Abstract
The origin and process of the domestication of wild animals have long fascinated scientists. However, there are no reliable methods to distinguish between tamed and wild animals. Here, we present a new method to identify tamed and wild juvenile brown bears (Ursus arctos) using retrospective isotope analysis of the femur. We used femurs from the nine bear cubs and the tibia from one domesticated dog excavated from the Nijibetsu Shuwan Kumaokuriba site, Hokkaido Islands, Japan (late 19th century–1939 AD). These bears were potentially tamed by indigenous Ainu people, and the domesticated dog was used as a reference of a tamed animal. We subdivided these bones into 10 sections along the growing axis, extracted collagen and measured the stable nitrogen isotope ratios (δ15N). The bone sections of the domesticated dog had constant δ15N values that were as high as that of salmon, suggesting that tamed animals exclusively consumed a marine diet fed to them by the Ainu. Notably, two of nine brown bear cubs showed a temporal elevation of δ15N to the similar isotope ratios of the dog tibia, which is unlikely to occur in the wild condition, strongly suggesting that they were tamed and fed by the Ainu people.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The domestication of animals, which is a foundation of our food supply today, has been a subject of scientists for at least 160 years (Darwin 1859; könyi 1976; Diamond 2002). Key findings about the history of domestication of animals have been supported by genetic analysis (e.g. Lindblad-Toh et al. 2005; Chessa et al. 2009; Larson et al. 2012), however, this method cannot distinguish between wild and tamed animals from ancient remains, although the taming of wild-born animals is believed to be the origin of animal domestication (e.g. Vila et al. 2001; Linseele et al. 2007). The lack of analytical framework to detect the taming of animals has often hampered the interpretation of ancient animal remains that are strongly suspected to have been bred by ancient people, and the taming status of the remains have been discussed based only on the circumstantial evidence of the burial conditions (e.g. Davis 1978; Vigne et al. 2004). Therefore, development of an analytical method to detect tamed wild animal individuals from archaeological sites could be a breakthrough in the field of domestication study.
Here, we present a new application of isotope analysis to distinguish between tamed and wild brown bears (Ursus arctos) from an archaeological site in Hokkaido Islands, Japan. Unlike genetic analysis, stable isotope analysis can reconstruct the individual life histories of ancient animals, such as feeding habits, temperature of the living environments, habitat and migration (e.g. Ambrose and DeNiro 1986; Amiot et al. 2011; Goedert et al. 2018). Therefore, stable isotope analysis can distinguish between tamed and wild animal individuals when they are raised in different environments. In the case of an omnivorous animal such as the brown bear, the reconstruction of diet history by stable carbon and nitrogen isotope ratios (e.g. Bocherens et al. 1994; Matsubayashi et al. 2015) can be a powerful tool to distinguish between tamed and wild bears when they had distinct feeding habits. The successful application of this technique to distinguish wild and captive-reared/domestic ancient Maya turkeys using carbon and nitrogen isotope ratios (Thornton et al. 2016) serves as a supporting example.
Bones of brown bear cubs are frequently excavated from archaeological sites of northern hemispheric peoples because such peoples have cultures that use bears for ceremonies and rituals (Fig. 1; Hallowell 1926; Watanabe 1994). They hunted a brown bear mother and weanling infant pair in the wild, brought back the infant to the village and raised it for several months or years (Akino 2006; Ikeda 2018; Sato 2019). After that, the human-raised infant bear is respectfully killed in the bear-sending ceremony (Akino 2006; Ikeda 2018; Sato 2019). Although bear cults are common in human populations living in the northern hemisphere, the bear-sending ceremony is specific to populations in the Far East, such as Hokkaido, Sakhalin, and regions along the Amur River (Goseki 2017; Ikeda 2018).
The traditions relating to the bear-sending ceremony in the Ainu, an indigenous hunter-gatherer population that lived around Sakhalin and Hokkaido during the 13 − 19th centuries, have been studied thoroughly in history, ethnography and archaeology (Utagawa 1999; Akino 2006; Ikeda 2018; Sato 2019). Although the Ainu society was non-literate, archaeological evidences suggest that this tradition dates back to the 13th century or earlier in the previous Okhotsk culture (Sato 2019). Within this period, tamed bears at an archaeological site have been identified based on the time lag in mortality seasons between maturing females and the cubs (Ohyi et al. 1980). This distinction is possible because the Okhotsk people hunted bears during hibernation (early spring) and raised captured cubs throughout the spring and summer months. However, this approach cannot identify all of the tamed cubs in cases where the mortality seasons of maturing females ranged widely and when tamed cubs and maturing females died at the same time. Therefore, a new method to identify tamed bear individuals more accurately among ancient skeletal remains is important to deepen the knowledge of the bear-raising culture in these regions.
Ethnographic evidence from the Ainu people in the early 20th century shows that infant bears were fed carefully with human diets, such as boiled fish and mashed potatoes, but were never fed with animal meat (Goseki 2017; Hokkaido Government Board of Education 1987). Several historical pieces of literature have even described infant bears being breastfed by humans (Akino 2006) who were also dependent on marine resources (Minagawa 2001; Naito et al. 2010; Tsutaya et al. 2014). These studies strongly suggest that the primary protein source of bear cubs tamed by the Ainu people was marine animals. For this reason, if the tamed bears experienced such a diet in prehistory, isotope ratios of their bones would show a strong marine signal (Fuller et al. 2006; Schoeninger et al. 1983; Schoeninger and DeNiro 1984; Tsutaya and Yoneda 2015).
