, Chew YL, Lum JS et al (2020) Amyotrophic lateral sclerosis: proteins, proteostasis, prions, and promises. Front Cell Neurosci 14. https://doi.org/10.3389/fncel.2020.581907
McManus JJ, Charbonneau P, Zaccarelli E, Asherie N (2016) The physics of protein self-assembly. Curr Opin Colloid Interface 22:73–79. https://doi.org/10.1016/j.cocis.2016.02.011
Michael J, Carroll R, Swift HH, Steiner DF (1987) Studies on the molecular organization of rat insulin secretory granules. J Biol Chem 262:16531–16535. https://doi.org/10.1016/S0021-9258(18)49288-5
Milovanovic D, De Camilli P (2017) Synaptic vesicle clusters at synapses: a distinct liquid phase? Neuron 93:995–1002. https://doi.org/10.1016/j.neuron.2017.02.013
Milovanovic D, Wu Y, Bian X, De Camilli P (2018) A liquid phase of synapsin and lipid vesicles. Science 361:604–607. https://doi.org/10.1016/j.neuron.2017.02.013
Miskei M, Horvath A, Vendruscolo M, Fuxreiter M (2020) Sequence-based prediction of fuzzy protein interactions. J Mol Biol 432:2289–2303. https://doi.org/10.1016/j.jmb.2020.02.017
Mitchison TJ (2020) Beyond Langmuir: surface-bound macromolecule condensates. MBoC 31:2502–2508. https://doi.org/10.1091/mbc.E20-06-0393
Mohanty P, Kapoor U, Sundaravadivelu Devarajan D et al (2022) Principles governing the phase separation of multidomain proteins. Biochemistry 61:2443–2455. https://doi.org/10.1021/acs.biochem.2c00210
Molliex A, Temirov J, Lee J et al (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell 163:123–133. https://doi.org/10.1016/j.cell.2015.09.015
Mudogo CN, Falke S, Brognaro H et al (2020) Protein phase separation and determinants of in cell crystallization. Traffic 21:220–230. https://doi.org/10.1111/tra.12711
Muiznieks LD, Sharpe S, Pomès R, Keeley FW (2018) Role of liquid–liquid phase separation in assembly of elastin and other extracellular matrix proteins. J Mol Biol 430:4741–4753. https://doi.org/10.1016/j.jmb.2018.06.010
Mukherjee A, Sudrik C, Hu Y et al (2020) CL6mN: rationally designed optogenetic photoswitches with tunable dissociation dynamics. ACS Synth Biol 9:2274–2281. https://doi.org/10.1021/acssynbio.0c00362
Muschol M, Rosenberger F (1997) Liquid–liquid phase separation in supersaturated lysozyme solutions and associated precipitate formation/crystallization. J Chem Phys 107:1953–1962. https://doi.org/10.1063/1.474547
Nassar R, Dignon GL, Razban RM, Dill KA (2021) The protein folding problem: the role of theory. J Mol Biol 433:167126. https://doi.org/10.1016/j.jmb.2021.167126
Nielsen L, Khurana R, Coats A et al (2001) Effect of environmental factors on the kinetics of insulin fibril formation: elucidation of the molecular mechanism. Biochemistry 40:6036–6046. https://doi.org/10.1021/bi002555c
Nikfarjam S, Ghorbani M, Adhikari S et al (2019) Irreversible nature of mesoscopic aggregates in lysozyme solutions. Colloid J 81:546–554. https://doi.org/10.1134/S1061933X19050090
Nilsson MR (2016) Insulin amyloid at injection sites of patients with diabetes. Amyloid 23:139–147. https://doi.org/10.1080/13506129.2016.1179183
Noji M, Samejima T, Yamaguchi K et al (2021) Breakdown of supersaturation barrier links protein folding to amyloid formation. Commun Biol 4:1–10. https://doi.org/10.1038/s42003-020-01641-6
Nusse R, Clevers H (2017) Wnt β–catenin signaling, disease, and emerging therapeutic modalities. Cell 169:985–999. https://doi.org/10.1016/j.cell.2017.05.016
Oxtoby DW (1992) Homogeneous nucleation: theory and experiment. J Phys Condens Matter 4:7627. https://doi.org/10.1088/0953-8984/4/38/001
