Abstract
The species Mulinia lateralis (Say, 1822) is native to the western North Atlantic Ocean and was first documented in European coastal waters in 2017. Since then, M. lateralis was reported several times in large abundances in the coastal waters of the Netherlands, Belgium, and more scattered in Germany. While the introduction vector is still unclear, we assume that dispersal in the southern North Sea is driven by larval drift related to anti-clockwise residual tidal currents. To test this hypothesis and to document its current status in the central Wadden Sea, individuals were sampled systematically from intertidal flats along 10 transects ranging from the outer Ems River estuary in the west to the outer Elbe River estuary in the east (German North Sea coast) between February and May 2022. In total, 897 specimens of M. lateralis were sampled from 392 stations (mean abundance 2.3 ± 5.0 ind./m2). The shell length ranged between 4.0 and 23.6 mm. Regarding the increasing number of records of M. lateralis at multiple sites in Europe since 2017 and based on the data of this study, the species can be considered as established in the western and central Wadden Sea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Wadden Sea stretches from the Netherlands to Denmark and represents, with 4700 km2 emerging tidal flat area during low tide, the largest coherent dynamic intertidal system in the world (Wehrmann 2016). Due to its young post-glacial evolution and complex structure (barrier islands, tidal flats, estuaries, salt marshes), it provides numerous, so far unoccupied niches (Wolff 1999) for species immigrating by expansion or shift of their biogeographic range. Although species dispersal is an ongoing natural process increasing the species richness (Beukema and Dekker 2011), rapid changes in community compositions can have a drastic influence on the balance within this ecosystem. Increasing globalisation, maritime transport and expanded aquaculture favour the unintended introduction of non-indigenous species (NIS) and despite monitoring and management programmes, the rate at which new introductions are observed is rising (Reise et al. 1999; Büttger et al. 2022).
Annually, about two additional NIS are introduced into the German North Sea and a total of 92 macrobiota species were officially registered in this area until today (Büttger et al. 2022). Nevertheless, a complete local extinction of native species was not documented yet and most NIS remain neutral and additive to the ecosystem (Gollasch and Nehring 2006; Buschbaum et al. 2012; Reise et al. 2023). The manifold burdens and benefits and their role in biodiversity changes and ecosystem services are intensely discussed (Schlaepfer et al. 2011; Boltovskoy et al. 2022; Kourantidou et al. 2022). For example, the Pacific oyster Magallana gigas (Thunberg, 1793), which was introduced to the German North Sea in 1991 (Reise 1998; Wehrmann et al. 2000), was suspected to outcompete the blue mussel Mytilus edulis Linnaeus, 1758. It was revealed that although the condition indices of both species were reduced, neither their growth rates nor mortality were affected by their coexistence (Joyce et al. 2021). However, a potential negative impact of NIS cannot be ruled out. Invasive species can alter the biotic community, habitats, and trophic structure (Crooks 1998; Kolar and Lodge 2001; Markert et al. 2010; Büttger et al. 2022); provide a basis for subsequent invasions (Markert et al. 2014); or cause economic damages like the Chinese mitten crab Eriocheir sinensis H. Milne Edwards, 1853 by undermining coastal protection dikes of the estuaries and infesting fish nets and traps (Gollasch 2011). Especially when facing future species distribution shifts and ecosystem changes in the context of the climate crisis and resulting socio-economic changes, monitoring of NIS is inevitable (Essl et al. 2020; Simões et al. 2021).
The majority of NIS in the Wadden Sea originates from the Western Atlantic or Pacific (Wolff 2005; Büttger et al. 2022). This is also the case for the dwarf surf clam Mulinia lateralis (Say, 1822) examined in this study. Its distribution range is originally in the Western North Atlantic from the Gulf of St. Lawrence to the Gulf of Mexico (Walker and Tenore 1984; Montagna and Kalke 1995; Brunel et al. 1998; Turgeon et al. 2009). Since August 2017, it was detected outside its native range along the Dutch coast by Craeymeersch et al. (2019) and Klunder et al. (2019). However, it was initially (mis-)identified as Spisula subtruncata (da Costa, 1778), as this species is native to the North Sea. M. lateralis was able to establish rapidly high densities of up to 5872.4 ind./m2 in the Dutch Voordelta (Craeymeersch et al. 2019) and was recorded annually from 2017 to 2021 at multiple locations (Wood et al. 2022). In Belgium, M. lateralis has been detected at several sites from Knokke to de Panne (Kerckhof 2019; Walles et al. 2020; Wood et al. 2022). Since 2017, M. lateralis has also been recorded in the German part of the Ems Dollard estuary (Klunder et al. 2019; G. Scheiffarth pers. comm.). Additional isolated records were reported further east at the JadeWeserPort (IfAÖ 2020), near Tossens, and in the inter- and subtidal around the Island of Sylt (K. Reise pers. comm.; U. Schückel pers. comm.).
The aim of the present study is (i) to test the hypothesis of a stepwise spread of the Mulinia lateralis larvae by anti-clockwise residual tidal currents as known from previous invasions (Brandt et al. 2008; Markert et al. 2014) by (ii) documenting the initial distribution based on population parameters (i.e., abundances, length-frequency distributions, biomasses) from a systematic survey along the coast which allows (iii) to define the present status of this non-native species in the central Wadden Sea.
