Abstract
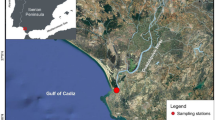
The ecology and population structure of the corophid amphipod Chelicorophium madrasensis (Nayar, 1950) was studied for 1 year in the Cochin estuary (CE), a tropical monsoonal estuary along the southwest coast of India. The incidence of Chelicorophium madrasensis in CE was a new distributional record from the south-west coast of India. Among the macrobenthic population, this species contributed in substantially higher density (> 90%) and biomass at the sampling location. This species showed a statistically significant positive correlation with salinity, benthic chlorophyll a, and fine sediment texture (clay and silt). The species also exhibited a significant seasonality in population dynamics, with a marked decline during the southwest monsoon which is characterized by remarkable decline in salinity and substantial changes in the substratum stability. The sex ratio was skewed towards dominance of females throughout the year. Though C. madrasensis is a continuous breeder, its peak breeding in the CE coincided with the pre-monsoon, which is characterized by mesohaline salinity and availability of surplus food. The study also showed that the high density of C. madrasensis in turn supported the sustenance of two carnivorous isopods. This study points to the fact that a strong and intricate abiotic-biotic interaction between the flora, fauna, and physical environment contributed to higher standing crop of the amphipod C. madrasensis at the study site.






Similar content being viewed by others
References
Aller RC (1994) Bioturbation and remineralization of sedimentary organic matter: effects of redox oscillation. Chem Geol 114:331–345
Aller RC, Aller JY (1998) The effect of biogenic irrigation intensity and solute exchange on diagenetic reaction rates in marine sediments. J Mar Res 56:905–936
APHA (2005) Standard methods for the examination of water and wastewater, in: edn., s. (Ed.). American Public Health Association/American Water Works Association/Water Environment Federation Washington DC
Aravind NP, Sheeba P, Nair KKC, Achuthankutty CT (2007) Life history and population dynamics of an estuarine amphipod, Eriopisa chilkensis Chilton (Gammaridae). Estuar Coast Shelf Sci 74:87–95
Balachandran KK, Raj CL, Nair M, Joseph T, Sheeba P, Venugopal P (2005) Heavy metal accumulation in a flow restricted, tropical estuary. Estuar Coast Shelf Sci 65:361–370
Bernard JL, Karaman GS (1991) The families and genera of marine Gammaridean Amphipoda (except marine Gammaroids)—part 1. Rec Aust Mus Supplement 13:419–866
Beukema J, Flach E (1995) Factors controlling the upper and lower limits of the intertidal distribution of two Corophium species in the Wadden Sea. Mar Ecol Prog Ser:117–126
Birkett L, McIntyre AD (1971) Treatment and sorting of samples methods for the study of marine benthos. IBP Handbook:156–168
Bousfield EL, Hoover P (1997) The amphipod superfamily Corophioidea on the Pacific coast of North America: 5. Family Corophiidae: Corophiinae, new subfamily: systematics and distributional ecology Amphipacifica
Boyden C, Little C (1973) Faunal distributions in soft sediments of the Severn estuary. Estuar Coast Mar Sci 1:203–223
Bragadeeswaran S, Rajasegar M, Srinivasan M, Rajan UK (2007) Sediment texture and nutrients of Arasalar estuary, Karaikkal, south-east coast of India. J Environ Biol 28:237–240
Brusca RC, Coelho V, Taiti S (2001) A guide to the coastal isopods of California Internet address: http://tolweb.org/notes
Clarke KR, Warwick RM (2001) An approach to statistical analysis and interpretation. Change Marine Communities 2
Coles S (1979) Benthic microalgal populations on intertidal sediments and their role as precursors to salt marsh development. Ecol Proc Coastal Environ:25–42
Conlan K (1994) Amphipod crustaceans and environmental disturbance: a review. J Nat Hist 28:519–554
Cunha M, Moreira M, Sorbe J (2000) The amphipod Corophium multisetosum (Corophiidae) in Ria de Aveiro (NW Portugal). II. Abundance, biomass and production. Mar Biol 137:651–660