Pan W, Galkin O, Filobelo L et al (2007) Metastable mesoscopic clusters in solutions of sickle-cell hemoglobin. Biophys J 92:267–277. https://doi.org/10.1529/biophysj.106.094854
Pan W, Vekilov PG, Lubchenko V (2010) Origin of anomalous mesoscopic phases in protein solutions. J Phys Chem B 114:7620–7630. https://doi.org/10.1021/jp100617w
Parchure A, Tian M, Stalder D et al (2022) Liquid–liquid phase separation facilitates the biogenesis of secretory storage granules. J Cell Biol 221:e202206132. https://doi.org/10.1083/jcb.202206132
Parry TL, Melehani JH, Ranek MJ, Willis MS (2015) Functional amyloid signaling via the inflammasome, necrosome, and signalosome: new therapeutic targets in heart failure. Front Cardiovasc Med 2:25. https://doi.org/10.3389/fcvm.2015.00025
Patel A, Lee HO, Jawerth L et al (2015) A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162:1066–1077. https://doi.org/10.1016/j.cell.2015.07.047
Pattanayak GK, Liao Y, Wallace EWJ et al (2020) Daily cycles of reversible protein condensation in cyanobacteria. Cell Rep 32:108032. https://doi.org/10.1016/j.celrep.2020.108032
Pauly T, Zhang T, Sternke-Hoffmann R et al (2023) Differentiation of subnucleus-sized oligomers and nucleation-competent assemblies of the A β peptide. Biophys J 122:269–278. https://doi.org/10.1016/j.bpj.2022.12.020
Pedrote MM, Motta MF, Ferretti GDS et al (2020) Oncogenic gain of function in glioblastoma is linked to mutant p53 amyloid oligomers. Iscience 23:100820. https://doi.org/10.1016/j.isci.2020.100820
Pekar AH, Frank BH (1972) Conformation of proinsulin. Comparison of insulin and proinsulin self-association at neutral pH. Biochemistry 11:4013–4016. https://doi.org/10.1021/bi00772a001
Peran I, Holehouse AS, Carrico IS et al (2019) Unfolded states under folding conditions accommodate sequence-specific conformational preferences with random coil-like dimensions. Proc Natl Acad Sci U S A 116:12301–12310. https://doi.org/10.1073/pnas.1818206116
Petronilho EC, Pedrote MM, Marques MA et al (2021) Phase separation of p53 precedes aggregation and is affected by oncogenic mutations and ligands. Chem Sci 12:7334–7349. https://doi.org/10.1039/D1SC01739J
Posey AE, Ruff KM, Harmon TS et al (2018) Profilin reduces aggregation and phase separation of huntingtin N-terminal fragments by preferentially binding to soluble monomers and oligomers. J Biol Chem 293:3734–3746. https://doi.org/10.1074/jbc.RA117.000357
Poudyal M, Patel K, Sawner AS et al (2022) Liquid condensate is a common state of proteins and polypeptides at the regime of high intermolecular interactions. bioRxiv. https://doi.org/10.1101/2021.12.31.474648
Protter DSW, Parker R (2016) Principles and properties of stress granules. Trends Cell Biol 26:668–679. https://doi.org/10.1016/j.tcb.2016.05.004
Ray S, Singh N, Kumar R et al (2020) α-Synuclein aggregation nucleates through liquid–liquid phase separation. Nat Chem 12:705–716. https://doi.org/10.1038/s41557-020-0465-9
Ray S, Mason TO, Boyens-Thiele L et al (2023) Mass photometric detection and quantification of nanoscale α-synuclein phase separation. Nat Chem 1–11. https://doi.org/10.1038/s41557-023-01244-8
Rey T, Zaganelli S, Cuillery E et al (2020) Mitochondrial RNA granules are fluid condensates positioned by membrane dynamics. Nat Cell Biol 22:1180–1186. https://doi.org/10.1038/s41556-020-00584-8
Riback JA, Zhu L, Ferrolino MC et al (2020) Composition-dependent thermodynamics of intracellular phase separation. Nature 581:209–214. https://doi.org/10.1038/s41586-020-2256-2