Material and methods
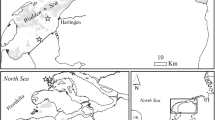
To document the present occurrence status of Mulinia lateralis along the central Wadden Sea coast, we developed a survey design, which allows easy access to the potential intertidal habitats. As known from some other non-native species (e.g. Magallana gigas, Hemigrapsus takanoi Asakura & Watanabe, 2005), invasion into the central Wadden Sea is often driven by an eastward drift of larvae due to residual tidal currents (Brandt et al. 2008; Markert et al. 2014). Therefore, sampling was carried out between February and May 2022 along 10 shore-normal transects (T1 to T10) in the Lower Saxon Wadden Sea National Park distributed regularly from the outer Ems River estuary in the west to the outer Elbe River estuary in the east (Fig. 1; Table 1).
Location of the surveyed transects in the central Wadden Sea and position of previous findings (asterisks) of Mulinia lateralis since August 2017 (Craeymeersch et al. 2019; Klunder et al. 2019; IfAÖ 2020; G. Scheiffarth pers. comm.). Greenish: above mean high-water; yellowish: intertidal; bluish: subtidal
Salinity in the study area covers a broad range from 13 to 22 in the outer Ems River estuary (T1 and T2) over 28 to 31 in the central tidal basins (T3), 30 to 34 in the Jade channel (T7 and T8) to 16 to 22 in the outer Elbe River estuary (T10) (Reineck and Flemming 1990; Becker 1998; Kaiser and Niemeyer 1999; Glorius et al. 2021). The tidal range of the semidiurnal tide varies between 2.9 m in the outer Ems River estuary (T1), 2.5 m in the backbarrier tidal flats (T3), 3.8 m in the Jade (T8), and 2.9 m in Cuxhaven (T10) (BSH 2022).
Each transect was 1 km in length covering 40 sampling stations of 1 m2, each 25 m apart from the other, starting close to the mean high-water line of the mainland coast following the topographic gradient down close to the low-water line. Bathymetric data along the transects were taken from the 2016 digital terrain model (10 m grid length) of the EasyGSH-DB platform (https://mdi-dienste.baw.de/geoserver/EasyGSH_Bathymetrie/wms; https://doi.org/10.48437/02.2020.K2.7000.0002). Bathymetric data refer to the standard elevation datum NHN. The top sediment layer (uppermost 3–4 cm) inside the 1 × 1 m-frame was sieved through a 1mm-sieve in the field. All specimens of M. lateralis were collected and stored for 1–5 days at 4 °C until further identification and measurements in the lab. Shell length was measured with a digital caliper rule (accuracy: 0.01 mm) from the posterior margin to the anterior margin. Live-wet weight (i.e. shell, flesh and mantle water) was measured with a KERN EW 220-3NM scale (accuracy: 1 mg). The specimens were identified using a Leica M205 C stereomicroscope with the identification key provided by Craeymeersch et al. (2019). All specimens were preserved in 96% denatured Ethanol. The occurrence data were summarised and uploaded as a new dataset to the open source platform EurOBIS (Gismann et al. 2023, https://doi.org/10.14284/602).
Statistical analyses were calculated in RStudio (R Core Team 2022, version 4.2.1; RStudio Team 2022) using the package ggplot2 (Wickham 2016).
Condition indices were calculated separately for all sampled specimens of M. lateralis (n = 897; Fig. 5), with the formula (Jakob et al. 1996; Marzec et al. 2010; Smith et al. 2000):
with W as the measured live-wet weight and L as the shell length. The exponent b is the slope calculated in the power relationship between live-wet weight and shell length (Fig. 5). To test the condition indices between the transects, the Kruskal-Wallis test (1952) and post hoc Dunn test (1964) were performed, using the Benjamini-Hochberg correction (1995).
The bimodality of the length distribution was analysed in two ways, using the function is.bimodal() available in the package LaplacesDemon (Statisticat 2021) and the function bimodality_coefficient() of the package mousetrap (Wulff et al. 2021).
In addition to the characteristic morphological features, we confirmed the species determination using DNA-based analyses of three specimens from each of the transects T1, T2 and T4 to T9 and two specimens from T3. The targeted COI gene region was amplified by polymerase chain reactions (PCRs) in Mastercycler pro S thermocycler (Eppendorf, Hamburg, Germany) with a final reaction volume of 20 μL, comprising 10 μL of Accustart II PCR SuperMix, 0.5 μL of each species-specific primers Mul2L (5′-TTATTCGAATGGAGTTAACATC-′3) and Mul1R (5′-GAACCTCTTTCCGCATAGGT-′3; Hare et al. 2000), 8 μL of ultrapure water, and 1 μL DNA extract. The PCR reactions were performed by an initial denaturation at 94 °C for 3 min, followed by 38 cycles consisting of 30 s of denaturation at 94 °C, 45 s of annealing at 45 °C, and 1 min of elongation at 72 °C, and a final extension for 3 min at 72 °C. PCR products were Sanger sequenced by Macrogen Europe (Amsterdam, Netherlands) in both directions. Resulting sequences were processed in Geneious™ (version R7.1.9.) and quality checked using the BLAST tool of NCBI (Altschul et al. 1997).