Davis WR (1993) The role of bioturbation in sediment resuspension and its interaction with physical shearing. J Exp Mar Bio Ecol 171:187–200
Day JH (1967) A monograph on the Polychaeta of Southern Africa,Part I, Errantia and II, Sedentaria. Nat Hist, London: Trustees of the British museum:878 pp
Day JW (1989) Estuarine ecology. John Wiley & Sons
Day RW, Quinn GP (1989) Comparisons of treatments after an analysis of variance in ecology. Ecol Monogr 59:433–463
Dyer KR, Christie MC, Feates N, Fennessy MJ, Pejrup M, Van der Lee W (2000) An investigation into processes influencing the morphodynamics of an intertidal mudflat, the Dollard Estuary, the Netherlands: I. Hydrodynamics and suspended sediment. Estuar Coast Shelf Sci 50(5):607–625
El Wakeel SK, Riley JP (1957) The determination of organic carbon in marine muds. Journal du conseil International Exploration 22:180–183
Elton C (1927) Animal ecology. Sidgwick & Jackson, LTD, London, p 56
Eriksson SP, Wennhage H, Norkko J, Norkko A (2005) Episodic disturbance events modify predator–prey interactions in soft sediments. Estuar Coast Shelf Sci 64:289–294
Fauvel P (1953) The fauna of India (Annelida, Polychaeta), 507 pp Allahabad: Indian Press Ltd
Fenchel T, Kofoed L, Lappalainen A (1975) Particle size-selection of two deposit feeders: the amphipod Corophium volutator and the prosobranch Hydrobia ulvae. Mar Biol 30:119–128
Fincham A (1969) Amphipods of the shallow-water sand community in the northern Irish Sea. J Mar Biol Assoc UK 49:1003–1024
Fuller SL, Hart C (1979) Pollution ecology of estuarine invertebrates. Academic Press
Gee J (1961) Ecological studies in South Benfleet Creek with special reference to the amphipod genus Corophium. Essex Nat 30:291–309
Gerdol V, Hughes R (1994a) Effect of Corophium volutator on the abundance of benthic diatoms, bacteria and sediment stability in two estuaries in southeastern England. Mar Ecol Prog Ser Oldendorf 114:109–115
Gerdol V, Hughes R (1994b) Feeding behaviour and diet of Corophium volutator in an estuary in southeastern England. Mar Ecol Prog Ser 114:103–103
Gopalan U, Vengayil DT, Udayavarma V, Krishnankutty M (1983) The shrinking backwaters of Kerala. J Mar Biol Assoc India 25:131–141
Gosner KL (1971) Guide to identification ofmarine and estuarine invertebrates. Wiley-Interscience, John Wiley and Sons, Inc., New York
Grant J, Daborn G (1994) The effects of bioturbation on sediment transport on an intertidal mudflat. Netherlands J Sea Res 32:63–72
Grasshoff K, Ehrhardt M, Kremling K et al (1983) Methods of seawater analysis (2nd ed.). Deerfield Beach. Weinheim
Gupta GVM, Thottathil SD, Balachandran KK, Madhu NV, Madeswaran P, Nair S (2009) CO2 supersaturation and net heterotrophy in a tropical estuary (Cochin, India): influence of anthropogenic effect. Ecosystems 12:1145–1157
Hawkins C (1985) Population carbon budgets and the importance of the amphipod Corophium volutator in the carbon transfer on a Cumberland Basin mudflat, upper Bay of Fundy, Canada. J Sea Res 19:165–176
Hughes R (1988) Dispersal by benthic invertebrates: the in situ swimming behaviour of the amphipod Corophium volutator. J Mar Biol Assoc UK 68:565–579
Kinne O (1966) Physiological aspects of animal life in estuaries with special reference to salinity. Neth J Sea Res 3:222–244
Krishnan L, John P (1974) Observations on the breeding biology of Melita zeylanica Stebbing, a brackish water amphipod. Hydrobiologia 44:413–430
Krumbein WC, Pettijohn FJ (1938) Manual of sedimentary petrography
Lopez GR, Levinton JS (1978) The availability of microorganisms attached to sediment particles as food for Hydrobia ventrosa Montagu (Gastropoda: Prosobranchia). Oecologia 32:263–275
Lorenzen CJ (1967) Determination of chlorophyll and pheo-pigments: spectrophotometric equations. Limnol Oceanogr 12:343–346
Lowe S, Thompson B (1997) Identifying benthic indicators for San Francisco bay, regional monitoring program, annual report. San Francisco Estuary Institute, Oakland
Madhu NV et al (2007) Monsoonal impact on planktonic standing stock and abundance in a tropical estuary (Cochin Backwaters- India). Estuar Coast Shelf Sci 73:54–64