Roden C, Gladfelter AS (2021) RNA contributions to the form and function of biomolecular condensates. Nat Rev Mol Cell Biol 22:183–195. https://doi.org/10.1038/s41580-020-0264-6
Rohli KE, Boyer CK, Blom SE, Stephens SB (2022) Nutrient regulation of pancreatic islet β-cell secretory capacity and insulin production. Biomolecules 12:335. https://doi.org/10.3390/biom12020335
Rouaud F, Sluysmans S, Flinois A et al (2020) Scaffolding proteins of vertebrate apical junctions: structure, functions and biophysics. Biochim Biophys Acta Biomembr 1862:183399. https://doi.org/10.1016/j.bbamem.2020.183399
Ruff KM, Roberts S, Chilkoti A, Pappu RV (2018) Advances in understanding stimulus-responsive phase behavior of intrinsically disordered protein polymers. J Mol Biol 430:4619–4635. https://doi.org/10.1016/j.jmb.2018.06.031
Sabari BR, Dall’Agnese A, Boija A et al (2018) Coactivator condensation at super-enhancers links phase separation and gene control. Science 361:eaar3958. https://doi.org/10.1126/science.aar3958
Safari MS, Byington MC, Conrad JC, Vekilov PG (2017) Polymorphism of lysozyme condensates. J Phys Chem B 121:9091–9101. https://doi.org/10.1021/acs.jpcb.7b05425
Safari MS, Vorontsova MA, Poling-Skutvik R et al (2015) Differential dynamic microscopy of weakly scattering and polydisperse protein-rich clusters. Phys Rev E 92:42712. https://doi.org/10.1103/PhysRevE.92.042712
Safari MS, Wang Z, Tailor K et al (2019) Anomalous dense liquid condensates host the nucleation of tumor suppressor p53 fibrils. Iscience 12:342–355. https://doi.org/10.1016/j.isci.2019.01.027
Sansevrino R, Hoffmann C, Milovanovic D (2023) Condensate biology of synaptic vesicle clusters. Trends Neurosci 46:293–306. https://doi.org/10.1016/j.tins.2023.01.001
Sawaya MR, Hughes MP, Rodriguez JA et al (2021) The expanding amyloid family: Structure, stability, function, and pathogenesis. Cell 184:4857–4873. https://doi.org/10.1016/j.cell.2021.08.013
Sawyer IA, Sturgill D, Dundr M (2019) Membraneless nuclear organelles and the search for phases within phases. WIREs RNA 10:e1514. https://doi.org/10.1002/wrna.1514
Schaefer KN, Peifer M (2019) Wnt/Beta-catenin signaling regulation and a role for biomolecular condensates. Dev Cell 48:429–444. https://doi.org/10.1016/j.devcel.2019.01.025
Schmidt HB, Görlich D (2016) Transport selectivity of nuclear pores, phase separation, and membraneless organelles. Trends Biochem Sci 41:46–61. https://doi.org/10.1016/j.tibs.2015.11.001
Schubert R, Meyer A, Baitan D et al (2017) Real-time observation of protein dense liquid cluster evolution during nucleation in protein crystallization. Cryst Growth Des 17:954–958. https://doi.org/10.1021/acs.cgd.6b01826
Schwayer C, Shamipour S, Pranjic-Ferscha K et al (2019) Mechanosensation of tight junctions depends on ZO-1 phase separation and flow. Cell 179:937–952. https://doi.org/10.1016/j.cell.2019.10.006
Seviour T, Wong LL, Lu Y et al (2020) Phase transitions by an abundant protein in the anammox extracellular matrix mediate cell-to-cell aggregation and biofilm formation. MBio 11:e02052–e02020. https://doi.org/10.1128/mbio.02052-20
Shapiro DM, Ney M, Eghtesadi SA, Chilkoti A (2021) Protein phase separation arising from intrinsic disorder: first-principles to bespoke applications. J Phys Chem B 125:6740–6759. https://doi.org/10.1021/acs.jpcb.1c01146
Shin Y, Brangwynne CP (2017) Liquid phase condensation in cell physiology and disease. Science 357:eaaf4382. https://doi.org/10.1126/science.aaf4382
