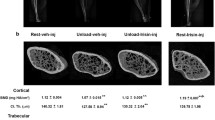
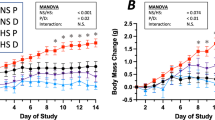
Abstract
The hindlimb-suspended or unloading rodent model was developed to mimic the microgravity of a spaceflight environment and has been used extensively to study the physiological responses to various aspects of musculoskeletal loading and unloading. This study investigated a compensating response to skeletal muscle loss in a hindlimb-suspended model that involves up-regulation of mTOR phosphorylation at four weeks. Body weight and muscle volume significantly decreased over four weeks in the hindlimb-suspended group compared with the sham control. Expressions of p-mTOR, raptor protein, p-p70S6K, p-Akt, and p-ERK, anti- or pro-apoptotic p53, Bcl-2, Bcl-XL, Bax, and Bak proteins were significantly increased in the hindlimb-suspended group compared to the sham control at four weeks. These results indicate that p-mTOR and p-Akt/p-ERK proteins might be up-regulated to compensate for the loss of skeletal muscle in the hindlimb-suspended model. Expressions of p-AMPK, IRS1, and IRS2 proteins were significantly increased, but that of p-eIF2α was significantly reduced in the hindlimb-suspended group compared to the sham control at four weeks. Our results suggest that the loss of skeletal muscle in a hindlimb-suspended model induces activation of p-mTOR and p-Akt/p-ERK proteins, and that these signaling pathways may preserve protein synthesis and cell growth in skeletal muscles of hindlimb-suspended animals.
Similar content being viewed by others
References
Morey-Holton E., Globus R. K., Kaplansky A., and Durnova G., “The Hindlimb Unloading Rat Model: Literature Overview, Technique Update and Comparison with Space Flight Data,” Advances in Space Biology and Medicine, Vol. 10, pp. 7–40, 2005.
Morey-Holton E. R. and Globus R. K., “Hindlimb Unloading Rodent Model: Technical Aspects,” Journal of Applied Physiology, Vol. 92, No. 4, pp. 1367–1377, 2002.
Bunn J. A., Buford T. W., Serra M. C., Kreider R. B., and Willoughby D. S., “Protein and Amino Acid Supplementation Does Not Alter Proteolytic Gene Expression Following Immobilization,” Journal of Nutrition and Metabolism, Vol. 2011, Article ID: 539690, 2011.
De Boer M. D., Maganaris C. N., Seynnes O. R., Rennie M. J., and Narici M. V., “Time Course of Muscular, Neural and Tendinous Adaptations to 23 Day Unilateral Lower-Limb Suspension in Young Men,” The Journal of Physiology, Vol. 583, No. 3, pp. 1079–1091, 2007.
Hornsby, G., Watt, P., Murdoch, G., and Renniei, M., “Decrease in Human Quadriceps Muscle Protein Turnover Consequent Upon Leg Immobilization,” Clinical Science, Vol. 72, No. 4, pp. 503–509, 1987.
Akima, H., Hotta, N., Sato, K., Ishida, K., Koike, T., and Katayama, K., “Cycle Ergometer Exercise to Counteract Muscle Atrophy during Unilateral Lower Limb Suspension,” Aviation, Space, and Environmental Medicine, Vol. 80, No. 7, pp. 652–656, 2009.
Ploutz-Snyder, L. L., Tesch, P. A., Crittenden, D. J., and Dudley, G. A., “Effect of Unweighting on Skeletal Muscle Use during Exercise,” Journal of Applied Physiology, Vol. 79, No. 1, pp. 168–175, 1995.
Biolo, G., Ciocchi, B., Lebenstedt, M., Barazzoni, R., Zanetti, M., et al., “Short Term Bed Rest Impairs Amino AcidInduced Protein Anabolism in Humans,” The Journal of Physiology, Vol. 558, No. 2, pp. 381–388, 2004.
de Boer, M. D., Seynnes, O. R., di Prampero, P. E., Pišot, R., et al., “Effect of 5 Weeks Horizontal Bed Rest on Human Muscle Thickness and Architecture of Weight Bearing and Non-Weight Bearing Muscles,” European Journal of Applied Physiology, Vol. 104, No. 2, pp. 401–407, 2008.
