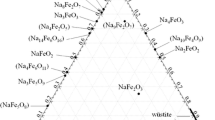
Abstract
Thermodynamic optimization of phase diagrams and thermodynamic properties of the FeO–Fe2O3–MnO–Mn2O3–TiO2–Ti2O3 system at 1 atm pressure was performed based on the literature data and new phase diagram data in this study. Isothermal phase diagrams of the system at 1250–1600 °C in air atmosphere were experimentally determined for the first time using the equilibration and quenching method. The liquid phase and complex solid solutions like pseudobrookite, ilmenite and spinel were described using the Modified Quasichemical Model and Compound Energy Formalism, respectively. A set of optimized model parameters of all phases was obtained which reproduces all reliable thermodynamic data and phase equilibria within 3 mol% in composition and ± 25 °C in temperature. The present thermodynamic results can help the prediction of complex thermodynamic data and phase equilibria of the Fe–Mn–Ti–O system at 1 atm total pressure from 25 °C to above the liquidus temperatures over the entire range of composition and \(p_{{O_{2} }}\) from metallic saturation to 1 atm.
Graphic abstract















Similar content being viewed by others
References
K. Seo, K. Kim, H.J. Kim, H. Ryoo, G.M. Evans, C. Lee, Met. Mater. Int. 26, 1226 (2020)
B. Wang, J. Li, Met. Mater. Int. (2020). https://doi.org/10.1007/s12540-020-00617-9
A. Feenstra, T. Peters, Contrib. Mineral. Petrol. 126, 109 (1996)
K.N. Raymond, H.R. Wenk, Contrib. Mineral. Petrol. 30, 135 (1971)
A.M. Hofmeister, Eur. J. Mineral. 5, 281 (1993)
N. Tomioka, K. Fujino, Science 277, 1084 (1997)
T. Yamanaka, Y. Komatsu, M. Sugahara, T. Nagai, Am. Miner. 90, 1301 (2005)
A.F. Buddington, D.H. Lindsley, J. Petrol. 5, 310 (1964)
K. Spencer, D.H. Lindsley, Am. Mineral. 66, 1189 (1981)
D.J. Andersen, D.H. Lindsley, Am. Mineral. 73, 714 (1988)
S.A. Degterov, E. Jak, P.C. Hayes, A.D. Pelton, Metall. Mater. Trans. B 31, 651 (2000)
Y.-B. Kang, I.-H. Jung, Metall. Mater. Trans. E. 3, 156 (2016)
S.K. Panda. Montréal. McGill University. (2019)
S.K. Panda, P. Hudon, I.-H. Jung, Calphad 66, 101639 (2019)
Y.-B. Kang, I.-H. Jung, J. Phys. Chem. Solids 98, 237 (2016)
C.W. Bale, E. Bélisle, P. Chartrand, S.A. Decterov, G. Eriksson, A.E. Gheribi, K. Hack, I.-H. Jung, Y.-B. Kang, J. Melançon, A.D. Pelton, S. Petersen, C. Robelin, J. Sangster, P. Spencer, M.-A. Van Ende, Calphad 54, 35 (2016)
I.E. Grey, A.F. Reid, D.G. Jones, Trans. Inst. Min. Metall., Sect. C 83, 105 (1974)
I.E. Grey, Ph.D. thesis, University of Melbourne (1975)
R.R. Merritt, J. Solid State Chem. 43, 267 (1982)
R.R. Merritt, J. Solid State Chem. 53, 254 (1985)
I.C. Smith, H.B. Bell, Trans. Inst. Min. Metall., Sect. C 80, 55 (1971)
Y. Morioka, K. Morita, F. Tsukihashi, N. Sano, Tetsu to Hagane 81, 40 (1995)
K. Morita, Y. Morioka, F. Tsukihashi, N. Sano, Ironmak. Conf. Proc. 55, 775 (1996)