Results
Morphological and genetic identification of Mulinia lateralis
Following the identification key provided by Craeymeersch et al. (2019), we could exclude other species of the family Mactridae occurring in the NE Atlantic Ocean with similar morphological characteristics (most similar to Spisula subtruncata (da Costa, 1778)). The following morphological features were decisive for the identification of M. lateralis (Fig. 2): (1) the triangular shell outline; (2) the distinct radial ridge along the posterior end of the valves; (3) the ligament was exclusively internal; (4) accessory lamella well developed; (5) anterior lateral teeth in the right valve of different sizes, the ventral one longer; two posterior lateral teeth similar in size; (6) shell colour whitish to cream; (7) shell surface smooth; (8) shell distinctly convex and (9) the cardial area between beaks was broad in large specimens.
Morphological identification characteristics of Mulinia lateralis; a Inside left valve with posterior lateral tooth (PLT), anterior lateral tooth (ALT), cardinal teeth (CT), and accessory lamella (AL); b Inside right valve with internal ligament (IL). Scale bar for a and b 5 mm; c Inside view with pallial sinus and pallial line; d Outside view with posterior radial ridge (RR). Scale bar for c and d 10 mm
All sequenced specimens matched 100% with prior uploaded sequences from Klunder et al. (2019) (MN207099.1; MN207097.1) and with sequences submitted by the Smithsonian Environmental Research Center (KT959410.1; KT959381.1). Sequence vouchers of specimens investigated in this study are publicly accessible through GenBank (Accession No. OP575829 to OP575848).
Abundance and spatial distribution
In the sampling area of 392 m2, 897 specimens of M. lateralis were found at 9 of 10 transects (Table 1; Fig. 3). No specimens of M. lateralis were found in the easternmost transect T10. The majority of individuals (86%) was found in three transects (Fig. 3), namely T1 (n = 343), T5 (n = 144), and T6 (n = 284). The highest mean abundance was recorded in T1 (10.72 ± 9.78 ind./m2), followed by T6 (7.10 ± 5.96 ind./m2) and T5 (3.60 ± 4.29 ind./m2). Recorded abundances in T2, T4, and T8 were lower, ranging between 0.63 ± 1.27 ind./m2 and 1.48 ± 1.50 ind./m2. Few individuals were found in T3, T7, and T9, resulting in a mean abundance of 0.1 ind./m2 or less (Table 1; Fig. 3).
M. lateralis is mainly distributed in an elevation range from + 0.35 m to − 0.40 m NHN (Fig. 4). Specimens in transects T1, T4, T5, and T8 are distributed in a depth range from + 0.25 m to − 0.43 m NHN, with the highest abundances at a depth below 0 m NHN (Fig. 3). In transect T1, the abundance increases with depth, with an average abundance of 30.25 ± 4.03 ind./m2 between − 0.33 m (station 29) and − 0.39 m NHN (station 32). In T2 and T6, the individuals are distributed in shallower depth ranges of + 0.52 m to − 0.24 m NHN (Fig. 3). All individuals found in transect T9 (n = 4) and two individuals in T7 (n = 3) are distributed in an elevation range from + 0.44 m to + 0.26 m NHN. The third individual in T7 was found at a higher elevation of + 0.94 m NHN.
Population structure
In total, the specimens of M. lateralis ranged in size between 3.98 and 23.55 mm. 3.57% (n = 32) of the individuals found in this study exceed the previously known maximum size of 21.2 mm (Craeymeersch et al. 2019). Live-wet weight ranged between 0.016 and 3.115 g. No pattern in length distribution along the elevation gradient was observed (Fig. 1 supplementary material).
Shell length and live-wet weight show a power relationship (Fig. 5). The log–log relationship is linear (linear model, R-squared: 0.95, p-value: < 2.2e-16) with log(intercept) = − 7.874 and slope = 2.846. This results in the formula:
with W as live-wet weight and L as shell length.
The condition indices calculated with the Formula (1) range from 0.51 × 10−4 in T6 to 7.18 × 10−4 in T8 (Fig. 6). The Kruskal-Wallis and the following post hoc Dunn test of the condition index per transect confirm a significantly lower condition index in T1 (\(\widetilde{x}\) = 3.52 × 10−4) than every other transect tested (Figs. 5 and 6). Transect T8 (\(\widetilde{x}\) = 4.16 × 10−4) shows a significant higher condition index than T1, T5, and T6 (Fig. 6).
The probability density function on shell length for all sampled individuals is bimodal, suggesting the presence of two size classes. Bimodality is supported by the function is.bimodal(), but did not reach the threshold criterium for the bimodality coefficient according to Pfister et al. (2013).
Separate testing of bimodality in T1, T2, T4, T5, T6, and T8, with n ≥ 25, reveals a bimodal shell length distribution fulfilling the threshold criterion of at least one bimodality test at every transect except T4. The density curves of transects T1, T2, T6, and T8 are similar, with a first increase in density ranging from 8 to 14 mm shell length and a second increase at a shell length of 16 to 20 mm. The bimodal distribution in transect T5 is shifted towards higher shell lengths, with a first peak at a shell length of 12.5 to 17.5 mm and a second peak at 20 to 22.5 mm (Fig. 7). The density curve of T4 shows a single peak at a shell length of 15 mm to 20 mm (Fig. 7).
