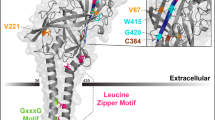
Abstract
The low-density lipoprotein receptor (LDLR) and its homologs capture and internalize lipoproteins into the cell. Due to the fact that LDLR family members possess a modular ectodomain that undergoes dynamic conformational changes, multi-scale structural analysis has been performed so as to understand the ligand capture and release mechanism. For example, crystallographic analyses have provided models for both the entire ectodomain and high-resolution structures of individual modules. In addition, nuclear magnetic resonance spectroscopic analyses have shown the rigidity and flexibility of inter-module linkers to restrict the mobility of ectodomain. Accumulated structural data suggest that the ectodomains of LDLR family members are flexible at the cell surface and switch between two metastable conformations, that is, the extended and contracted conformations. Recent structural analysis of ApoER2, a close homolog of LDLR, raised the possibility that the receptor binds with the ligand in the contracted conformation. After transport to an endosome by endocytosis, the receptor undergoes a conformational change to the closed conformation for completion of ligand release. In contrast, LDLR has been reported to adopt the extended conformation when it binds with a inhibitory regulator that recruits LDLR toward the degradation pathway. These findings support a mechanism of different ectodomain conformations for binding the ligand versus binding the regulatory protein. In this review, I provide an overview of studies that analyze the structural and biophysical properties of the ectodomains of LDLR family members and discuss a hypothetical model for ligand uptake and receptor recycling that integrates the known ectodomain conformational variability.








Similar content being viewed by others
References
Abifadel M, Varret M, Rabès JP et al (2003) Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 34:154–156. https://doi.org/10.1038/ng1161
Beglova N, Jeon H, Fisher C, Blacklow SC (2004) Cooperation between fixed and low pH-inducible interfaces controls lipoprotein release by the LDL receptor. Mol Cell 16:281–292. https://doi.org/10.1016/j.molcel.2004.09.038
Bottomley MJ, Cirillo A, Orsatti L et al (2009) Structural and biochemical characterization of the wild type PCSK9-EGF(AB) complex and natural familial hypercholesterolemia mutants. J Biol Chem 284:1313–1323. https://doi.org/10.1074/jbc.M808363200
Brown MS, Goldstein JL (1974) Expression of the familial hypercholesterolemia gene in heterozygotes: mechanism for a dominant disorder in man. Science 185:61–63
Brown MS, Goldstein JL (1986) A receptor-mediated pathway for cholesterol homeostasis. Science 232:34–47
Chen S, Bubeck D, MacDonald BT et al (2011) Structural and functional studies of LRP6 ectodomain reveal a platform for Wnt signaling. Dev Cell 21:848–861. https://doi.org/10.1016/j.devcel.2011.09.007
Cheng Z, Biechele T, Wei Z et al (2011) Crystal structures of the extracellular domain of LRP6 and its complex with DKK1. Nat Struct Mol Biol 18:1204–1210. https://doi.org/10.1038/nsmb.2139
Daly NL, Scanlon MJ, Djordjevic JT, Kroon PA, Smith R (1995) Three-dimensional structure of a cysteine-rich repeat from the low-density lipoprotein receptor. Proc Natl Acad Sci USA 92:6334–6338
D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T (1995) A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature 374:719–723. https://doi.org/10.1038/374719a0
Davis CG, Goldstein JL, Sudhof TC, Anderson RG, Russell DW, Brown MS (1987) Acid-dependent ligand dissociation and recycling of LDL receptor mediated by growth factor homology region. Nature 326:760–765. https://doi.org/10.1038/326760a0