Drummond, M. J., Dickinson, J. M., Fry, C. S., Walker, D. K., Gundermann, D. M., et al., “Bed Rest Impairs Skeletal Muscle Amino Acid Transporter Expression, mTORC1 Signaling, and Protein Synthesis in Response to Essential Amino Acids in Older Adults,” American Journal of Physiology-Endocrinology and Metabolism, Vol. 302, No. 9, pp. E1113–E1122, 2012.
Ferrando, A. A., Lane, H. W., Stuart, C. A., Davis-Street, J., and Wolfe, R. R., “Prolonged Bed Rest Decreases Skeletal Muscle and Whole Body Protein Synthesis,” American Journal of Physiology-Endocrinology and Metabolism, Vol. 270, No. 4, pp. E627–E633, 1996.
Goodman, C. A., Mayhew, D. L., and Hornberger, T. A., “Recent Progress Toward Understanding the Molecular Mechanisms that Regulate Skeletal Muscle Mass,” Cellular Signalling, Vol. 23, No. 12, pp. 1896–1906, 2011.
Hornberger, T. A., Sukhija, K. B., and Chien, S., “Regulation of mTOR By Mechanically Induced Signaling Events in Skeletal Muscle,” Cell Cycle, Vol. 5, No. 13, pp. 1391–1396, 2006.
Kelleher, A. R., Kimball, S. R., Dennis, M. D., Schilder, R. J., and Jefferson, L. S., “The mTORC1 Signaling Repressors Redd1/2 are Rapidly Induced and Activation of P70s6k1 by Leucine is Defective in Skeletal Muscle of an Immobilized Rat Hindlimb,” American Journal of Physiology-Endocrinology and Metabolism, Vol. 304, No. 2, pp. E229–E236, 2013.
Banerjee, A. and Guttridge, D. C., “Mechanisms for Maintaining Muscle,” Current Opinion in Supportive and Palliative Care, Vol. 6, No. 4, pp. 451–456, 2012.
Phillips, B. E., Hill, D. S., and Atherton, P. J., “Regulation of Muscle Protein Synthesis in Humans,” Current Opinion in Clinical Nutrition & Metabolic Care, Vol. 15, No. 1, pp. 58–63, 2012.
Phillips, S. M., Glover, E. I., and Rennie, M. J., “Alterations of Protein Turnover Underlying Disuse Atrophy in Human Skeletal Muscle,” Journal of Applied Physiology, Vol. 107, No. 3, pp. 645–654, 2009.
Booth, F. and Seider, M., “Early Change in Skeletal Muscle Protein Synthesis after Limb Immobilization of Rats,” Journal of Applied Physiology, Vol. 47, No. 5, pp. 974–977, 1979.
Glover, E. I., Phillips, S. M., Oates, B. R., Tang, J. E., Tarnopolsky, M. A., et al., “Immobilization Induces Anabolic Resistance in Human Myofibrillar Protein Synthesis with Low and High Dose Amino Acid Infusion,” The Journal of Physiology, Vol. 586, No. 24, pp. 6049–6061, 2008.
Krawiec, B. J., Frost, R. A., Vary, T. C., Jefferson, L. S., and Lang, C. H., “Hindlimb Casting Decreases Muscle Mass in Part by Proteasome-Dependent Proteolysis but Independent of Protein Synthesis,” American Journal of Physiology-Endocrinology and Metabolism, Vol. 289, No. 6, pp. E969–E980, 2005.
Magne, H., SavaryAuzeloux, I., Migné, C., Peyron, M. A., Combaret, L., Rémond, D., and Dardevet, D., “Contrarily to whey and High Protein Diets, Dietary Free Leucine Supplementation Cannot Reverse the Lack of Recovery of Muscle Mass after Prolonged Immobilization during Ageing,” The Journal of Physiology, Vol. 590, No. 8, pp. 2035–2049, 2012.
Dillon, E. L., “Nutritionally Essential Amino Acids and Metabolic Signaling in Aging,” Amino Acids, Vol. 45, No. 3, pp. 431–441, 2013.
Ge, Y. and Chen, J., “Mammalian Target of Rapamycin (mTOR) Signaling Network in Skeletal Myogenesis,” Journal of Biological Chemistry, Vol. 287, No. 52, pp. 43928–43935, 2012.