Discussion
Mulinia lateralis occurs in high densities in its native range along the North American East Coast (Flint and Younk 1983; 74022 ind./m2 in the Hillsborough Bay, USA (Santos and Simon 1980); 63168 ind./m2 in the intertidal area of the estuarine Wassaw Sound Bay, USA (Walker and Tenore 1984)), as well as in the non-native European coastal waters (5872 ind./m2 in the sublittoral of the Dutch Voordelta (Craeymeersch et al. 2019)). Craeymeersch et al. (2019) documented lower densities in the intertidal, ranging from 2.4 to 9.8 ind./m2, with exception of the intertidal in the Dutch Westerschelde (820.0 ind./m2).
This study confirms the occurrence of M. lateralis from the outer Ems River estuary up to the Dorumer Watt at the outer Weser River estuary. The maximum abundance of 36 ind./m2 was recorded at station 31 (− 0.37 m NHN) in transect T1 in the outer Ems River estuary, exceeding the maximum abundance of 9.8 ind./m2 known so far for this area (Craeymeersch et al. 2019) and the German North Sea.
The maximum shell length of 23.55 mm recorded in this study exceeds previous reports. Say (1822) first described a maximum shell length of 12.7 mm. Others state a maximum length of 20 mm (Calabrese 1969; Zettler and Alf 2021). Craeymeersch et al. (2019) reported a maximum shell length of 21.2 mm within the Dutch Wadden Sea area. In our study, 3.57% (n = 32) of all individuals investigated exceed the currently known maximum size of 21.2 mm (Craeymeersch et al. 2019).
Based on a life expectancy of 2 years known for M. lateralis (Calabrese, 1969), it can be assumed that the bimodal distribution of the length frequency (Fig. 7) shows the presence of two cohorts. This leads to the assumption that M. lateralis is able to survive and successfully reproduce.
Differences in condition indices between transects may be influenced by the time of sampling (Marzec et al. 2010). Over the past 50 years, there has been a consistent rise in monthly mean temperatures during all seasons in the Wadden Sea (Beukema et al. 2009; van Aken 2008). In cold-blooded animals, metabolism is regulated by outside temperatures. During a mild winter, metabolic rates are high and food availability low as the main food source, unicellular phytoplankton, is dependent on light. This results in a negative energy balance and greater weight loss (Beukema 1992). Therefore, the significantly lower condition index in T1 could be due to greater weight loss during winter. Sampling started in February (T1) and March (T2, T4, T6, T7), and lasted until April (T3, T5) and May (T8–T10). Following previous studies, the growing season coincides with the phytoplankton cycle from April to September (Dörjes 1992; Ramón 2003). Since the other transects (T2–T10) were sampled later in the year when food availability is greater, animals in these transects had time to regain the weight lost during the winter, resulting in a higher condition index compared to T1. This is supported by the finding of the highest condition index sampled in transect T8 (Table 1; Fig. 6). The sampling time may influence the shift in shell length distribution between the transect (Fig. 7).
Spatial differences in abundance may be dependent on hydrodynamics and sediment composition (Rosenberg 1995; Kröncke 2006; Schückel et al. 2013). Strong currents prevent larvae and small juveniles from settling and thus prevent a successful recruitment. Higher abundances of M. lateralis are reported in sandy mud and mud compared to coarser sediment (Walker and Tenore 1984; Klunder et al. 2019). Since no data about the sediment structure and hydrodynamics was collected, no statement about its influence on the recorded abundances can be made.
M. lateralis tolerates a wide range of salinity (Parker 1975) and occurs in mixohaline waters (Brunel et al. 1998; McKeon et al. 2015). In the Dutch Wadden Sea area, no distinct effect of salinity on the distribution of M. lateralis has been reported (Klunder et al. 2019). This can be supported by our study where the salinities of the transects in which M. lateralis was found are in a broad range from brackish (salinity 13) to normal marine (salinity 34; Reineck and Flemming 1990; Becker 1998; Kaiser and Niemeyer 1999; Glorius et al. 2021).
As known from some other NIS (e.g. Magallana gigas, Hemigrapsus takanoi), the spreading into the central Wadden Sea is often driven by an eastward drift of larvae due to anti-clockwise residual tidal currents (Brandt et al. 2008; Markert et al. 2014). Decreasing abundance from T1 (outer Ems River estuary; 10.72 ± 9.78 ind./m2) to T9 (outer Weser River estuary; < 1 ind./m2) and the absence of M. lateralis in transect T10 (outer Elbe River estuary) clearly indicate an eastward spread.
It cannot be excluded that the low abundances in T7, T9, and T10 are due to the sampled elevation range (+ 1.75 to + 0.21 m NHN) not covering the entire main distribution range of M. lateralis (+ 0.35 to − 0.40 m NHN; Fig. 3).