Wild brown bears in Hokkaido have large individual variations in diet within the population (Matsubayashi et al. 2015), and therefore, common isotope analysis using the entire compact bone thickness is not effective to distinguish between tamed and wild brown bears. Consequently, we focused on the differences in the patterns of temporal changes in isotope ratios between the two groups. Matsubayashi and Tayasu (2019) presented a new analytical protocol for sequential isotope analysis of the growing layer in cortical bone that can retrospectively reconstruct time-series changes in diet. This protocol is potentially applicable to fine-scale analysis during skeletal growth because the growth section in cortical bone can provide a large amount of collagen.
We analyzed right femurs of nine archaeological bear cub skeletons, which were potentially tamed by the Ainu, from the Nijibetsu Shuwan Kumaokuriba site (hereafter the Nijibetsu site) in eastern Hokkaido, Japan. This site was used by the Ainu people from the late 19th century until 1939 (Sato 2003). These bones were used for stable nitrogen isotope analysis, which is a powerful tool to evaluate the contribution of marine food resources to a consumer’s diet (e.g., Schoeninger et al. 1983; Schoeninger and DeNiro 1984), whereas the stable carbon isotope ratio is suitable to estimate the contributions of a C3 and C4 plant based diet (e.g. Kellner and Schoeninger 2007; Matsubayashi et al. 2014; 2015). The sequential stable isotope analysis developed by Matsubayashi and Tayasu (2019) was applied to reconstruct time-series changes of marine protein contribution during the growing period of the cubs. This study only focused on the nitrogen stable isotope ratios but not of carbon due to the higher concentration of 15N in salmon, a primary marine food source for wild brown bears in Hokkaido (e.g. Matsubayashi et al. 2015; Jimbo et al. 2022). This makes nitrogen isotopes a more sensitive indicator of salmon consumption compared to carbon stable isotope ratios. A tibia of a domesticated archaeological dog, which is an only dog excavated from the site, is used as a reference of tamed animal.
Materials and methods
Sample collection and preparation
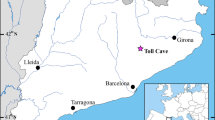
The Nijibetsu site is located in the eastern area of the Hokkaido Islands, Japan (Fig. 2). Multiple bear remains were excavated from the Nijibetsu site, and we identified nine right femurs of bear cubs from these bones based on the bone shapes and sizes (Sato 2003). We also sampled a tibia of a domestic dog from the same site. Then, we extracted collagen from multiple sections of the femur based on the protocol developed by Matsubayashi and Tayasu (2019). Briefly, we excised a longitudinal sections (20–30 mm long, 2–3 mm wide) from the mid-shaft of the femur, immersed the bone fragments in a methanol : chloroform mixture (1 : 1, vol : vol) for 12 h. After discarding the solvent, the bone fragments were allowed to air dry, and were immersed in 1.0 M HCL for 1–3 days until complete demineralization and softening were achieved. Once demineralization was complete, the specimens were rinsed twice with pure water and then immersed in 0.1 M NaOH for 12 h. Subsequently, the samples were rinsed twice with pure water, frozen at -20 °C, and cut into 10 sections using a freezing microtome (MC-802 A Electro Freeze, Yamato Kohki Industrial Co., Ltd., Saitama, Japan; Fig. 3). The sectioning process followed the height from the inner (marrow side) to the outer edge of each longitudinal section. Finally, all samples were heated in Milli-Q water at 90 °C for approximately 12 h and lyophilized.
Stable isotope analysis
We measured stable carbon and nitrogen isotope ratios of bone collagen with a Delta XP mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) connected to a Flash EA 2000 elemental analyser (Thermo Fisher Scientific). Stable isotope ratios are expressed in delta (δ) notation in accordance with international standard scales as follows:
δ15N = (Rsample / Rstandard) – 1, (1)
where Rsample is the 15N/14N ratio of the sample; and Rstandard is 15N/14N of atmospheric nitrogen. The elemental concentrations and isotope ratios of carbon and nitrogen were calibrated against those of alanine (δ15N = 22.71‰) and glycine (δ15N = 2.18‰) laboratory standards (Tayasu et al. 2011), which had been calibrated in turn against the international standards. The analytical standard deviations (SDs) of δ15N of the alanine standard (n = 15) were 0.25‰ and those of the glycine standard (n = 13) were 0.11‰.
δ 15N baselines of marine and terrestrial diets
As above, brown bears in Hokkaido primarily consume salmon in the wild (Matsubayashi et al. 2015; Jimbo et al. 2022). For tamed bears, they were fed a variety of fish including salmon by Ainu (Inukai and Natori 1939; Hokkaido Government Board of Education 1987), and studies showed that the δ15N of salmon muscle is slightly lower than other coastal fish around northern Japan (range from 10.4‰ to 15.4‰; Ishikawa et al. 2021). Consequently, we chose salmon to represent marine food sources in our study. The δ15N of dietary items for brown bears in the study area (eastern Hokkaido) were 3.7‰ (SD: ±1.5‰) for terrestrial animals and 11.0‰ (± 0.9‰) for salmon, according to Matsbayashi et al. (2015). Isotopic data for these endmembers were derived from isotope ratios of modern deer and salmon muscle, with corrections applied to account for historical changes in δ15N caused by human activity and variations in bone collagen isotope ratios between past and present. To correct for the trophic discrimination factor (TDF) between brown bear bone collagen and diet, we added 3.0‰ (± 1.5‰) to the δ15N values of these diets (see Matsbayashi et al. 2015). The suitability of a TDF can be assessed by examining whether the isotope ratios of the target organism fall well within a mixing polygon, which is defined by the isotope ratios of potential diet sources (Morgenthaler et al. 2021). The TDF of 3.0‰ for δ15N in bone collagen of brown bears allowed the highly-variable δ15N of past and modern Hokkaido brown bears (range of TDF-corrected δ15N: -3.8 to 10.6‰) to fall well within the isotopic range of their diet (from − 4.1‰ to 11.0‰), indicating their appropriateness (Matsubayashi et al. 2015). We also estimated the potential range for δ15N of each diet by adding up the SD of their δ15N and TDF. We determined the primary diet of each bear cub by comparing the overlap between the δ15N of the bone sections and the range formed by the δ15N of the food and the cumulated SD.