Madhu NV, Jyothibabu R, Balachandran KK (2010a) Monsoon-induced changes in the size-fractionated phytoplankton biomass and production rate in the estuarine and coastal waters of southwest coast of India. Environ Monit Assess 166:521–528
Madhu NV et al (2010b) Short-term variability of water quality and its implications on phytoplankton production in a tropical estuary (Cochin backwaters—India). Environ Monit Assess 170:287–300
Martin G et al (2011) Eutrophication induced changes in benthic community structure of a flow-restricted tropical estuary (Cochin backwaters), India. Environ Monit Assess 176:427–438
Mathew K et al (1994) Studies on the infestation of an isopod crustacean, Cirolana fluviatilis in some parts of the Cochin backwaters, Kerala. Mar Fish Inf Serv Tech Ext Ser 131:1–10
Mattila J, Bonsdorff E (1989) The impact of fish predation on shallow soft bottoms in brackish waters (SW Finland); an experimental study. J Sea Res 23:69–81
McLusky DS (1993) Marine and estuarine gradients—an overview. Aquat Ecol 27:489–493
McLusky DS, Elliott M (2004) The estuarine ecosystem: ecology, threats and management. Oxford University Press
Meadows P, Tait J (1989) Modification of sediment permeability and shear strength by two burrowing invertebrates. Mar Biol 101:75–82
Meadows P, Tait J, Hussain S (1990) Effects of estuarine infauna on sediment stability and particle sedimentation. Hydrobiologia 190:263–266
Menon NN, Balchand AN, Menon NR (2000) Hydrobiology of the Cochin backwater system–a review. Hydrobiologia 430:149–183
Miranda J, Balachandran K, Ramesh R, Wafar M (2008) Nitrification in Kochi backwaters. Estuar Coast Shelf Sci 78:291–300
Morino H (1978) Studies on the Talitridae (Amphipoda, Crustacea) in Japan-III. Life history and breeding activity of Orchestia platensis Krφyer
Nair KK, Anger K (1979) Life cycle of Corophium insidiosum (Crustacea, Amphipoda) in laboratory culture. Helgol Wiss Meeres 32:279
Nair K, Gopalakrishnan T, Venugopal P, Peter MG, Jayalakshmi K, Rao T (1983) Population dynamics of estuarine amphipods in Cochin backwaters. Mar Ecol Prog Ser Oldendorf 10:289–295
Nayar K (1950) Description of a new species of amphipod of the genus Corophium from Adyar, Madras, India. J Wash Acad Sci 40:225–228
Nayar KN (1959) Amphipoda of the Madras Coast Bulletin of the Madras Government Museum 6:15–76
Newman BK, Wooldridge TH, Cockcroft AC (2007) Aspects of the biology and ecology of the estuarine cirolanid isopod, Cirolana fluviatilis. Afr Zool 42:12–22
Prato E, Biandolino F (2006) Life history of the amphipod Corophium insidiosum (Crustacea: Amphipoda) from Mar Piccolo (Ionian Sea, Italy). Sci Mar 70:355–362
Qasim SZ (2003) Indian estuaries. Allied publishers
Qasim S, Gopinathan C (1969) Tidal cycle and the environmental features of Cochin Backwater (a tropical estuary). In: Proceedings of the Indian Academy of Sciences-Section B 336–348
Queiroga H (1990) Corophium multisetosum (Amphipoda: Corophiidae) in Canal de Mira, Portugal: some factors that affect its distribution. Mar Biol 104:397–402
Ramamirtham C, Muthusamy S (1986) Estuarine oceanography of the Vembanad lake part II: the region between Cochin and Azhikode. Indian J Fish 33:218–224
Rehitha T, Ullas N, Vineetha G, Benny P, Madhu N, Revichandran C (2017) Impact of maintenance dredging on macrobenthic community structure of a tropical estuary. Ocean Coast Manage 144:71–82
Revichandran C, Srinivas K, Muraleedharan K, Rafeeq M, Amaravayal S, Vijayakumar K, Jayalakshmy K (2012) Environmental set-up and tidal propagation in a tropical estuary with dual connection to the sea (SW Coast of India). Environ Earth Sci 66:1031–1042
Rhoads DC (1974) Organism-sediment relations on the muddy sea floor. Mar Biol Ann Rev 12:263–300
Rhoads DC, Young DK (1971) Animal-sediment relations in Cape Cod Bay, Massachusetts II. Reworking by Molpadia oolitica (Holothuroidea). Mar Biol 11:255–261
Sanilkumar M, Saramma A, Joseph K (2009) Vertical distribution of microphytobenthos in Cochin estuary. J Mar Biol Assoc India 51:61–68