High densities and high fecundity (Santos and Simon 1980; Flint and Younk 1983; Walker and Tenore 1984; Lu et al. 1996; Craeymeersch et al. 2019), the ability to reproduce several times a year (Calabrese 1970), and the ability to survive high environmental adversity (Parker 1975) indicate a high potential for M. lateralis to become invasive (Craeymeersch et al. 2019; Gittenberger et al. 2019; Klunder et al. 2019), meaning that by definition their introduction threatens the biological diversity of the ecosystem (Büttger et al. 2022). M. lateralis is sensitive to competition (Parker 1975; Klunder et al. 2019) but known to increase rapidly in abundance in areas that have recently been affected by a disturbance event (e.g. dredging (Kaplan et al. 1974)). Construction work in the Ems River estuary led to tidal amplification, increased fine sediment transport (van Maren et al. 2015) and, thus, may have allowed M. lateralis to quickly multiply. Flint and Younk (1983) observed a similar rapid recolonisation of M. lateralis after a disturbance event in the Corpus Christi Bay (Texas). M. lateralis was able to recolonize the disturbed area faster than the competing species. With the recurrence of the competing species, the abundance of M. lateralis decreased. As M. lateralis is similar to the native cockle Cerastoderma edule (Linnaeus, 1758) with respect to size, habitat preference, feeding mode, and food source, a competition is most likely. However, Craeymeersch et al. (2019) noted a disadvantage of M. lateralis in prolonged phases of starvation, making M. lateralis a weak competitor.
The assumption of IfAÖ (2020), Zettler and Alf (2021), and Wood et al. (2022), that M. lateralis is already or will become established in the German Wadden Sea, can be confirmed by this study. Whether the species will become invasive cannot be assessed at this early stage of bioinvasion.
Conclusion
After the first findings of the non-native bivalve Mulinia lateralis in the westernmost part of the central Wadden Sea in 2017, we expected an eastward directed dispersal due to larval drift by residual tidal currents. Therefore, we designed a survey of 10 shore-normal transects distributed in tidal flats of the central Wadden Sea between the outer Ems River estuary in the west and the outer Elbe River estuary in the east to document the actual invasion status.
The survey took place from February to May 2022. In total, we sampled 897 living specimens of M. lateralis from 392 sampling stations (each 1 × 1 m2) which reflects a mean abundance of 2.3 ind./m2. Highest abundance was observed at a station in the most western part with 36 ind./m2. There is also a clear trend of high abundances in the mid-tidal level (+ 0.35 m and − 0.40 m NHN; Fig. 4). The shell length varies between 3.98 and 23.55 mm, which exceeds the so far known maximum shell length of 20–21 mm. M. lateralis was absent in the most eastern transect. Bimodality of shell length distribution indicates the presence of at minimum two cohorts within the population.
Besides the characteristic morphological features, the presence of M. lateralis was also confirmed by DNA-based analyses where all sequenced specimens matched 100% with prior uploaded sequences.
Except for the most eastern transect, M. lateralis was found at each transect of the central Wadden Sea which supports the hypothesis of an eastward directed dispersal by larval drift. Due to the maximum lifespan of 2 years based on earlier studies, the evidence of a high number of small individuals, and the continuous distribution, reproduction of M. lateralis must have taken place in the German North Sea. As some findings were recently also reported from the northern part of the Wadden Sea, the present status of this non-indigenous species can be classified as established.
Further studies should focus on detailed population and reproduction dynamics, the genetic diversity of the newly established population, and the competitive traits to native species like Cerastoderma edule with respect to space and food.
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Becker G (1998) Der Salzgehalt im Wattenmeer. Nordfriesisches Und Dithmarscher Wattenmeer Umweltatlas Wattenmeer 1:60–61
Benjamini Y, Hochberg Y (1995) Controlling the als discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300. https://doi.org/10.2307/2346101
Beukema JJ (1992) Expected changes in the Wadden sea benthos in a warmer world lessons from periods with mild winters. Neth J Sea Res 30:73–79. https://doi.org/10.1016/0077-7579(92)90047-I
Beukema JJ, Dekker R, Jansen JM (2009) Some like it cold: populations of the tellinid bivalve Macoma balthica (L.) suffer in various ways from a warming climate. Mar Ecol Prog Ser 384:135–145. https://doi.org/10.3354/meps07952
Beukema JJ, Dekker R (2011) Increasing species richness of the macrozoobenthic fauna on tidal flats of the Wadden Sea by local range expansion and invasion of exotic species. Helgol Mar Res 65(2):155–164