Day IN, Whittall RA, O’Dell SD et al (1997) Spectrum of LDL receptor gene mutations in heterozygous familial hypercholesterolemia. Hum Mutat 10:116–127. https://doi.org/10.1002/(SICI)1098-1004(1997)10:2<116::AID-HUMU4>3.0.CO;2-I
Fass D, Blacklow S, Kim PS, Berger JM (1997) Molecular basis of familial hypercholesterolaemia from structure of LDL receptor module. Nature 388:691–693. https://doi.org/10.1038/41798
Fisher C, Beglova N, Blacklow SC (2006) Structure of an LDLR-RAP complex reveals a general mode for ligand recognition by lipoprotein receptors. Mol Cell 22:277–283. https://doi.org/10.1016/j.molcel.2006.02.021
Handford PA, Mayhew M, Baron M, Winship PR, Campbell ID, Brownlee GG (1991) Key residues involved in calcium-binding motifs in EGF-like domains. Nature 351:164–167. https://doi.org/10.1038/351164a0
Herz J, Beffert U (2000) Apolipoprotein E receptors: linking brain development and Alzheimer’s disease. Nat Rev Neurosci 1:51–58. https://doi.org/10.1038/35036221
Hirai H, Yasui N, Yamashita K et al (2017) Structural basis for ligand capture and release by the endocytic receptor ApoER2. EMBO Rep 18:982–999. https://doi.org/10.15252/embr.201643521
Hopkins PN, Wu LL, Stephenson SH et al (1999) A novel LDLR mutation, H190Y, in a Utah kindred with familial hypercholesterolemia. J Hum Genet 44:364–367. https://doi.org/10.1007/s100380050179
Jeon H, Meng W, Takagi J, Eck MJ, Springer TA, Blacklow SC (2001) Implications for familial hypercholesterolemia from the structure of the LDL receptor YWTD-EGF domain pair. Nat Struct Biol 8:499–504. https://doi.org/10.1038/88556
Kim DH, Iijima H, Goto K et al (1996) Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J Biol Chem 271:8373–8380
Kim DH, Magoori K, Inoue TR et al (1997) Exon/intron organization, chromosome localization, alternative splicing, and transcription units of the human apolipoprotein E receptor 2 gene. J Biol Chem 272:8498–8504
Kwon HJ, Lagace TA, McNutt MC, Horton JD, Deisenhofer J (2008) Molecular basis for LDL receptor recognition by PCSK9. Proc Natl Acad Sci USA 105:1820–1825. https://doi.org/10.1073/pnas.0712064105
Lagace TA, Curtis DE, Garuti R et al (2006) Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J Clin Invest 116:2995–3005. https://doi.org/10.1172/JCI29383
Li J, Tumanut C, Gavigan JA et al (2007) Secreted PCSK9 promotes LDL receptor degradation independently of proteolytic activity. Biochem J 406:203–207. https://doi.org/10.1042/BJ20070664
Lo Surdo P, Bottomley MJ, Calzetta A et al (2011) Mechanistic implications for LDL receptor degradation from the PCSK9/LDLR structure at neutral pH. EMBO Rep 12:1300–1305. https://doi.org/10.1038/embor.2011.205
Malby S, Pickering R, Saha S, Smallridge R, Linse S, Downing AK (2001) The first epidermal growth factor-like domain of the low-density lipoprotein receptor contains a noncanonical calcium binding site. Biochemistry 40:2555–2563
McNutt MC, Kwon HJ, Chen C, Chen JR, Horton JD, Lagace TA (2009) Antagonism of secreted PCSK9 increases low density lipoprotein receptor expression in HepG2 cells. J Biol Chem 284:10561–10570. https://doi.org/10.1074/jbc.M808802200
McNutt MC, Lagace TA, Horton JD (2007) Catalytic activity is not required for secreted PCSK9 to reduce low density lipoprotein receptors in HepG2 cells. J Biol Chem 282:20799–20803. https://doi.org/10.1074/jbc.C700095200
Natarajan P, Kathiresan S (2016) PCSK9 inhibitors. Cell 165:1037. https://doi.org/10.1016/j.cell.2016.05.016
Novak S, Hiesberger T, Schneider WJ, Nimpf J (1996) A new low density lipoprotein receptor homologue with 8 ligand binding repeats in brain of chicken and mouse. J Biol Chem 271:11732–11736
Perrey S, Ishibashi S, Kitamine T et al (2001) The LDL receptor is the major pathway for beta-VLDL uptake by mouse peritoneal macrophages. Atherosclerosis 154:51–60