Siezen RJ, Fisch MR, Slingsby C, Benedek GB (1985) Opacification of gamma-crystallin solutions from calf lens in relation to cold cataract formation. Proc Natl Acad Sci U S A 82:1701–1705. https://doi.org/10.1073/pnas.82.6.1701
Silva-Jr H, Araújo TS, da Silva AM et al (2022) Formation of subvisible particles in commercial insulin formulations. Colloids Surf B: Biointerfaces 216:112566. https://doi.org/10.1016/j.colsurfb.2022.112566
Sleutel M, Van Driessche AES (2014) Role of clusters in nonclassical nucleation and growth of protein crystals. Proc Natl Acad Sci USA 111:E546–E553. https://doi.org/10.1073/pnas.1309320111
Snead WT, Gladfelter AS (2019) The control centers of biomolecular phase separation: how membrane surfaces, post-translational modifications, and active processes regulate condensation. Mol Cell 76:295. https://doi.org/10.1016/j.molcel.2019.09.016
So M, Hall D, Goto Y (2016) Revisiting supersaturation as a factor determining amyloid fibrillation. Curr Opin Struct Biol 36:32–39. https://doi.org/10.1016/j.sbi.2015.11.009
Soranno A (2020) Physical basis of the disorder-order transition. Arch Biochem Biophys 685:108305. https://doi.org/10.1016/j.abb.2020.108305
Sosa L, Torkko JM, Primo ME et al (2016) Biochemical, biophysical, and functional properties of ICA512/IA-2 RESP18 homology domain. Biochim Biophys Acta, Proteins Proteomics 1864:511–522. https://doi.org/10.1016/j.bbapap.2016.01.013
Spruijt E, Westphal AH, Borst JW et al (2010) Binodal compositions of polyelectrolyte complexes. Macromolecules 43:6476–6484. https://doi.org/10.1021/ma101031t
Su X, Ditlev JA, Hui E et al (2016) Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352:595–599. https://doi.org/10.1126/science.aad9964
Taniue K, Akimitsu N (2022) Aberrant phase separation and cancer. FEBS J 289:17–39. https://doi.org/10.1111/febs.15765
Thomson JA, Schurtenberger P, Thurston GM, Benedek GB (1987) Binary liquid phase separation and critical phenomena in a protein/water solution. Proc Natl Acad Sci U S A 84:7079–7083. https://doi.org/10.1073/pnas.84.20.7079
Tiwary AK, Zheng Y (2019) Protein phase separation in mitosis. Curr Opin Cell Biol 60:92–98. https://doi.org/10.1016/j.ceb.2019.04.011
Toledo PL, Torkko JM, Müller A et al (2019) ICA512 RESP18 homology domain is a protein-condensing factor and insulin fibrillation inhibitor. J Biol Chem 294:8564–8576. https://doi.org/10.1074/jbc.RA119.007607
Toledo PL, Vazquez DS, Gianotti AR et al (2023) Condensation of the β-cell secretory granule luminal cargoes pro/insulin and ICA512 RESP18 homology domain. Protein Sci 32:e4649. https://doi.org/10.1002/pro.4649
Turoverov KK, Kuznetsova IM, Fonin AV et al (2019) Stochasticity of biological soft matter: emerging concepts in intrinsically disordered proteins and biological phase separation. Trends Biochem Sci 44:716–728. https://doi.org/10.1016/j.tibs.2019.03.005
Updike DL, Hachey SJ, Kreher J, Strome S (2011) P granules extend the nuclear pore complex environment in the C. elegans germ line. J Cell Biol 192:939–948. https://doi.org/10.1083/jcb.201010104
Urosev I, Lopez Morales J, Nash MA (2020) Phase separation of intrinsically disordered protein polymers mechanically stiffens fibrin clots. Adv Funct Mater 30:2005245. https://doi.org/10.1002/adfm.202005245
Uversky VN (2017) Protein intrinsic disorder-based liquid--liquid phase transitions in biological systems: complex coacervates and membrane-less organelles. Adv Colloid Interf Sci 239:97–114. https://doi.org/10.1016/j.cis.2016.05.012
Van Der Lee R, Buljan M, Lang B et al (2014) Classification of intrinsically disordered regions and proteins. Chem Rev 114:6589–6631. https://doi.org/10.1021/cr400525m
