Abstract
Lacewings (Neuroptera) have predatory larvae with highly specialised mouthparts. Larvae of many groups within Neuroptera are well represented as fossils preserved in ambers; however, larvae of some groups are less often reported in the literature. Here we report such a rare case, a larva of the group Hemerobiidae, an aphidlion, preserved in a piece of Eocene Baltic amber (about 40 million years old). It is preserved together with three possible prey items, wingless aphids, most likely representatives of Germaraphis (or at least closely related to this group). The aphidlion can be identified based on the morphology of the antennae, simple curved and toothless stylets, well developed labial palps, and the absence of other mouth-part structures such as a protruding labrum or maxillary palps. A long, club-shaped distal element of the labial palps identifies the specimen as a larva of Hemerobiidae. The aphids can be identified based on their very long, beak-like mouth parts. This find is, to our knowledge, the first example of a lacewing larva preserved together with its potential prey. We briefly discuss other cases in which fossils preserved in amber allow us to reconstruct aspects of behaviour and interactions of fossil lacewing larvae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
“Aphidlion” is a term used to address larval stages of certain lacewings that are specialised for feeding on aphids. More rarely, the term has also been used for the larval stages of ladybugs, which also feed on aphids, but we will concentrate here on the aphidivorous lacewing larvae (Neuroptera) and use the term aphidlion accordingly. Neuropteran species with aphidlions as larval stages do not form a monophyletic group, instead they form two distinct groups: that of green lacewings (Chrysopidae) and that of brown lacewings (Hemerobiidae). While earlier phylogenetic reconstructions resolved Chrysopidae and Hemerobiidae as sister groups (Aspöck and Aspöck 2007), newer reconstructions do not further support this view (Winterton et al. 2018; Vasilikopoulos et al. 2020; Badano et al. 2021).
Aphidlions, as well as (most) lacewing larvae in general, are fierce predators with highly specialised mouth-part morphology (MacLeod 1964; Aspöck and Aspöck 2007; Zimmermann et al. 2019): 1) Each upper jaw (mandible) forms with its corresponding lower jaw (maxilla) a so-called stylet. Hence, each larva has a pair of stylets; these inject venom into their prey, then saliva, and finally allow sucking out dissolved tissues from the prey. 2) Lower jaws (maxillae) lack palps. 3) The proximal part of the lower lip (labium) is largely continuous with the head capsule, therefore it is mostly recognisable by its well developed palps.
Aphidlions have simple curved stylets, in contrast to curved stylets with teeth in many antlion-like larvae (MacLeod 1964; Badano et al. 2018; Haug et al. 2019a, 2021a, b) or more or less straight stylets (Haug et al. 2021c) as, for example, in larvae of many mantis lacewings (Mantispidae; Redbord and MacLeod 1985; Hoffman and Brushwein 1992), beaded lacewings (Berothidae; Gurney 1947; Möller et al. 2006) or lance lacewings (Osmylidae; Matsuno and Yoshitomi 2016; Winterton et al. 2017). Furthermore, aphidlions often have a rather spindle-shaped body (see discussion in Haug et al. 2019a, b).
Aphidlions play an important role in modern ecosystems as controls of aphid populations. Therefore, aphidlions have been employed as discrete pest control instruments (Senior and McEwen 2001; Weihrauch 2012), with this even playing an economical role. Aphidlions and aphidlion-like larvae have also been found in the fossil record, yet so far only as fossils in amber (Pérez-de la Fuente et al. 2020). An unequivocal larval representative of Hemerobiidae has been reported from Baltic amber (Makarkin et al. 2012) as was a larva of Chrysopidae (Weitschat et al. 2009 fig. 45 p. 254). Older, Cretaceous ambers have provided a wealth of larvae of aphidlion-like appearance, generally suggested to be closely related to Chrysopidae (Pérez-de la Fuente 2012, 2016, 2018, 2019, 2020; Liu et al. 2016, 2018; Wang et al. 2016).
In fossil larvae, including those of lacewings, we generally assume the lifestyle based on comparison to extant relatives, the functional morphology, or mostly a combination of both. More rarely a more direct interaction can be observed preserved in amber (see discussion in Hörnig et al. 2020, 2022).
Here we report a possible case of co-occurrence of a predator with its presumed prey items in a single piece of Baltic amber, the first possible case of a co-occurrence of predator and prey for lacewing larvae. The amber piece includes an aphidlion and three aphid specimens. We discuss implications of this find.
Material and methods
Material
A single piece of Eocene Baltic amber (ca. 40 million years old) is in the centre of this study. It was legally purchased from Jonas Damzen, Vilnius (amberinclusions.eu). The amber piece was bought in a polished condition, further additional changes (preparations, embeddings) were not done by the authors. The specimen is now deposited in the Palaeo-Evo-Devo Research Group Collection of Arthropods, Ludwig-Maximilians-University of Munich, Germany, under repository number PED 0229. The amber piece has several inclusions, most prominently an aphidlion and three aphids.