Time-window of each femur section
In young animals, the fractions of forming and resorbing areas of the periosteal and cortical-endosteal surfaces are comparable (Sontag 1986). Thus, time required to form the entire thickness of the cortical bone can be estimated using the growth curve and the differences between the internal and external diameters of the femoral mid-shaft. The transverse external diameter and total thickness of the cortical bone of each femur were measured, and the internal diameter was calculated as follows:
Internal diameter = External diameter – 2 × thickness, (2)
The growth curve for the total length of the brown bear femur, which was determined in a previous study (Matsubayashi et al. 2016a), is positively correlated with that of the transverse diameter (Fosse and Cregut-Bonnoure 2014). Therefore, we estimated the minimum duration to form the entire thickness of the femoral mid-shaft for each bear cub by the growth curve for the total femur length as follows:
tEx = T – log(1 + Ex / In × exp(–K × (T – tIn) – 1)) / K, (3)
where tEx and tIn are the ages at which the periosteal and cortical-endosteal surfaces were formed, respectively (years); T is a fitting constant (years); Ex and In are the external and internal diameters of the mid-shaft femur, respectively (mm); K is the rate at which the asymptotic femur length was reached (years− 1). Here, tEx is minimized when these parameters are set for the bear with the maximum growth rate (i.e., the youngest male bear); therefore, we use − 1.01 for T, 0.45 for K, which corresponds to the growth curve for the total length of the male bear femur, and 0.11 for tIn which is the youngest age included in the estimation of the growth curve (see Matsubayashi et al. 2016a).
Results
δ 15N of bone sections of ancient animals
The δ15N (± cumulated SD) of potential dietary items of brown bears corrected for the TDF were 6.7 (± 3.0) ‰ for terrestrial animals and 14.0 (± 2.4) ‰ for salmon (Fig. 4). Some collagen samples from the bone sections of the studied animals are outside the established range of the C/N ratio for pure bone collagen samples (2.9–3.6; DeNiro 1985); as a result, these data are excluded and shown for reference purposes only (Fig. 4).
Nitrogen isotope ratios (δ15N) in femur sections of the brown bear (Ursus arctos, sample) cubs and tibia sections of a domestic dog (Canis lupus) excavated from the Nijibetsu site, Hokkaido Island, Japan. The mean and cumulated SD of δ15N values for potential diet items are also shown. The bears are classified into three groups: individuals with consistently high δ15N (A, IDs: Nijibetsu-03 and 05), individuals with seasonal δ15N increases (B, Nijibetsu-01, 02 and 04), and individuals with consistently low δ15N (C, Nijibetsu-06, 07, 08 and 09). Points with an open circle and triangle indicate that these samples are out of the established range of pure bone collagen samples (2.9–3.6; DeNiro 1985)
The tibia sections of the domestic dog showed a high and constant δ15N trajectory (mean ± SD: 14.4 ± 0.29‰), and all of its bone sections had similar values (range: 13.5 to 14.4‰) within the range of salmon. Based on the overlap of δ15N values between the bear cubs and the discrete ranges of the two dietary items, δ15N trajectories along the femur sections of the bear cubs are divided into 3 patterns: (i) δ15N values of all the femur sections were within or lower than the higher range of terrestrial animals (IDs: Nijibetsu-06, 07, 08 and 09); (ii) unimodal shifts in δ15N where the peak exceed the higher range of terrestrial animals (IDs: Nijibetsu-01, 02 and 04) and (iii) δ15N values of all the femur sections are higher than the lower range of salmon (IDs: Nijibetsu-03 and 05).
Time-window of isotopic values in femurs
The mean (± SD) thickness of the vertical sections and external and internal diameters of the mid-shaft femurs of the brown bear cubs are 3.33 (± 0.68), 19.65 (± 2.06) and 13.00 (± 1.56) mm, respectively (Appendix Table S2). The estimated minimum elapsed time to form the entire thickness of the cortical bone for each bear cub ranges from 0.73 to 1.53 years (Appendix Table S2).