Rutter, G., Dasilvaxavier, G., and Leclerc, I., “Roles of 5-Amp-Activated Protein Kinase (Ampk) in Mammalian Glucose Homoeostasis,” Biochemical Journal, Vol. 375, No. pp. 1–16, 2003.
Schiaffino, S., Dyar, K. A., Ciciliot, S., Blaauw, B., and Sandri, M., “Mechanisms Regulating Skeletal Muscle Growth and Atrophy,” FEBS Journal, Vol. 280, No. 17, pp. 4294–4314, 2013.
Witczak, C., Sharoff, C., and Goodyear, L., “Amp-Activated Protein Kinase in Skeletal Muscle: from Structure and Localization to Its Role as a Master Regulator of Cellular Metabolism,” Cellular and Molecular Life Sciences, Vol. 65, No. 23, pp. 3737–3755, 2008.
Glass, D. J., “Pi3 Kinase Regulation of Skeletal Muscle Hypertrophy and Atrophy,” in: Phosphoinositide 3-Kinase in Health and Disease, Rommel, C., Vanhaesebroeck, B., and Vogt, P., (Eds.), Springer, pp. 267–278, 2011.
Latres, E., Amini, A. R., Amini, A. A., Griffiths, J., Martin, F. J., et al., “Insulin-like Growth Factor-1 (Igf-1) Inversely Regulates Atrophy-Induced Genes via the Phosphatidylinositol 3-Kinase/Akt/Mammalian Target of Rapamycin (Pi3k/Akt/mTOR) Pathway,” Journal of Biological Chemistry, Vol. 280, No. 4, pp. 2737–2744, 2005.
Musi, N. and Goodyear, L. J., “Insulin Resistance and Improvements in Signal Transduction,” Endocrine, Vol. 29, No. 1, pp. 73–80, 2006.
Bertrand, L., Ginion, A., Beauloye, C., Hebert, A. D., Guigas, B., et al., “Ampk activation Restores the Stimulation of Glucose Uptake in an in Vitro Model of Insulin-Resistant Cardiomyocytes Via the Activation of Protein Kinase B,” American Journal of Physiology-Heart and Circulatory Physiology, Vol. 291, No. 1, pp. H239–H250, 2006.
Long, Y. C., Cheng, Z., Copps, K. D., and White, M. F., “Insulin Receptor Substrates Irs1 and Irs2 Coordinate Skeletal Muscle Growth and Metabolism Via the Akt and Ampk Pathways,” Molecular and Cellular Biology, Vol. 31, No. 3, pp. 430–441, 2011.
Longnus, S., Segalen, C., Giudicelli, J., Sajan, M., Farese, R., and Van Obberghen, E., “Insulin Signalling Downstream of Protein Kinase B is Potentiated by 5’ Amp-Activated Protein Kinase in Rat Hearts in Vivo,” Diabetologia, Vol. 48, No. 12, pp. 2591–2601, 2005.
Qiao, L. Y., Zhande, R., Jetton, T. L., Zhou, G., and Sun, X. J., “In Vivo Phosphorylation of Insulin Receptor Substrate 1 at Serine 789 by a Novel Serine Kinase in Insulin-Resistant Rodents,” Journal of Biological Chemistry, Vol. 277, No. 29, pp. 26530–26539, 2002.
Tzatsos, A. and Tsichlis, P. N., “Energy Depletion Inhibits Phosphatidylinositol 3-Kinase/Akt Signaling and Induces Apoptosis Via Amp-Activated Protein Kinase-Dependent Phosphorylation of Irs-1 at Ser-794,” Journal of Biological Chemistry, Vol. 282, No. 25, pp. 18069–18082, 2007.
Wang, C., Mao, X., Wang, L., Liu, M., Wetzel, M. D., et al., “Adiponectin Sensitizes Insulin Signaling by Reducing P70 S6 Kinase-Mediated Serine Phosphorylation of Irs-1,” Journal of Biological Chemistry, Vol. 282, No. 11, pp. 7991–7996, 2007.
Bloomfield, S. A., Girten, B. E., and Weisbrode, S. E., “Effects of Vigorous Exercise Training and B-Agonist Administration on Bone Response to Hindlimb Suspension,” Journal of Applied Physiology, Vol. 83, No. 1, pp. 172–178, 1997.
Park, E. and Schultz, E., “A Simple Hindlimb Suspension Apparatus,” Aviation, Space, and Environmental Medicine, Vol. 64, No. 5, pp. 401–404, 1993.