Baltic amber is one of the oldest mined for ambers with deposits along the shores of the Baltic sea in a wide geological area (Weitschat and Wichard 2010, summarised e.g. in Jouault et al. 2021 and Chény et al. 2019). The majority of Baltic amber is found in the Blue-Earth Formation (or Blue-Earth layer of the Prussian Formation) in Kaliningrad Peninsula, Russia, which contains a majority of Baltic amber. It is the largest deposit of amber in the world (Eskov 2002, Weitschat and Wichard 2010). At first the age of Baltic amber was assumed to be of early Oligocene age (ca. 28 mya) based on the composition of the marine fauna of the glauconite layer within which the Baltic amber can be found and assumed to be of early Oligocene (ca. 28 mya) age (e.g. Noetling 1883, 1888a, b and references therein). This was long left unchallenged. Later it was dated to Priabonian Age age (ca. 34-38 mya, late Eocene) based on microfaunistical data (Kaplan et al. 1977 in Perkovsky et al. 2007, Kosmoswska-Ceranowicz et al. 1997 in Chény et al. 2019 and Jouault et al. 2021, Aleksandrova and Zaporozhets 2008a, b). It was also assumed to be of older age (Lutetian, ca. 41-48 mya) based on radiometrically dating the glauconite-layer (Ritzkowski 1997 in Chény et al. 2019 and Jouault et al. 2021) of the Blue-Earth Formation, Russia; though this method can overestimate the age (compare Clauer et al. 2005) and the amber and the glauconite layer have been assumed to be re-deposited (e.g. Standke 2008 in Chény et al. 2019 and Jouault et al. 2021, Weitschat and Wichard 2010). The age of Baltic amber is still not unanimously agreed upon, but is assumed to be in its widest range ca. 23-43 mya (Early Oligocene to Mid-Eocene; compare e.g. Sadowski et al. 2017 and references therein), though many authors have (tentatively) accepted its age as late Eocene (ca. 34-38 mya; e.g. Eskov 2002, Perkovsky et al. 2007, Dlussky and Rasnitsyn 2009, Chény et al. 2019, Jouault et al. 2021).
Documentation method
The amber piece was documented on a Keyence VHX-6000 digital microscope equipped with a ZST 20–2000 objective. The entire piece as well as the inclusions in detail were documented with different settings (black and white background, cross-polarised coaxial illumination; Haug et al. 2013a; unpolarised low-angle ring illumination). Resulting images with the best contrast were used for presentation. Each image is a composite image (Haug et al. 2011). In order to overcome limitations of depth of field, a stack of images with shifting levels of focus was recorded and fused to a sharp image with the built-in software. To overcome limitation of field of view, several adjacent image details were recorded and stitched to a larger panorama with the built-in software (Haug et al. 2018). Each image was recorded with several exposure times in order to avoid too dark or too bright areas (HDR; Haug et al. 2013b). Resulting images were further processed in Adobe Photoshop CS2.
Results
Description of inclusions within the amber piece
General
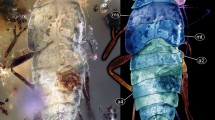
The amber piece includes many syn-inclusions; some of these are rather incomplete remains or strongly deformed (and therefore unidentifiable) exuviae. Yet, four specimens (Fig. 1a, b) are well preserved, an elongate, comparably large specimen (aphidlion, Fig. 1c) and three significantly smaller specimens that have prominent hemipteran-type beaks (aphids, Figs. 1d–f, 2d–g).
Specimen PED 0229, Baltic amber, continued. a-c Aphidlion. a In ventro-lateral view. b In (largely) ventral view; note that the head is seen in anterior view. c Close-up of head in frontal view (top), same image colour-marked (bottom). d Aphid specimen 2 in ventral view. e Aphid specimen 3 in ventral view. f Aphid specimen 3 in lateral view. g Close-up of aphid 2 in lateral view. Abbreviations: at antenna; hc head capsule; lp labial palp; sy stylet
Larger specimen (aphidlion)
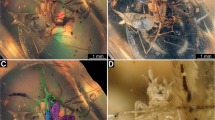
The large specimen is strongly ‘verlumt’ (Figs. 1c, 2a, b). Many details are concealed, but several aspects are accessible: Body overall elongate to spindle-shaped, differentiation into anterior head region and trunk apparent. Head bears three apparent pairs of structures (Fig. 2a, b). These are interpreted as antennae, stylets and labial palps (Fig. 2c). Antennae elongate, with three distinct elements. Proximal element 1 rather short, about as long as wide. Element 2 narrower, but significantly longer; exact length difficult to infer due to the perspective, but estimated to have 4–5x the length of element 1. Distal element shorter, tapering distally, exact length difficult to infer due to the perspective, but estimated to have about 2–3x the length of element 1.
Stylets (compound structures of upper jaw, mandible, and lower jaw, maxilla) curved, simple, no teeth apparent. Slightly shorter than antennae, but wider proximally, tapering distally.
Labial palps with two apparent elements. Supposedly a further proximal element is not accessible. First visible element stout, comparable to proximal antenna element. Distal element longer, 2–3x the length of the visible proximal element, club-shaped, i.e. proximally narrower than visible proximal element, then widening but tapering distally to a more rounded tip.
Trunk spindle-shaped, exact number of segments not accessible. Anterior trunk with prominent appendages (legs) protruding from the Verlumung, yet no details accessible.
Smaller specimens (aphids)
Three smaller specimens sub-similar in overall appearance, yet differentiated in size. Specimen 1 large, about 1 mm (Fig. 1d), specimen 2 smaller, about 0.6 mm (Fig. 1e) and specimen 3 also about 0.6 mm (Fig. 1f).