Van Driessche AES, Ling WL, Schoehn G, Sleutel M (2022) Nucleation of glucose isomerase protein crystals in a nonclassical disguise: the role of crystalline precursors. Proc Natl Acad Sci U S A 119:e2108674119. https://doi.org/10.1073/pnas.2108674119
Van Driessche AES, Van Gerven N, Bomans PHH et al (2018) Molecular nucleation mechanisms and control strategies for crystal polymorph selection. Nature 556:89–94. https://doi.org/10.1038/nature25971
Vazquez DS, Toledo PL, Gianotti AR, Ermácora MR (2022) Protein conformation and biomolecular condensates. Curr Res Struc Biol 4:285–307. https://doi.org/10.1016/j.crstbi.2022.09.004
Vecchi G, Sormanni P, Mannini B et al (2020) Proteome-wide observation of the phenomenon of life on the edge of solubility. Proc Natl Acad Sci U S A 117:1015–1020. https://doi.org/10.1073/pnas.1910444117
Vekilov PG (2004) Dense liquid precursor for the nucleation of ordered solid phases from solution. Cryst Growth Des 4:671–685. https://doi.org/10.1021/cg049977w
Vekilov PG (2016) Nucleation of protein crystals. Prog Cryst Growth Charact Mater 62:136–154. https://doi.org/10.1016/j.pcrysgrow.2016.04.007
Vekilov PG (2010) The two-step mechanism of nucleation of crystals in solution. Nanoscale 2:2346–2357. https://doi.org/10.1039/C0NR00628A
Vernon RM, Forman-Kay JD (2019) First-generation predictors of biological protein phase separation. Curr Opin Struct Biol 58:88–96. https://doi.org/10.1016/j.sbi.2019.05.016
Vorontsova MA, Chan HY, Lubchenko V, Vekilov PG (2015a) Lack of dependence of the sizes of the mesoscopic protein clusters on electrostatics. Biophys J 109:1959–1968. https://doi.org/10.1016/j.bpj.2015.09.025
Vorontsova MA, Maes D, Vekilov PG (2015b) Recent advances in the understanding of two-step nucleation of protein crystals. Faraday Discuss 179:27–40. https://doi.org/10.1039/C4FD00217B
Vorontsova MA, Vekilov PG, Maes D (2016) Characterization of the diffusive dynamics of particles with time-dependent asymmetric microscopy intensity profiles. Soft Matter 12:6926–6936. https://doi.org/10.1039/C6SM00946H
Walker AA, Holland C, Sutherland TD (2015) More than one way to spin a crystallite: multiple trajectories through liquid crystallinity to solid silk. Proc R Soc B 282:20150259. https://doi.org/10.1098/rspb.2015.0259
Walker FO (2007) Huntington’s disease. Lancet 369:218–228. https://doi.org/10.1016/S0140-6736(07)60111-1
Wang J, Choi J-M, Holehouse AS et al (2018) A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 174:688–699. https://doi.org/10.1016/j.cell.2018.06.006
Wang M, Barra ALC, Brognaro H, Betzel C (2022) Exploring nucleation pathways in distinct physicochemical environments unveiling novel options to modulate and optimize protein crystallization. Crystals 12:437. https://doi.org/10.3390/cryst12030437
Wei M-T, Elbaum-Garfinkle S, Holehouse AS et al (2017) Phase behaviour of disordered proteins underlying low density and high permeability of liquid organelles. Nat Chem 9:1118–1125. https://doi.org/10.1038/nchem.2803
Wilkaniec A, Czapski GA, Adamczyk A (2016) Cdk5 at crossroads of protein oligomerization in neurodegenerative diseases: facts and hypotheses. J Neurochem 136:222–233. https://doi.org/10.1111/jnc.13365
Wolf M, Roosen-Runge F, Zhang F et al (2014) Effective interactions in protein--salt solutions approaching liquid--liquid phase separation. J Mol Liq 200:20–27. https://doi.org/10.1016/j.molliq.2014.08.006