Rashid S, Curtis DE, Garuti R et al (2005) Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci USA 102:5374–5379. https://doi.org/10.1073/pnas.0501652102
Ren G, Rudenko G, Ludtke SJ, Deisenhofer J, Chiu W, Pownall HJ (2010) Model of human low-density lipoprotein and bound receptor based on cryoEM. Proc Natl Acad Sci USA 107:1059–1064. https://doi.org/10.1073/pnas.0908004107
Rudenko G, Henry L, Henderson K et al (2002) Structure of the LDL receptor extracellular domain at endosomal pH. Science 298:2353–2358. https://doi.org/10.1126/science.1078124
Schneider WJ, Nimpf J (2003) LDL receptor relatives at the crossroad of endocytosis and signaling. Cell Mol Life Sci 60:892–903. https://doi.org/10.1007/s00018-003-2183-Z
Springer TA (1998) An extracellular beta-propeller module predicted in lipoprotein and scavenger receptors, tyrosine kinases, epidermal growth factor precursor, and extracellular matrix components. J Mol Biol 283:837–862. https://doi.org/10.1006/jmbi.1998.2115
Sun XM, Patel DD, Webb JC et al (1994) Familial hypercholesterolemia in China. Identification of mutations in the LDL-receptor gene that result in a receptor-negative phenotype. Arterioscler Thromb 14:85–94
Takahashi S, Kawarabayasi Y, Nakai T, Sakai J, Yamamoto T (1992) Rabbit very low density lipoprotein receptor: a low density lipoprotein receptor-like protein with distinct ligand specificity. Proc Natl Acad Sci USA 89:9252–9256
Tissir F, Goffinet AM (2003) Reelin and brain development. Nat Rev Neurosci 4:496–505. https://doi.org/10.1038/nrn1113
Trommsdorff M, Gotthardt M, Hiesberger T et al (1999) Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell 97:689–701
Usifo E, Leigh SE, Whittall RA et al (2012) Low-density lipoprotein receptor gene familial hypercholesterolemia variant database: update and pathological assessment. Ann Hum Genet 76:387–401. https://doi.org/10.1111/j.1469-1809.2012.00724.x
Verdaguer N, Fita I, Reithmayer M, Moser R, Blaas D (2004) X-ray structure of a minor group human rhinovirus bound to a fragment of its cellular receptor protein. Nat Struct Mol Biol 11:429–434. https://doi.org/10.1038/nsmb753
Yamamoto T, Davis CG, Brown MS et al (1984) The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell 39:27–38
Yasui N, Nogi T, Kitao T, Nakano Y, Hattori M, Takagi J (2007) Structure of a receptor-binding fragment of reelin and mutational analysis reveal a recognition mechanism similar to endocytic receptors. Proc Natl Acad Sci USA 104:9988–9993. https://doi.org/10.1073/pnas.0700438104
Yasui N, Nogi T, Takagi J (2010) Structural basis for specific recognition of reelin by its receptors. Structure 18:320–331. https://doi.org/10.1016/j.str.2010.01.010
Zong Y, Zhang B, Gu S et al (2012) Structural basis of agrin-LRP4-MuSK signaling. Genes Dev 26:247–258. https://doi.org/10.1101/gad.180885.111
Acknowledgements
The author would like to congratulate Prof. Fumio Arisaka on the occasion of his 70th birthday and thank him for his long-standing contributions in the development and promulgation of biophysical methods. The author thanks Samuel Thompson for editing the manuscript and Prof. Junichi Takagi for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Terukazu Nogi declares that he has no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by the author.
Additional information
This article is part of a Special Issue on ‘Biomolecules to Bio-nanomachines—Fumio Arisaka 70th Birthday’ edited by Damien Hall, Junichi Takagi and Haruki Nakamura.
Rights and permissions
About this article
Cite this article
Nogi, T. How multi-scale structural biology elucidated context-dependent variability in ectodomain conformation along with the ligand capture and release cycle for LDLR family members. Biophys Rev 10, 481–492 (2018). https://doi.org/10.1007/s12551-017-0362-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12551-017-0362-7