Body differentiated into head and trunk. Head short, wider than long, dorsally partly concealed by anterior trunk (Fig. 1d–f). Head with few details accessible; prominent antennae protruding from head, antero-laterally. Slightly longer than width of head capsule, few elements, exact number not discernible (but about five). Mouth parts only well accessible in specimen 2 (Fig. 2d) forming elongate beak (rostrum) extending posteriorly beyond the trunk end.
Anterior three trunk segments prominent (thorax). Each about as long as head, but consecutively wider towards posterior. Ventrally each segment with a pair of prominent appendages (legs). Legs with distinct Z-shape; very proximal details of legs not well accessible. Proximal region of appendage (presumably coxa, trochanter and femur, subdivision partly visible in Fig. 2f), middle region (tibia) and distal region (tarsus) with indication of a pair of claws distally (Fig. 1d).
Posterior trunk (abdomen) with 9–10 visible units. Anterior units interpreted as true segments; most posterior unit presumably undifferentiated trunk end, compound structure of several segments. Anterior three abdomen segments slightly wider than thorax, but shorter than thorax segments, each about 50% of single thorax segment. Further posterior abdomen units similar in length, consecutively narrower, resulting in a gently rounded overall shape of abdomen.
Discussion
Identity of the large specimen (aphidlion)
The large specimen in the amber piece is largely concealed by ‘Verlumung’, yet quite some important details are accessible. The structures protruding from the head are strongly reminiscent of lacewing larvae (Gepp 1984). Some predatory beetle larvae show similar arrangements of head appendages with short antennae, prominent labial palps, and sickle-shaped mandibles, but in such larvae we usually additionally have, for example, a pair of maxillary palps.
Furthermore, the specific subdivision of the visible appendages is fully compatible with the larvae representing an aphidlion. The antennae with three elements and with element 2 being the longest are similar to the antennae of many extant larvae of Chrysopidae and Hemerobiidae (Díaz Aranda et al. 2001). The stylets are protruding forward from the head, prominent, and not covered by a labrum. They are simple sickle-shaped, inward curved, tapering distally, without any teeth, also in these aspects being similar to modern larvae of Chrysopidae and Hemerobiidae (Gepp 1984).
Although the labial palps are less completely accessible, they are very informative. Only in larvae of Hemerobiidae is the terminal element the most prominent one; also the general shape is very similar to that of modern larvae of Hemerobiidae (MacLeod 1960 pl. 5; Gepp 1984 fig. 16b pl. 7 p. 198).
Although the specimen is not perfectly accessible, all available observations clearly indicate that it is an aphidlion, more specific a larva of the group Hemerobiidae. According to Makarkin et al. (2012), adult representatives of Hemerobiidae are quite common compared to other winged lacewings in Baltic amber. Seven species of Hemerobiidae in Baltic amber have so far been formally described, several further specimens were illustrated (summarised in Makarkin et al. 2016, 2019). Remarkably, only a single larva of Hemerobiidae in Baltic amber was reported in the literature so far (Makarkin et al. 2012), further demonstrating the presence of such larvae in the Baltic amber forest.
Identity of the small specimens (aphids)
The smaller specimens are all sub-similar and presumably conspecific. The strong size difference is likely due to the fact that the larger specimen is a further advanced developmental stage (instar). The overall habitus of the specimens in combination with the strongly developed beak-like mouthparts clearly indicates that these specimens are aphids.
Representatives of Aphidoidea (aphids), commonly occur in Baltic amber (e.g. summarised in Weitschat and Wichard 2010; Gröhn 2015). While non-winged developmental stages of aphids are less easily identified, the specimens clearly have a strong resemblance to specimens of Germaraphis (e.g. Heie 1987 fig. 6.1.21 p. 379). Similarities include e.g. general habitus (see images in Heie 1971 fig. 2 p. 253, 1987 fig. 6.1.21 p. 379; Heie and Poinar 2011 fig. 2) size range (about 0.4 – 1.3 mm in different species of Germaraphis (Heie 1968, 1971), up to 2 mm in Germaraphis longula (= Lachnus longulus, Heie 1971)), general proportions of body parts and appendages, antenna with 4–6 elements, and an elongated beak (rostrum) which posteriorly protrudes beyond the trunk end (Heie 1968; Heie and Poinar 2011). Originally Germaraphis was interpreted as an ingroup of Pemphigidae, but this group seems no longer accepted as such (e.g. Zhang and Qiao 2008). As Germaraphis has, among others, been compared to Pachypappa (Heie 1987), an ingroup position of Pemphiginae or even Pemphigini seems likely. Due to the similarity, we suggest that also the three fossils are representatives of Germaraphis or at least closely related to this group.
Interpretation of the amber piece
As explained above, the amber piece studied preserves an aphidlion (in this case a larva of the group Hemerobiidae) together with three aphids. Representatives of Hemerobiidae today (with about 590 described species; Oswald and Machado 2018) have a raptorial lifestyle as adults and larvae (MacLeod and Stange 2021). Their preferred prey items are rather small, soft-bodied animals, especially aphids. Aphidlions, i.e. larvae of Hemerobiidae, are active predators – they use their piercing-sucking stylets to capture prey items, inject venom, and drain body fluids of their prey. Aphidlions are known to be very efficient in detecting and preying on a high number of aphid individuals (e.g. Cutright 1923), which leads to an important role in pest control (e.g. Senior and McEwen 2001; Weihrauch 2012).