Boltovskoy D, Guiaşu R, Burlakova L, Karatayev A, Schlaepfer MA, Correa N (2022) Misleading estimates of economic impacts of biological invasions: Including the costs but not the benefits. Ambio 51(8):1786–1799. https://doi.org/10.1007/s13280-022-01707-1
Brandt G, Wehrmann A, Wirtz KW (2008) Rapid invasion of Crassostrea gigas into the German Wadden Sea dominated by larval supply. J Sea Res 59(4):279–296. https://doi.org/10.1016/j.seares.2008.03.004
Brunel P, Bosse L, Lamarche G (1998) Catalogue of the marine invertebrates of the estuary and Gulf of St. Lawrence. Can Spec Publ Fish Aquat Sci 126:405
BSH (2022) Gezeitenkalender 2023 - Hoch- und Niedrigwasserzeiten für die Deutsche Bucht und deren Flussgebiete. 137 pp
Buschbaum C, Lackschewitz D, Reise K (2012) Nonnative macrobenthos in the Wadden Sea ecosystem. Ocean Coast Manag 68:89–101. https://doi.org/10.1016/j.ocecoaman.2011.12.011
Büttger H, Christoph S, Buschbaum C, Gittenberger A, Jensen K, Kabuta S, Lackschweitz D (2022) Alien species. In: Wadden Sea Quality Status Report. Wds. Kloepper S et al. Common Wadden Sea Secretariat, Wilhelmshaven, Germany. Last updated 06.09.2022. Downloaded 09.09.2022. https://qsr.waddensea-worldheritage.org/reports/alienspecies
Calabrese A (1969) Mulinia lateralis: Molluscan fruit fly? Pro Natl Shellfish Ass 5:65–66
Calabrese A (1970) Reproductive cycle of the coot clam, Mulinia lateralis (Say), in Long Island Sound. Veliger 12(3):265-269
Craeymeersch JA, Faasse MA, Gheerardyn H, Troost K, Nijland R, Engelberts A, Perdon KJ, van den Ende D, van Zwol J (2019) First records of the dwarf surf clam Mulinia lateralis (Say, 1822) in Europe. Mar Biodivers Rec 12(1):1–11
Crooks JA (1998) Habitat alteration and community-level effects of an exotic mussel, Musculista senhousia. Mar Ecol Prog Ser 162:137–152. https://doi.org/10.3354/meps162137
Dörjes J (1992) Zur Populationsdynamik von Cerastoderma edule (L.) nach dem Eiswinter 1978/79 am Beispiel zweier Stationen der Jadewatten (Nordsee) in der Zeit von 1979–1988. Senckenberg Marit 22(1/2): 21–28
Dunn OJ (1964) Multiple comparisons using rank sums. Technometrics 6(3):241–252. https://doi.org/10.2307/1266041
Essl F, Lenzner B, Bacher S, Bailey S, Capinha C, Daehler C, Dullinger S, Genovesi P, Hui C, Hulme PE, Jeschke JM, Katsanevakis S, Kühn I, Leung B, Liebhold A, Liu C, MacIsaac HJ, Meyerson LA, Nuñez MA, Pauchard A, Pyšek P, Rabitsch W, Richardson DM, Roy HE, Ruiz GM, Russell JC, Sanders NJ, Sax DF, Scalera R, Seebens H, Springborn M, Turbelin A, van Kleunen M, von Holle B, Winter M, Zenni RD, Mattsson BJ, Roura-Pascual N (2020) Drivers of future alien species impacts: an expert-based assessment. Glob Chang Biol 26(9):4880–4893. https://doi.org/10.1111/gcb.15199
Flint RW, Younk JA (1983) Estuarine benthos: long-term community structure variations, Corpus Christi Bay Texas. Estuaries 6(2):126–141. https://doi.org/10.2307/1351703
Gismann L, Wenke LK, Uhlir C, Martínez Arbizu P, Wehrmann A (2023) Status and occurrence of the non-indigenous Mulinia lateralis (Say, 1822) in the central Wadden Sea (southern North Sea). https://doi.org/10.14284/602
Gittenberger A, Rensing M, van der Veer HW, Philippart CJM, van der Hoom B, D’Hont A, Wesdorp KH, Schrieken N, Klunder L, Kleine-Schaars L, Holthuijsen S, Stengenga H (2019) Native and non-native species of the Dutch Wadden Sea in 2018. GiMaRIS report 2019 (09):79
Glorius ST, Meijboom AM, Gienapp T, Janssen T, Wehrmann A (2021) Initiating the formation of an intertidal mussel bed - a trial in the Ems-Dollard estuary. Wageningen Marine Research Report C090.21B: 87
Gollasch S, Nehring S (2006) National checklist for aquatic alien species in Germany. Aquat Invasions 1(4):245–269. https://doi.org/10.3391/ai.2006.1.4.8
Gollasch S (2011) NOBANIS – invasive alien species fact sheet – Eriocheir sinensis. – from: Online Database of the European Network on Invasive Alien Species – NOBANIS. www.nobanis.org. Accessed 30 May 2023
Hare MP, Palumbi SR, Butman CA (2000) Single-step species identification of bivalve larvae using multiplex polymerase chain reaction. Mar Biol 137(5):953–961. https://doi.org/10.1007/s002270000402
IfAÖ (2020) Neobiota-Erfassung an 'Hot spots' der Neubesiedlung in niedersächsischen Küstengewässern - Ergebnisbericht 2019. 67 pp
Jakob EM, Marshall SD, Uetz GW (1996) Estimating fitness: a comparison of body condition indices. Oikos 77(1):61–67. https://doi.org/10.2307/3545585
Joyce PWS, Smyth DM, Dick JTA, Kregting LT (2021) Coexistence of the native mussel, Mytilus edulis, and the invasive Pacific oyster, Crassostrea (Magallana) gigas, does not affect their growth or mortality, but reduces condition of both species. Hydrobiologia 848:1859–1871. https://doi.org/10.1007/s10750-021-04558-1
Kaiser R, Niemeyer HD (1999) Wasser-Beschaffenheit. Umweltatlas Wattenmeer 2:32-33
Kaplan EH, Welker JR, Kraus MG (1974) Some effects of dregding on populations of macrobenthic organisms. Fish Bull 72:445–480
