Abstract
The late Early Pleistocene archaeological site of Fuente Nueva 3 (Orce, Guadix-Baza Depression, SE Spain), dated to ~1.4 Ma, provides evidence on the subsistence strategies of the first hominin population that dispersed in Western Europe. The site preserves Oldowan tool assemblages associated with abundant remains of large mammals. A small proportion of these remains show cut marks and percussion marks resulting from defleshing and bone fracturing, and a small proportion of bones also show tooth marks. Previous taphonomic studies of FN3 suggested that the hominins had secondary access to the prey leftovers abandoned by sabretooth cats and other primary predators. However, a recent analysis by Yravedra et al. (2021) of the frequency of anthropogenic marks and tooth marks has concluded that the hominins had primary access to the carcasses of a wide variety of ungulate prey, even though the frequency of evisceration marks is strikingly low. In this rebuttal, we analyse the patterns of bone preservation in FN3, which show that the exploitation of bone marrow by the hominins after hammerstone breakage was a usual activity at the site. Our study also reviews the evidence available on the lesser abilities of sabretooth cats for carcass processing compared to pantherine felids. This reinforces the hypothesis that primary predators provided the hominins the opportunity to scavenge sizeable chunks of meat and bone marrow of their prey carcasses before the arrival of hyaenas. Finally, we also provide new inferences on resource availability and competition intensity among the members of the carnivore guild in FN3, which reinforce our interpretation that a secondary access by the Oldowan hominins to the prey leftovers of sabretooth cats was an optimal foraging strategy in the Guadix-Baza Depression.
Similar content being viewed by others
Introduction
This article is a rebuttal to Yravedra et al. (2021), who have recently published a reinterpretation of the taphonomy of the late Early Pleistocene archaeological site of Fuente Nueva 3 (FN3; Guadix-Baza Depression, SE Spain). In their paper, Yravedra et al. (2021) challenge our previous inferences (Espigares et al. 2013, 2019) on the mode and time of access of sabretooth cats, hominins, and hyaenas to ungulate carcasses, favouring a model of primary access by the hominins to these resources. In this rebuttal, we focus on the following issues, providing counterarguments to Yravedra et al. (2021) that include: (i) a review of the models of primary and secondary access to ungulate carcasses by the Oldowan hominins; (ii) a comparison of the frequencies of anthropogenic marks and tooth marks described by Espigares et al. (2019) and Yravedra et al. (2021) in the skeletal remains of large mammals unearthed from FN3 in different excavation seasons; (iii) new data on the patterns of skeletal completeness and bone survival in the faunal assemblage of FN3; (iv) a review of the craniodental and postcranial anatomy of sabretooth cats and their implications on the hunting habits of these predators, their limitations for carcass consumption, and the scavenging opportunities provided to the hominins and hyaenas; (v) a review of the bone cracking abilities and other ecomorphological inferences for the giant hyaena Pachycrocuta brevirostris; (vi) new estimates of resource abundance and competition intensity among the members of the carnivore guild of FN3; and (vii) a review of faunal turnover events in the late Early Pleistocene and their implications for hominin ecology.