Discussion
All of the bone sections from a domestic dog tibia had similar δ15N values to that of salmon (Fig. 4), suggesting that tamed animals at this site were exclusively fed a marine diet. Globally, salmon is the only marine prey constantly available to wild brown bears during specific seasons (e.g. Sato et al. 2005; López-Alfaro et al. 2015; Mangipane et al. 2020). In eastern Hokkaido, salmon availability is also restricted from late summer through fall (e.g., Sato et al. 2005; Shirane et al. 2021). While polar bears have year-round access to marine carcasses (Stirling 1974; Stirling and McEwan 1975; Stirling and Archibald 1977), brown bears living outside the Arctic rarely encounter carcasses of marine mammals (Stirling and Derocher 1990). Therefore, assuming no significant changes in salmon runs or marine mammal stranding patterns since the late 19th century, wild bears in Hokkaido would likely not rely solely on marine food sources for extended periods. Nevertheless, two bear cubs from the Nijibetsu site (IDs: Nijibetsu-03 and 05) had constantly high stable isotope ratios (Fig. 4A), which were within the marine range and clearly indicated that they heavily depended on marine food resources throughout the growing periods of the entire thickness of their femoral mid-shafts. Individuals with such elevated δ15N values have not been observed in modern Hokkaido (Matsubayashi et al. 2015) or on the neighboring, more pristine Etorofu Island, which boasts abundant salmon populations (Matsubayashi et al. 2016b). Given that Nijibetsu-03 and 05 took 0.96 and 1.53 years (Appendix Table S2), respectively, to form the entire thickness of the femur, our data strongly suggested that these two bears did not grow in a wild condition but were instead consumed artificial diets provided by the Ainu people for almost a year or more. The true duration of the marine-based diet might have been shorter due to the slower turnover time of bone collagen compared to soft tissues (Hedges et al. 2007; Matsubayashi and Tayasu 2019). Nonetheless, the dietary profile of these individuals clearly differed from that of wild bears, as evidenced by our ability to reconstruct seasonal salmon consumption patterns in other bears (IDs: Nijibetsu-01, 02 and 04) through sequential isotope analysis of femur sections. While the possibility of these bears scavenging leftovers of Ainu people cannot be entirely eliminated, the presence of a highly skilled hunter at the Nijibetsu site is worth considering. This individual, documented to have killed hundreds of bears (Inukai and Natori 1940), probably including specimens used in this study, makes it less likely that the bears could have consistently accessed food scraps near human settlements for such a prolonged period.
On the other hand, isotopic shifts in the femur sections of the bear cubs other than Nijibetsu-03 and 05 reflected the natural diet of Hokkaido brown bears. Bears that had almost the same or lower δ15N values as that of terrestrial animals (IDs: Nijibetsu-06, 07, 08 and 09; Fig. 4C) mainly depended on plants and terrestrial animals throughout their lives, which were considered the most common feeding habits in Hokkaido bears (Matsubayashi et al. 2014; 2015). The unimodal isotopic trajectories observed in femur sections of three bear individuals (IDs: Nijibetsu-01, 02 and 04; Fig. 4C) indicated that they were seasonal salmon feeders. We can roughly calculate the duration per section by dividing the duration of the isotopic information estimated based on the femur thickness of these individuals (0.73, 1.19 and 1.43 years, respectively) by the number of sections (10). It should be noted that the actual period of salmon predation may have been much shorter, given the long turnover time of bone collagen, and the period of salmon consumption estimated here is a maximum. Since the number of femur sections with peak δ15N values is approximately two for each individual, we can estimate the duration of intensive feeding on salmon to be about 1.8, 2.9, and 3.4 months, respectively. In eastern Hokkaido, the pink salmon run peaks from September to October (Iida et al. 2014) and the chum salmon run peaks from October to early December (Miyakoshi et al. 2012). Therefore, brown bears are likely to consume salmon for approximately four months of the year, which is consistent with the period of salmon consumption predicted from the femur time-series analysis. In addition, the lower δ15N values of the outermost layers of the femur of these individuals are also important in distinguishing captive and wild individuals. Given that the outer femoral sections of growing animals reflected isotopic values of their diets just before their deaths (Matsubayashi et al. 2019), tamed and finally sacrificed bears in the bear-sending ceremony would have shown high δ15N values at least on the outermost sections. However, the outermost sections (No. 10) of these bears’ bones did not have high δ15N values (Fig. 2), and therefore, we identified them to be wild bears.
This study provides the first geochemical evidence for the taming of brown bear cubs by the Ainu people of Hokkaido, Japan. As noted above, past studies have emphasized the mortality time lag between mature females and their cubs, as inferred from the length of the erupting teeth, as important archaeological evidence for bear taming (Ohyi et al. 1980). Physical observations are particularly valid for the ancient bear skeletons of the Okhotsk culture, which flourished in Hokkaido from the 5th to 13th centuries AD (Amano 2003). It is estimated that the Okhotsk people hunted bears during hibernation (early spring), and when they captured cubs by hunting, they raised them from early spring to autumn. Thus, there is a large time lag between the mortality of tamed and wild bears. On the other hand, the Ainu people who flourished in Hokkaido after the 13th century AD generally killed tamed bears in early spring, just before the main bear hunting season, so there is little time lag between the time of death of ancient Ainu bear skeletal specimens and it is difficult to distinguish between tamed and wild bear cubs through physical observation. Therefore, the results of this study make it possible to identify captive brown bears based on isotope analysis of limb bones, which will allow us to examine in more detail the extent of captive-breeding rituals among the ancient Ainu.