Kerckhof F (2019) Mulinia lateralis (Say, 1822) de Kleine Amerikaanse strandschelp nu ook in België. De Strandvlo 39(1):4–9
Klunder L, Lavaleye M, Schaars LK, Dekker R, Holthuijsen S, van Der Veer HW (2019) Distribution of the dwarf surf clam Mulinia lateralis (Say, 1822) in the Wadden Sea after first introduction. Bio Invasions Rec 8(4):818–827. https://doi.org/10.3391/bir.2019.8.4.10
Kolar CS, Lodge DM (2001) Progress in invasion biology: predicting invaders. Trends Ecol Evol 16(4):199–204. https://doi.org/10.1016/S0169-5347(01)02101-2
Kourantidou M, Haubrock PJ, Cuthbert RN, Bodey TW, Lenzner B, Gozlan RE, Nuñez MA, Salles JM, Diagne C, Courchamp F (2022) Invasive alien species as simultaneous benefits and burdens: trends, stakeholder perceptions and management. Biol Invasions 24:1905–1926. https://doi.org/10.1007/s10530-021-02727-w
Kröncke I (2006) Structure and function of macrofaunal communities influenced by hydrodynamically controlled food availability in the Wadden Sea, the open North Sea, and the Deep-sea. A synopsis. Senckenb Marit 36: 123–164. https://doi.org/10.1007/bf03043725
Kruskal WH, Wallis WA (1952) Use of ranks in one-criterion variance analysis. J Am Stat Assoc 47(260):583–621. https://doi.org/10.2307/2280779
Lu JK, Chen TT, Allen SK, Matsubara T, Burns JC (1996) Production of transgenic dwarf surfclams, Mulinia lateralis, with pantropic retroviral vectors. Proc Natl Acad Sci USA 93(8):3482–3486. https://doi.org/10.1073/pnas.93.8.348
Markert A, Wehrmann A, Kroencke I (2010) Recently established Crassostrea-reefs versus native Mytilus-beds: differences in ecosystem engineering affects the macrofaunal communities (Wadden Sea of Lower Saxony, German Bight). Biol Invasions 12(1):15–32. https://doi.org/10.1007/s10530-009-9425-4
Markert A, Raupach MJ, Segelken-Voigt A, Wehrmann A (2014) Molecular identification and morphological characteristics of native and invasive Asian brush-clawed crabs (Crustacea: Brachyura) from Japanese and German coasts Hemigrapsus penicillatus (De Haan, 1835) versus Hemigrapsus takanoi Asakura & Watanabe 2005. Org Divers Evol 14(4): 369–382. https://doi.org/10.1007/s13127-014-0176-4
Marzec RJ, Kim Y, Powell EN (2010) Geographical trends in weight and condition index of surfclams (Spisula solidissima) in the Mid-Atlantic Bight. J Shellfisch Res 29(1):117–128. https://doi.org/10.2983/035.029.0104
McKeon CS, Tunberg BG, Johnston CA, Barshis DJ (2015) Ecological drivers and habitat associations of estuarine bivalves. PeerJ 3:e1348. https://doi.org/10.7717/peerj.1348
Montagna PA, Kalke R (1995) Ecology of infauna Mollusca in South Texas estuaries. Am Malacol Bull 11:163–175
Parker RH (1975) The study of benthic communities: a model and a review. Mar Geol 20(2):183–184. https://doi.org/10.1016/0025-3227(76)90091-8
Pfister R, Schwarz K, Janczyk M, Dale R, Freeman J (2013) Good things peak in pairs: a note on the Bimodality Coefficient. Front Psychol 4:700. https://doi.org/10.3389/fpsyg.2013.00700
Ramón M (2003) Population dynamics and secondary production of the cockle Cerastoderma edule (L.) in a backbarrier tidal flat in the Wadden Sea. Sci Mar 67(4):429–443
Reineck HE, Flemming BW (1990) Salzgehalte der Restnässe auf oder in der obersten Sedimentschicht und der Porenwässer im Eu- und Supralitoral der Jadewatten in Relation zu denen des Jadewassers. Senckenberg Marit 21:33–54
Reise K (1998) Pacific oysters invade mussel beds in the European Wadden Sea. Senckenberg Marit 28(4):167–175. https://doi.org/10.1007/BF03043147
Reise K, Gollasch S, Wolff WJ (1999) Introduced marine species of the North Sea coasts. Helgol Mar Res 52:219–234. https://doi.org/10.1007/BF02908898
Reise K, Buschbaum C, Lackschewitz D, Thieltges DW, Waser AM, Wegner KM (2023) Introduced species in a tidal ecosystem of mud and sand: curse or blessing? Mar Biodivers 53(5). https://doi.org/10.1007/s12526-022-01302-3
Rosenberg R (1995) Benthic marine fauna structured by hydrodynamic processes and food availability. Neth J Sea Res 34:303–317. https://doi.org/10.1016/0077-7579(95)90040-3
RStudio Team (2022) RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA. http://www.rstudio.com/. Accessed 20 July 2022
Santos SL, Simon JL (1980) Response of soft-bottom benthos to annual catastrophic disturbance in a south Florida estuary. Mar Ecol Prog Ser 3:347–355
Say T (1822) An account of some of the marine shells of the United States. Proc Acad Nat Sci Phila 2(1): 221–248; 2(2): 257–276, 302–325
Schlaepfer MA, Sax DF, Olden JD (2011) The potential conservation value of non-native species. Conserv Biol 25(3):428–437. https://doi.org/10.1111/j.1523-1739.2010.01646.x
Schückel U, Beck M, Kröncke I (2013) Spatial variability in structural and functional aspects of macrofauna communities and their environmental parameters in the Jade Bay (Wadden Sea Lower Saxony, southern North Sea). Helgol Mar Res 67:121–136. https://doi.org/10.1007/s10152-012-0309-0