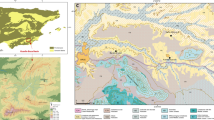
FN3 is located near the town of Orce in the north-eastern sector of the Guadix-Baza Depression, SE Spain (Fig. 1A). This inland basin extends over an area of ~4000 km2 and is surrounded by the highest relief of the Alpine-Betic orogeny, which rises to an altitude of ~3000 m. The basin preserves a relatively complete Plio-Pleistocene sedimentary record, which is composed of lacustrine and fluvial deposits (limestones, marls, shales, sands, and conglomerates) as well as dark clays and silexites originated from hot springs (García-Aguilar and Palmqvist 2011; García-Aguilar et al. 2014, 2015). During the Miocene, the basin was connected to the Mediterranean Sea by the Almanzora Corridor and to the Atlantic Ocean by the Guadalquivir Basin. The closure of these corridors took place between the end of the Tortonian and the Messinian, which led to a change from exorheic conditions to continental sedimentation (Soria et al. 1999; Hüsing et al. 2010). Isostatic uplifting, with an average uplift rate of ~200 m/Ma (estimated from Late Neogene coastal marine conglomerates and coral reefs; Braga et al. 2003), resulted in an elevation of the uppermost level of the basin to ~1000 m above sea level. During most of the Pliocene and Pleistocene, the Guadix-Baza Depression developed a network of endorheic drainage and was subject to intense tectonic subsidence, which allowed the accumulation of a thick (~550 m) and relatively continuous record of continental sediments. Tectonically induced hydrothermal activity provided a mild and productive environment for the terrestrial fauna during the Early Pleistocene (García-Aguilar et al. 2014, 2015; Palmqvist et al. 2022a). This allowed the establishment of a rich and diverse community of large mammals, whose remains are preserved in many archaeological and palaeontological localities across the entire sedimentary depression, especially in its north-eastern sector (Palmqvist et al. 1996, 2011; Arribas and Palmqvist 1998; Palmqvist and Arribas 2001; Espigares et al. 2013, 2019; Toro-Moyano et al. 2013; Rodríguez-Gómez et al. 2016a, 2017a; Ros-Montoya et al. 2017). The Orce area, a satellite basin in the NE sector of the Guadix-Baza Depression with an extent of ~170 km2, preserves a unique fossil record of the Early Pleistocene fauna, including two Late Villafranchian sites that mark the oldest presence of hominins in Western Europe, Barranco León (BL) and FN3 (Martínez-Navarro et al. 1997, 2014; Espigares et al. 2013, 2019; Toro-Moyano et al. 2013; Palmqvist et al. 2016).
A Geological context of the Guadix-Baza Depression in the Betic Cordillera, SE Spain. The rectangle encloses the Orce-Fuente Nueva sector of the Baza Basin. The position of the two late Early Pleistocene sites discussed in the text, Barranco León (BL) and Fuente Nueva 3 (FN3), is marked. B Stratigraphic series of the FN3 site. C View of FN3 during the summer excavation of 2015. D View of a tusk of elephant Mammuthus meridionalis, probably a male individual given the length (>3 m) and width (~30 cm) of this specimen
The archaeological sites of BL and FN3 preserve huge lithic assemblages of Oldowan tradition (Martínez-Navarro et al. 1997; Toro-Moyano et al. 2011, 2013; Barsky et al. 2010, 2015, 2016; Titton et al. 2018, 2021) together with an abundant fauna of large and small vertebrates, including a high diversity of carnivores: two mustelids (Martellictis ardea and Meles meles), three canis (Vulpes alopecoides, Canis mosbachensis, and Lycaon lycaonoides), one ursid (Ursus struscus), one hyaenid (Pachycrocuta brevirostris), and three felids (Lynx pardinus, Megantereon whitei, and Homotherium latidens) (Martínez-Navarro et al. 2010, 2021; Madurell-Malapeira et al. 2011; Boscaini et al. 2015; Medin et al. 2017; Ros-Montoya et al. 2021). This rich carnivore guild resembles those found in other Early Pleistocene archaeological and palaeontological sites of Eurasia, such as Dmanisi in Georgia, dated to ~1.8 Ma (Lordkipanidze et al. 2007; Bartolini-Lucenti et al. 2022), Pirro Nord in Italy, dated to 1.6–1.3 Ma (Petrucci et al. 2013), and Vallonnet in France, dated to ~1.1 Ma (Moullé et al. 2006), or the early Acheulian site of Ubeidiya in Israel, dated to 1.6–1.2 Ma (Ballesio 1986; Bar-Yosef and Goren-Inbar 1993; Martínez-Navarro et al. 2009). In Africa, carnivores are well represented at sites older than 1.8–1.7 Ma: for example, Shungura E-F-G, Lokalalei and Olduvai Bed I in East Africa, the lower levels of Swartkrans and Sterkfontein Member 5 in the South African cave complexes, and Ain Hanech in North Africa (Brain 1981; O’Regan 2007; O’Regan and Steininger 2017; Sahnouni and van der Made 2009; and references therein).