Sanilkumar M, Saramma A, Joseph K, Cahoon L (2011) Monsoon effects on biomass and composition of microphytobenthic diatoms in the Cochin Estuary, Southwest India. J North Carolina Acad Sci 127:1–12
Sankaranarayanan V, Qasim S (1969) Nutrients of the Cochin backwater in relation to environmental characteristics. Mar Biol 2:236–247
Sarkar SK, Bhattacharya A, Giri S, Bhattacharya B, Sarkar D, Nayak DC, Chattopadhaya AK (2005) Spatiotemporal variation in benthic polychaetes (Annelida) and relationships with environmental variables in a tropical estuary. Wetl Ecol Manag 13:55–67
Satyanarayana Murthy P, Rao KS (1959) Geol soci India 13:38
Sheeba P (2000) Distribution of benthic in fauna in the cochin backwaters in relation to environmental parameters. Cochin University of Science and Technology, Kochi
Shyamasundari K (1972) Studies on the tube building amphipod Corophium triaenonyx Stebbing from Visakhapatnam harbour. Annual life cycle. Riv Biol 65:203
Smith K, Baldwin R (1982) Scavenging deep-sea amphipods: effects of food odor on oxygen consumption and a proposed metabolic strategy. Mar Biol 68:287–298
Smith D, Hughes R, Cox EJ (1996) Predation of epipelic diatoms by the amphipod Corophium volutator and the polychaete Nereis diversicolor. Mar Ecol Prog Ser 145:53–61
Srinivas K, Revichandran C, Maheswaran P, Asharaf TM, Murukesh N (2003) Propagation of tides in the Cochin estuarine system, southwest coast of India. I J Mar Sci 32:14
Steele DH, Steele VJ (1991) Effects of salinity on the survival, growth rate, and reproductive output of Gammarus lawrencianus (Crustacea, Amphipoda). Mar Ecol Prog Ser 78:49–56
Van den Brink F, Van der Velde G, Bij de Vaate A (1993) Ecological aspects, explosive range extension and impact of a mass invader, Corophium curvispinum Sars, 1895 (Crustacea: Amphipoda), in the Lower Rhine (The Netherlands). Oecologia 93:224–232
Warwick R (2001) Evidence for the effects of metal contamination on the intertidal macrobenthic assemblages of the Fal Estuary. Mar Pollut Bull 42:145–148
Watkin EE (1941) The yearly life cycle of the amphipod, Corophium volutator. J Anim Ecol:77–93
Wongkamhaeng K, Nabhitabhata J, Towatana P (2015) Corophiine amphipods of the genera Chelicorophium and Paracorophium from the lower Gulf of Thailand (Crustacea, Amphipoda, Corophiidae, Corophiinae). ZooKeys:35
WoRMS Editorial Board (2016) World Register of Marine Species. Available from http:/www.marinespecies.org at VLIZ. Accessed 2016–12-01
Acknowledgements
The authors are grateful to the Director, CSIR-National Institute of Oceanography (NIO), Goa and Scientist-in-Charge, Regional Centre, CSIR-NIO, Kochi, for the facilities provided. They are also grateful to Dr. C. Revichandran, Senior Principal Scientist, Regional Centre, CSIR-NIO, Kochi, for the support and encouragement. This is NIO contribution number 6198.
Funding
The study was supported by the project SSP-2179 Funded by Cochin Port Trust (CPT), Kochi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained by the authors from the competent authorities and are mentioned in the acknowledgements.
Additional information
Communicated by S. Kaiser
Electronic supplementary material
Supplementary Fig. 1
Microphotographs of Distinguishing features of C. madrasensis; Male-(a) Antenna 1, (b) Antenna 2, (c) Gnathopod 1(d) Gnathopod 2; Female (e) Antenna 1&2, (f) Proximal tooth on 2nd Gnathopod (in 60X) (g) four spines the lower edge on the 4th segment of Antennae 2 (in 60X). (JPEG 99 kb)
Supplementary Fig. 2
Photograph showing the tube formation by C. madrasensis in the sampling locations (JPEG 173 kb)
Rights and permissions
About this article
Cite this article
Rehitha, T.V., Madhu, N.V., Vineetha, G. et al. Macrobenthic fauna with special reference to the ecology and population structure of a tubicolous amphipod, Chelicorophium madrasensis (Nayar, 1950) in a tropical estuary, southwest coast of India. Mar Biodiv 49, 1013–1026 (2019). https://doi.org/10.1007/s12526-018-0886-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12526-018-0886-5