As aphids are the preferred prey items of aphidlions in the modern fauna, we can expect that this was similar in the past, also in the Eocene amber forest (“extant phylogenetic bracket” concept, Witmer 1995, see also Hörnig et al. 2022). The inclusion of an aphidlion and three aphids in one amber piece seems less likely coincidental and further supports this expectation.
An aphidlion actively searching for aphids is to be expected in close proximity to them and hence may be preserved in such an arrangement. The fact that aphid specimens of different developmental stages are preserved together is well compatible with a natural group of aphids (e.g. Saberski et al. 2016). We therefore interpret the amber piece as preserving a natural situation of a predator (the aphidlion) among its potential prey items (the aphids).
Behaviour and interactions of fossil lacewing larvae
Lacewing larvae in general are relatively well represented in ambers in comparison to larvae of other groups of holometabolans, such as moth caterpillars (Haug et al. in review). It should therefore not be surprising that there are quite some examples that allow us to infer certain aspects of their behaviour, sometimes even in a kind of caught-in-the-act case, or also indications of interactions with other organisms:
-
1)
Hatching is a crucial action in the life of an animal, and so it is for lacewing larvae. This moment has been preserved in some cases in Cretaceous Lebanese and Myanmar ambers as well (Engel and Grimaldi 2008 figs. 12–14 pp. 57, 58; Pérez-de la Fuente et al. 2019).
-
2)
Some fossil lacewing larvae are known to hide themselves (unclear if hiding from predator or prey). Several specimens are known to use camouflaging cloaks, based on functional morphology for Miocene specimens (Engel and Grimaldi 2007 fig. 34 p. 32), but also actual cloaks on specimens in the Eocene (Weitschat et al. 2009), and even already in the Cretaceous (Wang et al. 2016; Pérez-de la Fuente et al. 2012, 2016, 2018). One special type of larva (known by two specimens) from the Cretaceous has been interpreted as having a morphology mimicking a co-occurring liverwort (Liu et al. 2018). While camouflaging cloaks are known in modern lacewing larvae (e.g. Aspöck and Aspöck 2007 figs. 127, 128 p. 499), a mimesis as in the fossil is not known in the modern fauna.
-
3)
A very peculiar lacewing larva from the Cretaceous (Liu et al. 2016) has been interpreted as interacting with web-spinning spiders based on its functional morphology and similarities to the likewise spider-interacting thread-legged bugs (Reduviidae: Emesinae). Similar to the case of the mimesis, such a type of interaction is not known in modern lacewing larvae.
-
4)
Some modern lacewing larvae are digging, and such a behaviour has been inferred for at least some fossils based on phylogenetic reasoning and functional morphology (Badano et al. 2018). Other larvae that are related to modern lineages with at least shallow digging larvae seem unlikely to have performed a similar action (Haug et al. 2021a).
-
5)
It is not well investigated which other animals feed on modern-day lacewing larvae (ants, wasps and caterpillars are mentioned as putative predators by Henry 1972). Yet, some fossil finds clearly indicate that also lacewing larvae in the Cretaceous have been prey items for other organisms (Hörnig et al. 2020).
-
6)
Lacewing larvae may be closely associated after hatching (see above), but are usually very aggressive against each other (Rojht et al. 2009 fig. 2 p. 9). Few exceptions are known in larvae of certain owl lacewings (owl “flies”; Ascalaphidae), in which groups of larvae perform group defence. A group of similar appearing fossils, which seem to have performed a similar type of group defence, have recently been reported (Hörnig et al. 2022).
-
7)
Concerning feeding habits of the fossil lacewing larvae, not many observations were so far possible. Based on comparison to extant larvae and their morphology, we can assume that most fossil lacewing larvae were predators, just as their modern counterparts, as there are only few exceptions in the modern fauna. Certain larvae of mantis lacewings (Mantispidae) climb on female spiders in their first larval stage and act as ecto-parasites. Direct observations of such an interaction were possible due to exceptional amber pieces from the Eocene (Ohl 2011) and Cretaceous (Haug et al. 2018).
Conclusions
The here reported piece is a valuable addition to the list of amber pieces providing hints for the possible predator-prey interactions of fossil lacewing larvae. While we could well assume that aphidlions were already feeding on aphids in the past, the new amber piece clearly demonstrates that they co-occurred in close proximity, making a feeding interaction very likely. This is to our knowledge the first record of a fossil lacewing larva preserved together with its potential prey, which is quite surprising given the rather high number of known fossil lacewing larvae (Pérez-de la Fuente 2020; Haug et al. 2021d). However, fossil larvae of Hemerobiidae are so far extremely rarely preserved, which is especially astonishing due to the comparably high number of adult forms in Baltic amber. The specimen presented here represents only the second report of an aphidlion of Hemerobiidae in Baltic amber and also in the fossil record.
Data availability
All data generated or analysed during this study are included in this published article.
References
Aleksandrova, G. N., & Zaporozhets, N. I. (2008a). Palynological characteristics of Upper Cretaceous and Paleogene deposits on the west of the Sambian Peninsula (Kaliningrad region), Part 1. Stratigraphy and Geological Correlation, 16(3), 295–316. https://doi.org/10.1134/S0869593808030052.