Sarbassov, D. D., Ali, S. M., and Sabatini, D. M., “Growing Roles for the mTOR Pathway,” Current Opinion in Cell Biology, Vol. 17, No. 6, pp. 596–603, 2005.
Steelman, L. S., Chappell, W. H., Abrams, S. L., Kempf, C. R., Long, J., et al., “Roles of the Raf/Mek/Erk and Pi3k/Pten/Akt/mTOR Pathways in Controlling Growth and Sensitivity to Therapy- Implications for Cancer and Aging,” Aging (Albany NY), Vol. 3, No. 3, pp. 192–222, 2011.
Ko, C. Y., Seo, D. H., and Kim, H. S., “Deterioration of Bone Quality in the Tibia and Fibula in Growing Mice during Skeletal Unloading: Gender-Related Differences,” Journal of Biomechanical Engineering, Vol. 133, No. 11, Paper No. 111003, 2011.
Waarsing, J., Day, J., Van der Linden, J., Ederveen, A., Spanjers, C., et al., “Detecting and Tracking Local Changes in the Tibiae of Individual Rats: A Novel Method to Analyse Longitudinal in Vivo Micro-Ct Data,” Bone, Vol. 34, No. 1, pp. 163–169, 2004.
Seo, D. H., Kim, H. S., Ko, C. Y., Schreiber, J., Jang, Y. S., and Bae, K., “The Effects of Circadian Disturbances Induced by Night Shifts on the Mouse Peripheral Tissues,” Animal Cells and Systems, Vol. 16, No. 5, pp. 357–365, 2012.
Luu, Y., Lublinsky, S., Ozcivici, E., Capilla, E., Pessin, J., et al., “In Vivo Quantification of Subcutaneous and Visceral Adiposity by Micro-Computed Tomography in a Small Animal Model,” Medical Engineering & Physics, Vol. 31, No. 1, pp. 34–41, 2009.
Garman, R., Gaudette, G., Donahue, L. R., Rubin, C., and Judex, S., “Low-Level Accelerations Applied in the Absence of Weight Bearing can Enhance Trabecular Bone Formation,” Journal of Orthopaedic Research, Vol. 25, No. 6, pp. 732–740, 2007.
Müller, R., Hahn, M., Vogel, M., Delling, G., and Rüegsegger, P., “Morphometric Analysis of Noninvasively Assessed Bone Biopsies: Comparison of High-Resolution Computed Tomography and Histologic Sections,” Bone, Vol. 18, No. 3, pp. 215–220, 1996.
Rüegsegger, P., Koller, B., and Müller, R., “A Microtomographic System for the Nondestructive Evaluation of Bone Architecture,” Calcified Tissue International, Vol. 58, No. 1, pp. 24–29, 1996.
Haddad, F., Adams, G., Bodell, P., and Baldwin, K., “Isometric Resistance Exercise Fails to Counteract Skeletal Muscle Atrophy Processes during the Initial Stages of Unloading,” Journal of Applied Physiology, Vol. 100, No. 2, pp. 433–441, 2006.
BiBodine, S. C., Stitt, T. N., Gonzalez, M., Kline, W. O., Stover, G. L., et al., “Akt/mTOR Pathway is a Crucial Regulator of Skeletal Muscle Hypertrophy and Can Prevent Muscle Atrophy in Vivo,” Nature Cell Biology, Vol. 3, No. 11, pp. 1014–1019, 2001.
Sandri, M., Sandri, C., Gilbert, A., Skurk, C., Calabria, E., et al., “Foxo Transcription Factors Induce the Atrophy-Related Ubiquitin Ligase Atrogin-1 and Cause Skeletal Muscle Atrophy,” Cell, Vol. 117, No. 3, pp. 399–412, 2004.
Zhao, J., Brault, J. J., Schild, A., Cao, P., Sandri, M., et al., “Foxo3 Coordinately Activates Protein Degradation by the Autophagic/ Lysosomal and proteasomal Pathways in Atrophying Muscle Cells,” Cell Metabolism, Vol. 6, No. 6, pp. 472–483, 2007.
Mammucari, C., Schiaffino, S., and Sandri, M., “Downstream of Akt: FoxO3 and mTOR in the Regulation of Autophagy in Skeletal Muscle,” Autophagy, Vol. 4, No. 4, pp. 524–526, 2008.