Based on the profiles of salmon consumption in the femur sections, this study has successfully reconstructed seasonal changes in isotope ratios by sequential analysis of femur sections. This high temporal resolution in isotope ratios may be due to the fact that the individuals analyzed in this study were juveniles with high femoral growth rates (Mastubayashi et al. 2016) and without active bone remodeling (Veitschegger et al. 2018). Although the seasonal variation in isotope ratios was an important factor that enabled us to distinguish between wild and tamed individuals, the bone sections formed at the juvenile stage would not have been preserved in the femur of adult mammals. Observations of the microstructure of the dense bone of brown bears have shown that lines of arrested growth (LAGs) appear in the femur of individuals after 10 months of age, and although the number of LAGs generally matches age in young individuals, the number of LAGs relative to age gradually decreases with aging, with a maximum number of LAGs of 8–10 (Babosová et al. 2022). In other words, bones formed at the juvenile stage are gradually resorbed and are not retained until after growth. Given the slow growth rate and active remodeling in bones of adult mammals (e.g. Caccia et al. 2016; Veitschegger et al. 2018), it would be challenging to achieve a time series analysis with as high a resolution as that of juvenile animals. Thus, the discrimination of tamed and wild individuals based on retrospective isotope analysis of femur may be more effective when targeting juveniles. Moreover, this method can only be applied when the target wild and tamed animals have different isotopic signatures due to different living conditions, such as diet and location, on a given time scale. Since human captivity dramatically alters the habitat and diet of wild animals, we believe that in many cases it is possible to distinguish between captive and wild individuals by using not only δ13C and δ15N, but also δ18O (e.g. Tomczyk et al. 2016; Pederzani and Britton 2019), which reflects the isotopic ratio of drinking water, and 87Sr/86Sr (e.g. Price et al. 2000; Takigami and Yoneda 2020), which reflects the geological features of the habitat.
Although the brown bear is not included in a small set of mammalian species that have been successfully domesticated (i.e. not involved selective breeding and adaptation to human environments like cats and dogs) in human history (Diamond 2002), taming of brown bear through the tradition relating to the bear-sending ceremony had significant effects on the Ainu society. Meat, fur, and organs of the raised bear were important sources of food, clothing, and traditional medicine, respectively, and were sometimes exported from the Ainu to other populations as precious trade goods (Ikeda 2018). The bear-sending ceremony was a kind of feast, as well as an important philosophical representation for the Ainu people and provided an opportunity for people living in distant villages to come together and communicate (Ikeda 2018). Although the taming of wild animals has been studied little compared with domestication, it is one of the most important relationships between humans and animals, and its philosophical, economic, and social impacts are by no means minor. This study shows that sequential stable isotope analysis of the growth layer in bear bone is an effective method to detect tamed individuals in a skeletal collection obtained from an archaeological site. Since we presented the results of the analysis from one site only, this method is widely applicable to other sites, especially those related to the bear-sending culture in Northeast Asia. Furthermore, this method can be used for other mammals (Matsubayashi and Tayasu 2019) and can be a breakthrough in understanding the domestication process for other important animals for humans such as wolves and boar.
Data availability
No datasets were generated or analysed during the current study.
References
Akino S (2006) Sending Ainu spirits ceremony in the Edo era: from the aspect of Japanese archives. J Ainu Pac Rim Cultures 5:1–26 (in Japanese)
Amano T (2003) Okhotsk-Bunka Toha Nanika. In: Nomura T, Utagawa H (eds) Zoku-Jomon, Okhotsk Bunka. Hokkaido Shimbun, Sapporo, pp 110–133. (in Japanese)
Ambrose SH, DeNiro MJ (1986) Reconstruction of African Human diet using bone collagen carbon and nitrogen isotope ratios. Nature 319:321. https://doi.org/10.1038/319321a0
Amiot R, Wang X, Zhou Z, Wang X, Buffetaut E, Lécuyer C, Ding Z, Fluteau F, Hibino T, Kusuhashi N, Mo J, Suteethorn V, Wang Y, Xu X, Zhang F (2011) Oxygen isotopes of east Asian dinosaurs reveal exceptionally cold early cretaceous climates. Proc Nat Acad Sci 108:5179–5183. https://doi.org/10.1073/pnas.1011369108
Babosová R, Zedda M, Belica A, Golej M, Chovancová G, Kalaš M, Vondráková M (2022) The enrichment of knowledge about the microstructure of brown bear compact bone tissue. Eur Zool J 89:615–624. https://doi.org/10.1080/24750263.2022.2068682