Aleksandrova, G. N., & Zaporozhets, N. I. (2008b). Palynological characteristics of Upper Cretaceous and Paleogene deposits on the west of the Sambian Peninsula (Kaliningrad region), Part 2. Stratigraphy and Geological Correlation, 16(5), 528–539. https://doi.org/10.1134/S0869593808050067.
Aspöck, U., & Aspöck, H. (2007). Verbliebene Vielfalt vergangener Blüte. Zur Evolution, Phylogenie und Biodiversität der Neuropterida (Insecta: Endopterygota). Denisia, 20, 451–516.
Badano, D., Engel, M. S., Basso, A., Wang, B., & Cerretti, P. (2018). Diverse Cretaceous larvae reveal the evolutionary and behavioural history of antlions and lacewings. Nature Communications, 9, 3257. https://doi.org/10.1038/s41467-018-05484-y.
Badano, D., Fratini, M., Maugeri, L., Palermo, F., Pieroni, N., Cedola, A., Haug, J. T., Weiterschan, T., Velten, J., Mei, M., Di Giulio, A., & Cerretti, P. (2021). X-ray microtomography and phylogenomics provide insights into the morphology and evolution of an enigmatic Mesozoic insect larva. Systematic Entomology, 46, 672–684. https://doi.org/10.1111/syen.12482.
Chény, C., Wang, B., & Perrichot, V. (2019). A new genus of myrmicine ant (Hymenoptera: Formicidae) from Eocene Baltic amber. Comptes Rendus Palevol, 18(6), 589–597. https://doi.org/10.1016/j.crpv.2019.05.005.
Clauer, N., Huggett, J. M., & Hillier, S. (2005). How reliable is the K-Ar glauconite chronometer? A case study of Eocene sediments from the Isle of Wight. Clay Minerals, 40(2), 167–176. https://doi.org/10.1180/0009855054020163.
Cutright, C. R. (1923). Life history of Micromus posticus Walker. Journal of Economic Entomology, 16(5), 448–456. https://doi.org/10.1093/jee/16.5.448.
Díaz Aranda, L. M., Monserrat, V. J., & Tauber, C. A. (2001). Recognition of larval Neuroptera. Recognition of early stages of Chrysopidae. In P. K. McEwen, T. R. New, & A. E. Whittington (Eds.), Lacewings in the crop environment (pp. 60–81, 546 pp). Cambridge University Press.
Dlussky, G. M., & Rasnitsyn, A. P. (2009). Ants (Insecta: Vespida: Formicidae) in the Upper Eocene amber of central and eastern Europe. Paleontological journal, 43(9), 1024–1042. https://doi.org/10.1134/S0031030109090056.
Engel, M. S., & Grimaldi, D. A. (2007). The Neuropterid Fauna of Dominican and Mexican Amber (Neuropterida: Megaloptera, Neuroptera). American Museum Novitates, 3587, 1–58. https://doi.org/10.1206/0003-0082(2007)3587[1:TNFODA]2.0.CO;2.
Engel, M. S., & Grimaldi, D. A. (2008). Diverse Neuropterida in Cretaceous amber, with particular reference to the paleofauna of Myanmar (Insecta). Nova Supplementa Entomologica, 20, 1–86.
Eskov, K. Y. (2002). Fossil Resins. In A. P. Rasnitsyn & D. L. J. Quicke (Eds.), History of Insects (pp. 444–446). Kluwer Academic Publishers.
Gepp, J. (1984). Erforschungsstand der Neuropteren-Larven der Erde (mit einem Schlüssel zur Larvaldiagnose der Familien, einer Übersicht von 340 beschriebenen Larven und 600 Literaturzitaten). In J. Gepp, H. Aspöck, & H. Hölzel (Eds.), Progress in World’s Neuropterology (pp. 183–239). Graz, Austria: Proceedings of the 1st International Symposium on Neuropterology, 22–26 September 1980,1–265.
Gröhn, C. (2015). Einschlüsse im Baltischen Bernstein (pp. 424). Hamburg: Wachholtz Verlag-Murmann Publishers.
Gurney, A. B. (1947). Notes on Dilaridae and Berothidae, with special reference to the immature stages of the Nearctic genera (Neuroptera). Psyche, 54, 145–169. https://doi.org/10.1155/1947/78317.
Haug, J. T., Haug, C., Kutschera, V., Mayer, G., Maas, A., Liebau, S., Castellani, C., Wolfram, U., Clarkson, E. N. K., & Waloszek, D. (2011). Autofluorescence imaging, an excellent tool for comparative morphology. Journal of Microscopy, 244, 259–272. https://doi.org/10.1111/j.1365-2818.2011.03534.x.
Haug, J. T., Müller, C. H. G., & Sombke, A. (2013a). A centipede nymph in Baltic amber and a new approach to document amber fossils. Organisms Diversity & Evolution, 13, 425–432. https://doi.org/10.1007/s13127-013-0129-3.
Haug, C., Shannon, K. R., Nyborg, T., & Vega, F. J. (2013b). Isolated mantis shrimp dactyli from the Pliocene of North Carolina and their bearing on the history of Stomatopoda. Bolétin de la Sociedad Geológica Mexicana, 65, 273–284. https://doi.org/10.18268/bsgm2013v65n2a9.
Haug, J. T., Müller, P., & Haug, C. (2018). The ride of the parasite: a 100-million-year old mantis lacewing larva captured while mounting its spider host. Zoological Letters, 4, 31. https://doi.org/10.1186/s40851-018-0116-9.