M. Hillert, B. Jansson, B. Sundman, Application of the compound-energy model to oxide systems. Z. Metallkd. 79, 81 (1988)
A.D. Pelton, Calphad 25, 319 (2001)
A.D. Pelton, S.A. Degterov, G. Eriksson, C. Robelin, Y. Dessureault, Metall. Mater. Trans. B 32, 643 (2000)
A.D. Pelton, P. Chartrand, Metal Mater. Trans. A. 32, 1355 (2001)
G. Eriksson, A.D. Pelton, Metall. Mater. Trans. B 24, 795 (1993)
P. Wu, G. Eriksson, A.D. Pelton, J. Am. Ceram. Soc. 76, 2065 (1993)
E. Ising, Z. Phys. 31, 253 (1925)
S.K. Panda, I.-H. Jung, ISIJ Int. 60, 31 (2019)
W.Q. Guo, S. Malus, D.H. Ryan, Z. Altounian, J. Phys. Condens. Matter. 11, 6337 (1999)
A.R. Lennie, K.S. Knight, C.M.B. Henderson, Am. Mineral. 92, 1165 (2007)
G. Seitz, N. Penin, L. Decoux, A. Wattiaux, M. Duttine, M. Gaudon, Inorg. Chem. 55, 2499 (2016)
R.H. Mitchell, R.P. Liferovich, Can. Metal. 42, 1871 (2004)
R.P. Liferovich, R.H. Mitchell, Can. Metal. 44, 1099 (2006)
R.P. Liferovich, R.H. Mitchell, Crystallogr. Rep. 51, 383 (2006)
R.J. Harrison, U. Becker, S.A.T. Redfern, Am. Mineral. 85, 1694 (2000)
R.J. Harrison, S.A.T. Redfern, R.I. Smith, Am. Mineral. 85, 194 (2000)
S.K. Panda, I.-H. Jung, J. Am. Ceram. Soc. 97, 3328 (2014)
A.N. Grundy, B. Hallstedt, L.J. Gauckler, J. Phase Equilib. 24, 21 (2003)
S.E. Dorris, T.O. Mason, J. Am. Ceram. Soc. 71, 379 (1988)
C.I. Pearce, C.M.B. Henderson, N.D. Telling, R.A.D. Pattrick, J.M. Charnock, V.S. Coker et al., Am. Mineral. 95, 425 (2010)
B.A. Wechsler, A. Navrotsky, J. Solid State Chem. 55, 165 (1984)
R.L. Millard, R.C. Peterson, B.K. Hunter, Am. Mineral. 80, 885 (1995)
E.F. Bertaut, H. Vincent, Solid State Commun. 6, 269 (1968)
V. Stevanovic, M. d’Avezac, A. Zunger, J. Am. Chem. Soc. 133, 11649 (2011)
I.-H. Jung, Ph.D. Thesis, École Polytechnique. Montréal (2003), p. 338
Y.-B. Kang, I.-H. Jung, H.-G. Lee, Calphad 30, 235 (2006)
G. Eriksson, A.D. Pelton, E. Woermann, A. Ender, Ber. Bunsenges. Phys. Chem. 100, 1839 (1996)
Acknowledgements
Financial supports from Tata Steel Europe, POSCO, Hyundai Steel, Nucor Steel, RioTinto Iron and Titanium, Nippon Steel and Sumitomo Metals Corp., JFE Steel, Voestalpine Stahl, RHI, Schott A.G., and the Natural Sciences and Engineering Research Council of Canada are gratefully acknowledged. One of the authors (S.K. Panda) would like to thank the McGill Engineering Doctorate Award (MEDA) from McGill University. This work was also supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government(MSIT)(No. 2020R1A5A6017701).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Panda, S.K., Jung, IH. Coupled Experimental Study and Thermodynamic Modeling of the Fe–Mn–Ti–O System. Met. Mater. Int. 27, 725–743 (2021). https://doi.org/10.1007/s12540-020-00815-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12540-020-00815-5