Bocherens H, Fizet M, Mariotti A (1994) Diet, physiology and ecology of fossil mammals as inferred from stable carbon and nitrogen isotope biogeochemistry: implications for pleistocene bears. Palaeogeogr Palaeocl Palaeoecol 107:213–225. https://doi.org/10.1016/0031-0182(94)90095-7
Bökönyi S (1976) Development of early stock rearing in the Near East. Nature 264:19–23. https://doi.org/10.1038/264019a0
Caccia G, Magli F, Tagi VM, Porta DGA, Cummaudo M, Márquez-Grant N, Cattaneo C (2016) Histological determination of the human origin from dry bone: a cautionary note for subadults. Int J Legal Med 130:299–307. https://doi.org/10.1007/s00414-015-1271-6
Chessa B, Pereira F, Arnaud F, Amorim A, Goyache F, Mainland I, Kao RR, Pemberton JM, Beraldi D, Stear MJ, Alberti A, Pittau M, Iannuzzi L, Banabazi MH, Kazwala RR, Zhang YP, Arranz JJ, Ali BA, Wang Z, Uzun M, Dione MM, Olsaker I, Holm LE, Saarma U, Ahmad S, Marzanov N, Eythorsdottir E, Holland MJ, Ajmone-Marsan P, Bruford MW, Kantanen J, Spencer TE, Palmarini M (2009) Revealing the history of sheep domestication using retrovirus integrations. Science 324:532–536. https://doi.org/10.1126/science.1170587
Darwin C (1859) On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. John Murray, London
Davis SJ, Valla FR (1978) Evidence for domestication of the dog 12,000 years ago in the Natufian of Israel. Nature 276:608. https://doi.org/10.1038/276608a0
DeNiro MJ (1985) Postmortem preservation and alteration of in vivo bone collagen isotope ratios in relation to palaeodietary reconstruction. Nature 317:806–809. https://doi.org/10.1038/317806a0
Diamond J (2002) Evolution, consequences and future of plant and animal domestication. Nature 418:700–707. https://doi.org/10.1038/nature01019
Fosse P, Cregut-Bonnoure E (2014) Ontogeny/growth of (sub)modern brown bear (Ursus arctos) skeleton: a guideline to appraise seasonality for cave bear (Ursus Spelaeus) sites? Quatern Intern 339:275–288. https://doi.org/10.1016/j.quaint.2014.03.046
Fuller BT, Fuller JL, Harris DA, Hedges RE (2006) Detection of breastfeeding and weaning in modern human infants with carbon and nitrogen stable isotope ratios. Am J Phys Anthropol 129:279–293. https://doi.org/10.1002/ajpa.20249
Goedert J, Lécuyer C, Amiot R, Arnaud-Godet F, Wang X, Cui L, Cuny G, Douay G, Fourel F, Panczer G, Simon L, Steyer JS, Zhu M (2018) Euryhaline ecology of early tetrapods revealed by stable isotopes. Nature 558:68. https://doi.org/10.1038/s41586-018-0159-2
Goseki M (2017) Research on the features of the iomante: a regional comparison. Bull Papers Showa Women’s Univ Graduate School Life Sci 26:43–60 (in Japanese)
Hallowell AI (1926) Bear Ceremonialism in the northern hemisphere. Am Anthropo 28:1–173. https://doi.org/10.1525/aa.1926.28.1.02a00020
Hedges RE, Clement JG, Thomas CDL, O’connell TC (2007) Collagen turnover in the adult femoral mid-shaft: modeled from anthropogenic radiocarbon tracer measurements. Am J Phys Anthropol 133:808–816. https://doi.org/10.1002/ajpa.20598
Hokkaido Government Board of Education (1987) Report on the ethnographic and cultural studies of Ainu. Hokkaido Government Board of Education, Hokkaido. (in Japanese)
Iida M, Miyakoshi Y, Kato T, Tokuda H, Fujiwara M, Ando D (2014) Natural spawning of pink salmon Oncorhynchus gorbuscha in rivers with and without weirs on the Okhotsk side of Hokkaido. Aquacult Sci 62:129–136 (in Japanese with English abstract). https://doi.org/10.11233/aquaculturesci.62.129
Ikeda T (2018) Belief to bears and bear ceremonies in Hokkaido. Biostory 30:28–35 (in Japanese)
Inukai T, Natori T (1939) Iomante (Ainu no Kuma Matsuri) no Bunnkateki igi to Sono Keishiki Nituite 1 [On the significance of the bear festival (iomante) in Ainu culture and its local forms, part 1]. Stud Res Inst North Arct Cult 2:237–271
Inukai T, Natori T (1940) Iomante (Ainu no Kuma Matsuri) no Bunnkateki igi to Sono Keishiki Nituite 2 [On the significance of the bear festival (iomante) in Ainu culture and its local forms, part 2]. Stud Res Inst North Arct Cult 3:79–135 (in Japanese)
Ishikawa NF, Ogawa NO, Chikaraishi Y, Yamaguchi MO, Fujikura K, Miyairi Y, Yokoyama Y, Nagata T, Ohkouchi N (2021) Influences of ocean currents on the diets of demersal fish communities in the western North Pacific revealed by their muscle carbon and nitrogen isotopic compositions. Front Mar Sci 8:641282. https://doi.org/10.3389/fmars.2021.641282
Jimbo M, Ishinazaka T, Shirane Y, Umemura Y, Yamanaka M, Uno H, Sashika M, Tsubota T, Shimozuru M (2022) Diet selection and asocial learning: Natal habitat influence on lifelong foraging strategies in solitary large mammals. Ecosphere 13:e4105. https://doi.org/10.1002/ecs2.4105
Kellner CM, Schoeninger MJ (2007) A simple carbon isotope model for reconstructing prehistoric human diet. Am J Phys Anthropol 133:1112–1127. https://doi.org/10.1002/ajpa.20618