Haug, C., Herrera-Flórez, A. F., Müller, P., & Haug, J. T. (2019a). Cretaceous chimera–an unusual 100-million-year old neuropteran larva from the “experimental phase” of insect evolution. Palaeodiversity, 12, 1–11. https://doi.org/10.18476/pale.v12.a1.
Haug, J. T., Müller, P., & Haug, C. (2019b). A 100-million-year old slim insectan predator with massive venom-injecting stylets – a new type of neuropteran larva from Burmese amber. Bulletin of Geosciences, 94, 431–440. https://doi.org/10.3140/bull.geosci.1753.
Haug, G. T., Baranov, V., Wizen, G., Pazinato, P. G., Müller, P., Haug, C., & Haug, J. T. (2021a). The morphological diversity of long-necked lacewing larvae (Neuroptera: Myrmeleontiformia). Bulletin of Geosciences, 96, 431–457. https://doi.org/10.3140/bull.geosci.1807.
Haug, G. T., Haug, C., & Haug, J. T. (2021b). The morphological diversity of spoon-winged lacewing larvae and the first possible fossils from 99 million-year-old Kachin amber, Myanmar. Palaeodiversity, 14, 133–152. https://doi.org/10.18476/pale.v14.a6.
Haug, J. T., Haug, G. T., Zippel, A., van der Wal, S., Müller, P., Gröhn, C., Wunderlich, J., Hoffeins, C., Hoffeins, H.-W., & Haug, C. (2021c). Changes in the morphological diversity of larvae of lance lacewings, mantis lacewings and their closer relatives over 100 million years. Insects, 12, art. 860. https://doi.org/10.3390/insects12100860.
Haug, C., Haug, G. T., Baranov, V. A., Solórzano-Kraemer, M. M., & Haug, J. T. (2021d). An owlfly larva preserved in Mexican amber and the Miocene record of lacewing larvae. Boletín de la Sociedad Geológica Mexicana, 73(3), A271220. https://doi.org/10.18268/BSGM2021v73n3a271220.
Heie, O. E. (1968). An aphid identified with Aphis transparens Germar et Berendt, 1856, and some other Baltic amber aphids in German collection. Stuttgarter Beiträge Naturkunde Serie A, 184, 1–7.
Heie, O. E. (1971). The rediscovered types of the fossil aphids described by Germar and Berendt in 1856. Deutsche Entomologische Zeitschrift, 18(1–3), 251–264. https://doi.org/10.1002/mmnd.19710180118.
Heie, O. E. (1987). Chapter 6 Evolution, 6.1 Palaeontology and Phylogeny. In A. K. Minks & P. Harrewijn (Eds.), Aphids: their biology, natural enemies and control (pp. 367–391). Elsevier.
Heie, O. E., & Poinar Jr., G. O. (2011). Using fossils to determine an amber source: aphids and crane flies in Chinese or Baltic amber? Historical Biology, 23(4), 431–433. https://doi.org/10.1080/08912963.2010.542031.
Henry, C. S. (1972). Eggs and rapagula of Ululodes and Ascaloptynx (Neuroptera: Ascalaphidae): a comparative study. Psyche: A Journal of Entomology, 79, 1–22. https://doi.org/10.1155/1972/54050.
Hoffman, K. M., & Brushwein, J. R. (1992). Descriptions of the larvae and pupae of some North American Mantispinae (Neuroptera: Mantispidae) and development of a system of larval chaetotaxy for Neuroptera. Transactions of the American Entomological Society, 118, 159–196.
Hörnig, M. K., Kiesmüller, C., Müller, P., Haug, C., & Haug, J. T. (2020). A new glimpse on trophic interactions of 100-million-year old lacewing larvae. Acta Palaeontologica Polonica, 65, 777–786. https://doi.org/10.4202/app.00677.2019.
Hörnig, M. K., Haug, C., Müller, P., & Haug, J. T. (2022). Not quite social – possible cases of gregarious behaviour of immatures of various lineages of Insecta preserved in 100 million-year-old amber. Bulletin of Geosciences, 97(1). https://doi.org/10.3140/bull.geosci.1818.
Jouault, C., Legendre, F., Condamine, F., & Nel, A. (2021). A new stonefly species (Plecoptera: Perlodidae) from Eocene Baltic amber and questions on the wing venation potential for species diagnostic of fossil Plecoptera. Palaeoentomology, 4(3), 243–256. https://doi.org/10.11646/palaeoentomology.4.3.12.
Liu, X., Zhang, W., Winterton, S. L., Breitkreuz, L. C., & Engel, M. S. (2016). Early morphological specialization for insect-spider associations in Mesozoic lacewing. Current Biology, 26(12), 1590–1594. https://doi.org/10.1016/j.cub.2016.04.039.
Liu, X., Shi, G., Xia, F., Lu, X., Wang, B., & Engel, M. S. (2018). Liverwort mimesis in a Cretaceous lacewing larva. Current Biology, 28(9), 1475–1481. https://doi.org/10.1016/j.cub.2016.04.039.
MacLeod, E. G. (1960). The immature stages of Boriomyia fidelis (Banks) with taxonomic notes on the affinities of the genus Boriomyia (Neuroptera: Hemerobiidae). Psyche, 67, 26–40. https://doi.org/10.1155/1960/53093.