Woodruff JB, Hyman AA, Boke E (2018) Organization and function of non-dynamic biomolecular condensates. Trends Biochem Sci 43:81–94. https://doi.org/10.1016/j.tibs.2017.11.005
Wu H (2013) Higher-order assemblies in a new paradigm of signal transduction. Cell 153:287–292. https://doi.org/10.1016/j.cell.2013.03.013
Wu X, Qiu H, Zhang M (2022a) Interactions between membraneless condensates and membranous organelles at the presynapse: a phase separation view of synaptic vesicle cycle. J Mol Biol 435:167629. https://doi.org/10.1016/j.jmb.2022.167629
Wu C, Holehouse AS, Leung DW et al (2022b) Liquid phase partitioning in virus replication: observations and opportunities. Ann Rev Virol 9:285–306. https://doi.org/10.1146/annurev-virology-093020-013659
Wunderlich B (1999) A classification of molecules, phases, and transitions as recognized by thermal analysis. Thermochim Acta 340:37–52. https://doi.org/10.1016/S0040-6031(99)00252-X
Xu S, Zhang H, Qiao B, Wang Y (2021) Review of liquid--liquid phase separation in crystallization: from fundamentals to application. Cryst Growth Des 21:7306–7325. https://doi.org/10.1021/acs.cgd.0c01376
Xu Y, Yan Y, Seeman D et al (2012) Multimerization and aggregation of native-state insulin: effect of zinc. Langmuir 28:579–586.https://doi.org/10.1021/la202902a
Yadav K, Yadav A, Vashistha P et al (2019) Protein misfolding diseases and therapeutic approaches. Curr Protein Pept Sci 20:1226–1245. https://doi.org/10.2174/1389203720666190610092840
Yamazaki T, Kimura Y, Vekilov PG et al (2017) Two types of amorphous protein particles facilitate crystal nucleation. Proc Natl Acad Sci U S A 114:2154–2159. https://doi.org/10.1073/pnas.1606948114
Yanagisawa H, Davis EC (2010) Unraveling the mechanism of elastic fiber assembly: the roles of short fibulins. Int J Biochem 42:1084–1093. https://doi.org/10.1016/j.biocel.2010.03.009
Yang DS, Saeedi A, Davtyan A et al (2021) Mesoscopic protein-rich clusters host the nucleation of mutant p53 amyloid fibrils. Proc Natl Acad Sci U S A 118:e2015618118. https://doi.org/10.1073/pnas.2015618118
Yoshizawa T, Nozawa R-S, Jia TZ et al (2020) Biological phase separation: cell biology meets biophysics. Biophys Rev 12:519–539. https://doi.org/10.1007/s12551-020-00680-x
Zhang F (2017) Nonclassical nucleation pathways in protein crystallization. J Phys Condens Matter 29:443002. https://doi.org/10.1088/1361-648X/aa8253
Zhao H, Wu D, Nguyen A et al (2021) Energetic and structural features of SARS-CoV-2 N-protein co-assemblies with nucleic acids. iScience 24:102523. https://doi.org/10.1016/j.isci.2021.102523
Zhao YG, Zhang H (2020) Phase separation in membrane biology: the interplay between membrane-bound organelles and membraneless condensates. Dev Cell 55:30–44. https://doi.org/10.1016/j.devcel.2020.06.033
Zihni C, Mills C, Matter K, Balda MS (2016) Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol 17:564–580. https://doi.org/10.1038/nrm.2016.80
Acknowledgements
We thank Professor Michele Solimena for stimulating discussions on protein condensation in secretory granules.
Funding
This work was supported by grants from Universidad Nacional de Quilmes (PUNQ2022 2272), Consejo Nacional de Investigaciones Científicas y Técnicas (PIP2021 1054), and Agencia Nacional de Promoción Científica y Tecnológica (PICT2016 0584), Argentina.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toledo, P.L., Gianotti, A.R., Vazquez, D.S. et al. Protein nanocondensates: the next frontier. Biophys Rev 15, 515–530 (2023). https://doi.org/10.1007/s12551-023-01105-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-023-01105-1