Larson G, Karlsson EK, Perri A, Webster MT, Ho SYW, Peters J, Stahl PW, Piper PJ, Lingaas F, Fredholm M, Comstock KE, Modiano JF, Schelling C, Agoulnik AI, Leegwater PA, Dobney K, Vigne J, Vilà C, Andersson L, Lindblad-Toh K (2012) Rethinking dog domestication by integrating genetics, archeology, and biogeography. Proc Nat Acad Sci 109:8878–8883. https://doi.org/10.1073/pnas.1203005109
Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, Clamp M, Chang JL, Kulbokas EJ III, Zody MC, Mauceli E, Xie X, Breen M, Wayne RK, Ostrander EA, Ponting CP, Galibert F, Smith DR, deJong PJ, Kirkness E, Alvarez P, Biagi T, Brockman W, Butler J, Chin CW, Cook A, Cuff J, Daly MJ, DeCaprio D, Gnerre S, Grabherr M, Kellis M, Kleber M, Bardeleben C, Goodstadt L, Heger A, Hitte C, Kim L, Koepfli KP, Parker HG, Pollinger JP, Searle SMJ, Sutter NB, Thomas R, Webber C, Broad Sequencing Platform members, Lander ES (2005) Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438:803–819. https://doi.org/10.1038/nature04338
Linseele V, Van Neer W, Hendrickx S (2007) Evidence for early cat taming in Egypt. J Archaeol Sci 34:2081–2090. https://doi.org/10.1016/j.jas.2007.02.019
López-Alfaro C, Coogan SC, Robbins CT, Fortin JK, Nielsen SE (2015) Assessing nutritional parameters of brown bear diets among ecosystems gives insight into differences among populations. PLoS ONE 10:e0128088. https://doi.org/10.1371/journal.pone.0128088
Mangipane LS, Lafferty DJ, Joly K, Sorum MS, Cameron MD, Belant JL, Hilderbrand GV, Gustine DD (2020) Dietary plasticity and the importance of salmon to brown bear (Ursus arctos) body size and condition in a low Arctic ecosystem. Polar biol 43:825–833. https://doi.org/10.1007/s00300-020-02690-7
Matsubayashi J, Tayasu I (2019) Collagen turnover and isotopic records in cortical bone. J Archaeol Sci 106:37–44. https://doi.org/10.1016/j.jas.2019.03.010
Matsubayashi J, Morimoto J, Mano T, Aryal A, Nakamura F (2014) Using stable isotopes to understand the feeding ecology of the Hokkaido brown bear (Ursus arctos) in Japan. Ursus 25:87–98. https://doi.org/10.2192/URSUS-D-12-00015.1
Matsubayashi J, Morimoto JO, Tayasu I, Mano T, Nakajima M, Takahashi O, Kobayashi K, Nakamura F (2015) Major decline in marine and terrestrial animal consumption by brown bears (Ursus arctos). Sci Rep 5:9203. https://doi.org/10.1038/srep09203
Matsubayashi J, Tayasu I, Morimoto JO, Mano T (2016a) Testing for a predicted decrease in body size in brown bears (Ursus arctos) based on a historical shift in diet. Can J Zool 94:489–495. https://doi.org/10.1139/cjz-2016-0046
Matsubayashi J, Otsubo K, Morimoto JO, Nakamura F, Nose T, Tayasu I (2016b) Feeding habits may explain the morphological uniqueness of brown bears on Etorofu Island, Southern Kuril Islands in East Asia. Biol J Linn Soc 119:99–105. https://doi.org/10.1111/bij.12798
Minagawa M (2001) Dietary pattern of prehistoric Japanese populations inferred from stable carbon and nitrogen isotopes in bone protein. Bull Natl Museum Japanese History 86:333–357
Miyakoshi Y, Urabe H, Saneyoshi H, Aoyama T, Sakamoto H, Ando D, Kasugai K, Mishima Y, Takada M, Nagata M (2012) The occurrence and run timing of naturally spawning chum salmon in northern Japan. Environ Biol Fishes 94:197–206. https://doi.org/10.1007/s10641-011-9872-5
Morgenthaler A, Millones A, Gandini P, Frere E (2021) Which trophic discrimination factors fit the best? A combined dietary study of a coastal seabird. J Ornithol 162:179–190. https://doi.org/10.1007/s10336-020-01813-5
Naito YI, Chikaraishi Y, Ohkouchi N, Mukai H, Shibata Y, Honch NV, Dodo Y, Ishida H, Amano T, Ono H, Yoneda M (2010) Dietary reconstruction of the Okhotsk Culture of Hokkaido, Japan, based on nitrogen isotopic composition of amino acids: implication for correction of 14C marine reservoir effects on human bones. Radiocarbon 52:671–681. https://doi.org/10.1017/S0033822200045690
Ohyi H, Ohtaishi N, Nishimoto T (1980) Determination of age, sex and mortals-season of Yezo Brown Bear, excavated from the Kabukai A site, Rebun Island, Hokkaido. Bulletin of the Institute for the Study of North Eurasian. Cultures Hokkaido Univ 13:43–74 (In Japanese with English summary)
Pederzani S, Britton K (2019) Oxygen isotopes in bioarchaeology: principles and applications, challenges and opportunities. Earth-Sci Rev 188:77–107
Price TD, Manzanilla L, Middleton WD (2000) Immigration and the ancient city of Teotihuacan in Mexico: a study using strontium isotope ratios in human bone and teeth. J Archaeol Sci 27:903–913. https://doi.org/10.1006/jasc.1999.0504
Sato T (2003) Faunal remains excavated from the Nijibetsu Shuwan Kumaokuriba site: a zooarchaeological study of Nusa site at Ainu Kotan. Bull Natl Museum Japnaese History 107:119–165 (in Japanese with English summary)
Sato T (2019) A zooarchaeological study of the formation process of the Ainu bear-sending ceremony. In: Mattila R, Iso S, Fink S (eds) Animals and their relation to gods, humans and things in the ancient world. Springer VS, Wiesbaden, pp 389–408