Simões MV, Saeedi H, Cobos ME, Brandt A (2021) Environmental matching reveals non-uniform range-shift patterns in benthic marine Crustacea. Clim Change 168(3):1–20. https://doi.org/10.1007/s10584-021-03240-8
Smith EB, Scott KM, Nix ER, Korte C, Fischer CR (2000) Growth and condition of seep mussels (Bathymodiolus childressi) at a Gulf of Mexico Brine Pool. Ecology 81(9):2392–2403. https://doi.org/10.2307/177462
Statisticat LLC (2021) LaplacesDemon: Complete Environment for Bayesian Inference. Bayesian-Inference.com. R package version 16.1.6. https://web.archive.org/web/20150206004624/http://www.bayesian-inference.com/software. Accessed 08 June 2022
Turgeon DD, Lyons W, Mikkelsen P, Rosenberg G, Moretzsohn F (2009) Bivalvia (Mollusca) of the Gulf of Mexico. In: Felder DL, Camp D (eds) Gulf of Mexico–origins, waters, and biota biodiversity. Texas A&M University Press, pp 711–744
van Aken HM (2008) Variability of the water temperature in the western Wadden Sea on tidal to centennial scales. J Sea Res 60:227–234. https://doi.org/10.1016/j.seares.2008.09.001
van Maren DS, Winerwerp JC, Vroom J (2015) Fine sediment transport into the hyper-turbid lower Ems River: the role of channel deepening and sediment-induced drag reduction. Ocean Dyn 65:589–605. https://doi.org/10.1007/s10236-015-0821-2
Walker RL, Tenore KR (1984) Growth and production of the Dwarf Surf Clam Mulinia lateralis (Say 1822) in a Georgia Estuary. Gulf Caribb Res 7(4):357–363. https://doi.org/10.18785/grr.0704.07
Walles B, Brummelhuis E, Ysebaert T (2020) T0 monitoring bodemdieren en slibgehalte bij het schor van Bath. Wageningen Marine Research rapport C050/20: 47 pp
Wehrmann A, Herlyn M, Bungenstock F, Hertweck G, Millat G (2000) The distribution gap is closed - first record of natural settled pacific oysters Crassostrea gigas in the East Frisian Wadden Sea, North Sea. Senckenberg Mar 30 (3/4): 153–160
Wehrmann A (2016) Wadden Sea. In: Harff J, Meschede M, Petersen S, Thiede J (eds) Encyclopedia of marine geosciences, pp 933–939, Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6238-1_143
Wickham H (2016) ggplot2: Elegant graphics for data analysis. Springer-Verlag New York. http://ggplot2.org. Accessed 20 July 2022
Wolff WJ (1999) Exotic invaders of the meso-oligohaline zone of estuaries in the Netherlands: why are there so many? Helgol Meeresunters 52:393–400
Wolff WJ (2005) Non-indigenous marine and estuarine species in the Netherlands. Zool Meded 79(1):1–116
Wood CA, Galanidi M, Beckmann B (2022) Mulinia lateralis (Say, 1822). In Study on Invasive Alien Species – Development of risk assessments to tackle priority species and enhance prevention. European Union 674–809
Wulff DU, Kieslich PJ, Henninger F, Haslbeck JMB, Schulte-Mecklenbeck M (2021) Movement tracking of cognitive processes: A tutorial using mousetrap. PsyArXiv. https://doi.org/10.31234/osf.io/v685r. Accessed 08 June 2022
Zettler ML, Alf A (2021) Bivalvia of German Marine Waters of the North and Baltic Sea. ConchBooks, Harxheim, pp 250-251
Acknowledgements
The ambitious monitoring programme of Mulinia lateralis would not have been possible without the help of numerous colleagues during field work, namely Gina Dambrowski, Eileen Deeken, Katharina Kniesz, Kai Pfennings, Fritz Schiller, and Katja Uhlenkott (all Senckenberg am Meer SaM), who are gratefully acknowledged by the authors. Thanks also to Torsten Janßen (SaM) for logistics and assistance during field work and Nicol Mahnken (SaM) for taking pictures of the specimens. We are also grateful to the two anonymous reviewers for their numerous comments and critical remarks.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All applicable international, national and/or institutional guidelines for animal testing, animal care and use of animals were followed by the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements, if applicable. The study is compliant with CBD and Nagoya protocols.
Data availability
The occurrence dataset generated and analysed during the current study is available in the open-source platform EurOBIS repository (Gismann et al. 2023, https://doi.org/10.14284/602). Sequence vouchers of specimens investigated in this study are publicly accessible through GenBank (Accession No. OP575829 to OP575848).
Author contribution
AW and PMA conceived the ideas and designed the research. AW, CU, LG, and LW performed the field work. CU, LG, and LW generated the lab data. LG and PMA analysed the data. LG wrote a first draft of the manuscript. All authors contributed to the manuscript revision, read and approved the final version of the submitted manuscript.
Additional information
Communicated by C. Buschbaum
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gismann, L., Wenke, LK., Uhlir, C. et al. Status and occurrence of the non-indigenous dwarf surf clam Mulinia lateralis (Say, 1822) in the central Wadden Sea (southern North Sea)—a systematic survey. Mar. Biodivers. 53, 83 (2023). https://doi.org/10.1007/s12526-023-01381-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-023-01381-w