MacLeod, E. G. (1964). A comparative morphological study of the head capsule and cervix of larval Neuroptera (Insecta) (pp. 1-528). Ph.D. dissertation. Cambridge, Massachusetts, USA: Harvard University.
MacLeod, E. G., & Stange, L. A. (2021). Brown Lacewings (of Florida) (Insecta: Neuroptera: Hemerobiidae). Entomology and Nematology Department, Florida Cooperative Extension Service, EENY-225. Retrieved October 14, 2021, from https://edis.ifas.ufl.edu/publication/IN382
Makarkin, V. N., Wedmann, S., & Weiterschan, T. (2012). First record of a fossil larva of Hemerobiidae (Neuroptera) from Baltic amber. Zootaxa, 3417(2), 53–63. https://doi.org/10.11646/zootaxa.3417.1.3.
Makarkin, V. N., Wedmann, S., & Weiterschan, T. (2016). A new genus of Hemerobiidae (Neuroptera) from Baltic amber, with a critical review of the Cenozoic Megalomus-like taxa and remarks on the wing venation variability of the family. Zootaxa, 4179(3), 345–370. https://doi.org/10.11646/zootaxa.4179.3.2.
Makarkin, V. N., Perkovsky, E. E., & Gröhn, C. (2019). Neotype designation and re-description of Prolachlanius resinatus (Hagen) (Neuroptera, Hemerobiidae) from Baltic amber, with the first record of the species from Rovno amber. Zootaxa, (1), 4688, zootaxa-4688. https://doi.org/10.11646/zootaxa.4688.1.2.
Matsuno, S., & Yoshitomi, H. (2016). Descriptions of three larvae of Osmylus species from Japan (Neuroptera: Osmylidae), with a proposed naming system for the larval sclerites. Zootaxa, 4189(2). https://doi.org/10.11646/zootaxa.4189.2.9.
Möller, A., Minter, L. R., & Olivier, P. A. S. (2006). Larval morphology of Podallea vasseana Navás and Podallea manselli Aspöck & Aspöck from South Africa (Neuroptera: Berothidae). African Entomology, 14, 1–12.
Noetling, F. (1883). Ueber das Alter des samländischen Tertiärs. Zeitschrift der Deutschen Geologischen Gesellschaft, 35, 671–694.
Noetling, F. (1888a). Die Fauna des samländischen Tertiärs. I. Theil. Abhandlungen zur geologischen Specialkarte von Preussen und den Thüringischen Staaten, 4(3), 1–216 [271–486].
Noetling, F. (1888b). Die Fauna des samländischen Tertiärs. II. Theil. Abhandlungen zur geologischen Specialkarte von Preussen und den Thüringischen Staaten, 4(4), 1–109 [487–495].
Ohl, M. (2011). Aboard a spider – a complex developmental strategy fossilized in amber. Naturwissenschaften, 98, 453–456. https://doi.org/10.1007/s00114-011-0783-2.
Oswald, J. D., & Machado, R. J. P. (2018). Biodiversity of the Neuropterida (Insecta: Neuroptera: Megaloptera, and Raphidioptera). In R. G. Foottit & P. H. Adler (Eds.), Insect biodiversity: science and society (vol. II, 2nd ed.pp. 627–671). Hoboken: John Wiley & Sons.
Pérez-de la Fuente, R., Delclòs, X., Peñalver, E., Speranza, M., Wierzchos, J., Ascaso, C., & Engel, M. S. (2012). Early evolution and ecology of camouflage in insects. Proceedings of the National Academy of Sciences, 109(52), 21414–21419. https://doi.org/10.1073/pnas.1213775110.
Pérez-de la Fuente, R., Delclos, X., Penalver, E., & Engel, M. S. (2016). A defensive behavior and plant-insect interaction in Early Cretaceous amber–the case of the immature lacewing Hallucinochrysa diogenesi. Arthropod Structure & Development, 45(2), 133–139. https://doi.org/10.1016/j.asd.2015.08.002.
Pérez-de la Fuente, R., Peñalver, E., Azar, D., & Engel, M. S. (2018). A soil-carrying lacewing larva in Early Cretaceous Lebanese amber. Scientific Reports, 8(1), 16663. https://doi.org/10.1038/s41598-018-34870-1.
Pérez-de la Fuente, R., Engel, M. S., Azar, D., & Peñalver, E. (2019). The hatching mechanism of 130-million-year-old insects: an association of neonates, egg shells and egg bursters in Lebanese amber. Palaeontology, 62(4), 547–559. https://doi.org/10.1111/pala.12414.
Pérez-de la Fuente, R., Engel, M. S., Delclòs, X., & Peñalver, E. (2020). Straight-jawed lacewing larvae (Neuroptera) from Lower Cretaceous Spanish amber, with an account on the known amber diversity of neuropterid immatures. Cretaceous Research, 106, 104200. https://doi.org/10.1016/j.cretres.2019.104200.
Perkovsky, E. E., Rasnitsyn, A. P., Vlaskin, A. P., & Taraschuk, M. V. (2007). A comparative analysis of the Baltic and Rovno amber arthropod faunas: representative samples. African invertebrates, 48(1), 229–245.
Redborg, K. E., & MacLeod, E. G. (1985). The developmental ecology of Mantispa uhleri Banks (Neuroptera: Mantispidae). Illinois Biological Monographs, 53, 1–130. https://doi.org/10.5962/bhl.title.49908.