Sato Y, Mano T, Takatsuki S (2005) Stomach contents of brown bears Ursus arctos in Hokkaido. Japan Wildl Biol 11:133–144. https://doi.org/10.2981/0909-6396(2005)11[133:SCOBBU]2.0.CO;2
Schoeninger MJ, DeNiro MJ (1984) Geochim Cosmochim ac 48(4):625–639. https://doi.org/10.1016/0016-7037(84)90091-7. Nitrogen and carbon isotopic composition of bone collagen from marine and terrestrial animals
Schoeninger MJ, DeNiro MJ, Tauber H (1983) Stable nitrogen isotope ratios of bone collagen reflect marine and terrestrial components of prehistoric human diet. Science 220(4604):1381–1383. https://doi.org/10.1126/science.6344217
Shirane Y, Jimbo M, Yamanaka M, Nakanishi M, Mori F, Ishinazaka T, Sashika M, Tsubota T, Shimozuru M (2021) Dining from the coast to the summit: Salmon and pine nuts determine the summer body condition of female brown bears on the Shiretoko Peninsula. Ecol Evol 11(10):5204–5219. https://doi.org/10.1002/ece3.7410
Sontag W (1986) Quantitative measurements of periosteal and cortical-endosteal bone formation and resorption in the midshaft of female rat femur. Bone 7(1):55–62. https://doi.org/10.1016/8756-3282(86)90152-3
Stirling I (1974) Midsummer observations on the behavior of wild polar bears (Ursus maritimus). Can J Zool 52(9):1191–1198
Stirling I, Archibald WR (1977) Aspects of predation of seals by polar bears. J Fisheries Board Can 34(8):1126–1129
Stirling I, Derocher AE (1990) Factors affecting the evolution and behavioral ecology of the modern bears. Bears: Their Biology Manage, 189–204
Stirling I, McEwan EH (1975) The caloric value of whole ringed seals (Phoca hispida) in relation to polar bear (Ursus maritimus) ecology and hunting behavior. Can J Zool 53(8):1021–1027
Takigami M, Yoneda M (2020) Stable Isotope Analysis in Archaeological Science and Mummy Studies. The Handbook of Mummy Studies: New Frontiers in Scientific and Cultural Perspectives, 1–14
Tayasu I, Hirasawa R, Ogawa NO, Ohkouchi N, Yamada K (2011) New organic reference materials for carbon- and nitrogen-stable isotope ratio measurements provided by Center for Ecological Research, Kyoto University, and Institute of Biogeosciences, Japan Agency for Marine-Earth Science and Technology. Limnology 12 (3), 261–266. https://doi.org/10.1007/s10201-011-0345-5
Tomczyk J, Wierzbowski H, Zalewska M (2016) Stable isotope record of human and sheep enamel carbonate from the Ancient Middle Euphrates Valley (Syria). Int J Osteoarchaeol 26(4):599–609. https://doi.org/10.1002/oa.2449
Tsutaya T, Yoneda M (2015) Reconstruction of breastfeeding and weaning practices using stable isotope and trace element analyses: a review. Yearb Physic Anthropol 156:2–21. https://doi.org/10.1002/ajpa.22657
Tsutaya T, Naito YI, Ishida H, Yoneda M (2014) Carbon and nitrogen isotope analyses of human and dog diet in the Okhotsk culture: perspectives from the Moyoro site. Japan Anthropol Sci 122(2):89–99. https://doi.org/10.1537/ase.140604
Utagawa H (1999) The Archaeology of Iyomante. In: Fitzhugh WW, Dubruil CO (eds) Ainu: Spitir of a Northern people. Arctic Studies Center, National Museum of Natural History, Smithsonian Institution in association with University of Washington Press, Washington, DC., US, pp 256–260
Veitschegger K, Kolb C, Amson E, Scheyer TM, Sanchez-Villagra MR (2018) Palaeohistology and life history evolution in cave bears, Ursus Spelaeus Sensu Lato. PLoS ONE 13:e0206791. https://doi.org/10.1371/journal.pone.0206791
Vigne JD, Guilaine J, Debue K, Haye L, Gérard P (2004) Early taming of the cat in Cyprus. Science 304(5668):259–259. https://doi.org/10.1126/science.1095335
Vila C, Leonard JA, Gotherstrom A, Marklund S, Sandberg K, Liden K, Wayne RK, Ellegren H (2001) Widespread origins of domestic horse lineages. Science 291(5503):474–477
Watanabe H (1994) The animal cult of Northern Huunter-Gatherers: patterns and their ecological implications. In: Irimoto T, Yamada T (eds) Circumpolar Religion and Ecology: an Anthropology of the North. University of Tokyo, Tokyo, Japan, pp 47–67
Acknowledgements
This study was conducted by the support of Joint Research Grant for the Environmental Isotope Study of Research Institute for Humanity and Nature.
Funding
This work was supported by Japan Society for the Promotion of Science KAKENHI Grant Numbers 17J04991, 19K20495, 21H04784 and 22H05028 to J.M.
Author information
Authors and Affiliations
Contributions
J.M., T.T., T.S. conceived this study; T.S. provided samples; J.M. performed isotope analyses; J.M. and T.T. wrote the first draft of the paper; all authors were involved in interpreting the results and contributed to the final draft of the paper.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Matsubayashi, J., Tsutaya, T. & Sato, T. Retrospective isotope analysis of ancient remains to distinguish between tamed and wild animals. Archaeol Anthropol Sci 16, 138 (2024). https://doi.org/10.1007/s12520-024-02042-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-024-02042-0