Zhao, J., Brault, J. J., Schild, A., and Goldberg, A. L., “Coordinate Activation of Autophagy and the Proteasome Pathway by Foxo Transcription Factor,” Autophagy, Vol. 4, No. 3, pp. 378–380, 2008.
Risson, V., Mazelin, L., Roceri, M., Sanchez, H., Moncollin, V., et al., “Muscle Inactivation of mTOR Causes Metabolic and Dystrophin Defects Leading to Severe Myopathy,” The Journal of Cell Biology, Vol. 187, No. 6, pp. 859–874, 2009.
Bentzinger, C. F., Romanino, K., Cloëtta, D., Lin, S., Mascarenhas, J. B., et al., “Skeletal Muscle-Specific Ablation of Raptor, but Not of Rictor, Causes Metabolic Changes and Results in Muscle Dystrophy,” Cell Metabolism, Vol. 8, No. 5, pp. 411–424, 2008.
Ohanna, M., Sobering, A. K., Lapointe, T., Lorenzo, L., Praud, C., et al., “Atrophy of S6k1/Skeletal Muscle Cells Reveals Distinct mTOR Effectors for Cell Cycle and Size Control,” Nature Cell Biology, Vol. 7, No. 3, pp. 286–294, 2005.
Park, I. H., Erbay, E., Nuzzi, P., and Chen, J., “Skeletal Myocyte Hypertrophy Requires mTOR Kinase Activity and S6k1,” Experimental Cell Research, Vol. 309, No. 1, pp. 211–219, 2005.
Rommel, C., Bodine, S. C., Clarke, B. A., Rossman, R., Nunez, L., et al., “Mediation of IGF-1-Induced Skeletal Myotube Hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 Pathways,” Nature Cell Biology, Vol. 3, No. 11, pp. 1009–1013, 2001.
Kumar, A., Harris, T. E., Keller, S. R., Choi, K. M., Magnuson, M. A., and Lawrence, J. C., “Muscle-Specific Deletion of Rictor Impairs Insulin-Stimulated Glucose Transport and Enhances Basal Glycogen Synthase Activity,” Molecular and Cellular Biology, Vol. 28, No. 1, pp. 61–70, 2008.
Shu, L. and Houghton, P. J., “The mTORC2 Complex Regulates Terminal Differentiation of C2C12 Myoblasts,” Molecular and Cellular Biology, Vol. 29, No. 17, pp. 4691–4700, 2009.
Gorres, B. K., Bomhoff, G. L., Morris, J. K., and Geiger, P. C., “In Vivo Stimulation of Oestrogen Receptor a Increases Insulin Stimulated Skeletal Muscle Glucose Uptake,” The Journal of Physiology, Vol. 589, No. 8, pp. 2041–2054, 2011.
Velders, M., Schleipen, B., Fritzemeier, K. H., Zierau, O., and Diel, P., “Selective Estrogen Receptor-B Activation Stimulates Skeletal Muscle Growth and Regeneration,” The FASEB Journal, Vol. 26, No. 5, pp. 1909–1920, 2012.
Johnson, B., White, M., Hathaway, M., Christians, C., and Dayton, W., “Effect of a Combined Trenbolone Acetate and Estradiol Implant on Steady-State IGF-I Mrna Concentrations in the Liver Of Wethers and the Longissimus Muscle of Steers,” Journal of Animal Science-Menasha Then Albany Then Champaign IllinoiS-, Vol. 76, No. pp. 491–497, 1998.
Tsai, W. J. A., McCormick, K. M., Brazeau, D. A., and Brazeau, G. A., “Estrogen Effects on Skeletal Muscle Insulin-Like Growth Factor-1 and Myostatin in Ovariectomized Rats,” Experimental Biology and Medicine, Vol. 232, No. 10, pp. 1314–1325, 2007.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yeong-Min Yoo and Ji Hyung Park contributed equally to this work
Rights and permissions
About this article
Cite this article
Yoo, YM., Park, J.H., Seo, DH. et al. Activation of mTOR for the loss of skeletal muscle in a hindlimb-suspended rat model. Int. J. Precis. Eng. Manuf. 16, 1003–1010 (2015). https://doi.org/10.1007/s12541-015-0130-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12541-015-0130-1