Rojht, H., Budija, F., & Trdan, S. (2009). Effect of temperature on cannibalism rate between green lacewings larvae (Chrysoperla carnea [Stephens], Neuroptera, Chrysopidae). Acta agriculturae Slovenica, 93(1), 5–9. https://doi.org/10.2478/v10014-009-0001-5.
Saberski, E. T., Diamond, J. D., Henneman, N. F., & Levitis, D. A. (2016). Post-reproductive parthenogenetic pea aphids (Acyrthosiphon pisum) are visually identifiable and disproportionately positioned distally to clonal colonies. PeerJ, 4, e2631. https://doi.org/10.7717/peerj.2631.
Sadowski, E.-M., Schmidt, A. R., Seyfullah, L. J., & Kunzmann, L. (2017). Conifers of the ‘Baltic amber forest’ and their palaeoecological significance. Stapfia, 106, 1–73.
Senior, L. J., & McEwen, P. K. (2001). The use of lacewings in biological control. In P. K. McEwan, T. R. New, & A. E. Whittington (Eds.), Lacewings in the Crop Environment (pp. 296–302). Cambridge University Press.
Vasilikopoulos, A., Misof, B., Meusemann, K., Lieberz, D., Flouri, T., Beutel, R. G., Niehuis, O., Wappler, T., Rust, J., Peters, R. S., Donath, A., Podsiadlowski, L., Mayer, C., Bartel, D., Böhm, A., Liu, S., Kapli, P., Greve, C., Jepson, J. E., et al. (2020). An integrative phylogenomic approach to elucidate the evolutionary history and divergence times of Neuropterida (Insecta: Holometabola). BMC Evolutionary Biology, 20, 64. https://doi.org/10.1186/s12862-020-01631-6.
Wang, B., Xia, F., Engel, M. S., Perrichot, V., Shi, G., Zhang, H., Chen, J., Jarzembowski, E. A., Wappler, T., & Rust, J. (2016). Debris-carrying camouflage among diverse lineages of Cretaceous insects. Science Advances, 2(6), e1501918. https://doi.org/10.1126/sciadv.1501918.
Weihrauch, F. (2012). The significance of Brown and Green Lacewings as aphid predators in the special crop hops (Neuroptera: Hemerobiidae, Chrysopidae). Mitteilungen der Deutschen Gesellschaft für Allgemeine und Angewandte Entomologie, 18, 587–590.
Weitschat, W., & Wichard, W. (2010). Baltic amber. In D. Penney (Ed.), Biodiversity of fossils in amber from the major world deposits (pp. 80–115). Rochdale: Siri Scientific Press.
Weitschat, W., Berning, B., & Podenas, S. (2009). Jäger, Gejagte, Parasiten und Blinde Passagiere–Momentaufnahmen aus dem Bernsteinwald. Denisia, 26, 253–256.
Winterton, S. L., Zhao, J., Garzón-Orduña, I. J., Wang, Y., & Liu, Z. (2017). The phylogeny of lance lacewings (Neuroptera: Osmylidae). Systematic Entomology, 42(3), 555–574. https://doi.org/10.1111/syen.12231.
Winterton, S. L., Lemmon, A. R., Gillung, J. P., Garzon, I. J., Badano, D., Bakkes, D. K., Breitkreuz, L. C. V., Engel, M. S., Moriarty Lemmon, E., Liu, X., Machado, R. J. P., Skevington, J. H., & Oswald, J. D. (2018). Evolution of lacewings and allied orders using anchored phylogenomics (Neuroptera, Megaloptera, Raphidioptera). Systematic Entomology, 43(2), 330–354. https://doi.org/10.1111/syen.12278.
Witmer, L. M. (1995). The extant phylogenetic bracket and the importance of reconstructing soft tissues in fossils. Functional Morphology in Vertebrate Paleontology, 1, 19–33.
Zhang, H. C., & Qiao, G. X. (2008). Molecular phylogeny of Pemphiginae (Hemiptera: Aphididae) inferred from nuclear gene EF-1α sequences. Bulletin of Entomological Research, 98(5), 499–507. https://doi.org/10.1017/S0007485308005828.
Zimmermann, D., Randolf, S., & Aspöck, U. (2019). From chewing to sucking via phylogeny – from sucking to chewing via ontogeny: mouthparts of Neuroptera. In H. Krenn (Ed.), Insect Mouthparts (pp. 361–385). Zoological Monographs, vol 5. Springer, Cham. https://doi.org/10.1007/978-3-030-29654-4_11
Acknowledgements
The Volkswagen Foundation kindly supported the work of JTH in the frame of a Lichtenberg professorship. The study has been supported by the German Research Foundation (DFG) under DFG HA 6300/6-1 granted to JTH. CK is currently funded by the Landesgraduiertenförderung MV. We thank all people providing low cost, open access and open source software. J. Matthias Starck, Munich, and Steffen Harzsch, Greifswald, are thanked for long-term support. We thank Corentin Jouault, Paris, and one anonymous reviewer for their helpful comments and suggestions to the manuscript. This is LEON publication #31.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Haug, J.T., Kiesmüller, C., Haug, G.T. et al. A fossil aphidlion preserved together with its prey in 40 million-year-old Baltic amber. Palaeobio Palaeoenv 103, 155–163 (2023). https://doi.org/10.1007/s12549-021-00521-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12549-021-00521-z