There are several age estimates for the archaeological sites of BL and FN3. For example, a combination of biostratigraphy, magnetostratigraphy, and the U-series/electron spin resonance (ESR) dating method applied to optically bleached quartz grains and fossil teeth allowed the estimation of their age as 1.43 ± 0.38 Ma for BL and 1.19 ± 0.21 for FN3 (Duval et al. 2012; Toro-Moyano et al. 2013). In addition, an age of 1.50 ± 0.31 Ma has been derived for FN3 based on cosmogenic nuclides (Álvarez et al. 2015). Other age estimates for these sites (1.26 ± 0.13 Ma for BL and 1.20 ± 0.12 Ma for FN3) were obtained with a biometric approach based on the size of the lower molar tooth of the arvicolid Mimomys savini (Lozano-Fernández et al. 2013, 2014). However, this “vole-clock” assumes a rectilinear pattern of change through time, which discourages its use in biostratigraphy (Martin 2014; Palmqvist et al. 2014, 2016). Moreover, the absence of suids from the fossil assemblages of BL and FN3 provides a useful biochronological marker (Martínez-Navarro et al. 2015): suids are not present in Europe in the interval between 1.8 and 1.2 Ma until the arrival of a derived form of Sus strozzi during the Epivillafranchian (Cherin et al. 2018, 2020). This species is first recorded in association with hominin remains at level TE9 of Sima del Elefante in the Atapuerca karstic complex, which is dated to 1.22 ± 0.16 Ma on the basis of cosmogenic nuclides (Carbonell et al. 2008), and later in other sites of Jaramillo age like Untermassfeld in Germany, Vallonnet in France, or Vallparadís in Spain (Moullé et al. 2006; Madurell-Malapeira et al. 2010, 2014; Cherin et al. 2018, 2020). According to these lines of evidence, the most parsimonious age estimate for BL and FN3 is ~1.4 Ma (Palmqvist et al. 2016).
The sub-horizontal stratigraphy of FN3 shows three sedimentary cycles deposited in a lutitic-carbonate, lacustrine-to-swampy environment, each with limestones at the top of the sequence separated by clays, fine sands, and marly lutites (Fig. 1B). The clays and calcilutites were deposited under a semi-permanent water table while the sands correspond to lake shore deposits. Microscopic analyses showed that the sands are poorly concretioned ostracodites, which indicates the presence of oligosaline waters (Anadón et al. 1986), with scarce detritic cobbles (10–15% of quartz grains). The fertile strata of FN3 comprise six layers, which cluster in two main archaeological levels (Turq et al. 1996; Martínez-Navarro et al. 1997; Espigares et al. 2013, 2019). The stratigraphy of BL is dominated by limestones, sandstones, carbonate silts, and dark mudstones deposited in a lacustrine system with an alternation of oligo- to mesohaline waters (Turq et al. 1996; Arribas and Palmqvist 2002; Anadón et al. 2015). These sediments are associated with a swampy environment except level D (formerly BL-5: Arribas and Palmqvist 2002), which shows fluvial features and encases most of the archaeological assemblage (Toro-Moyano et al. 2013).
BL and FN3 have provided assemblages of Oldowan tools that represent the whole reduction sequence, composed of abundant small flakes, cobbles (one-third with percussion marks), cores, and debitage made of flint, limestones, and calcarenites from the surroundings of the sites. Flint was largely exploited for flake production while limestones, although also used for flake production, were mostly employed as percussion tools (Tixier et al. 1995; Turq et al. 1996; Martínez-Navarro et al. 1997; Oms et al. 2000; Palmqvist et al. 2005; Barsky et al. 2010, 2015, 2016; Toro-Moyano et al. 2011, 2013; Titton et al. 2018, 2021). The tools are associated with abundant remains of large mammals. These rich lithic assemblages, together with the inferences on the availability of animal resources and the intensity of competition among carnivores (including hominins) for these resources, provide interesting clues on the subsistence strategies of the earliest hominin population of Western Europe (Rodríguez-Gómez et al. 2016a; Espigares et al. 2019).
A small proportion of bones show cut marks and percussion marks resulting from defleshing and bone fracturing for accessing their marrow contents (Espigares et al. 2019; Yravedra et al. 2021). This provides useful information on the subsistence strategies of the first hominins that colonized Western Europe in the late Early Pleistocene (Espigares et al. 2013, 2019; Toro-Moyano et al. 2013). Cut marks are relatively short (length range: 1.8–13.0 mm) and are predominantly represented by incisions, although scrapes, sawing marks, and chop marks are also documented. They mostly appear on the bone remains of animals of medium-to-large and very large size (e.g., horses Equus altidens and E. suessenbornensis, megacerine deer Praemegaceros cf. verticornis, bison Bison sp., rhino Stephanorhinus hundseimensis, hippo Hippopotamus antiquus, and elephant Mammuthus meridionalis). Cut marks evidence patterns of skinning, disarticulation, defleshing, evisceration, and periosteum removal. Evidence of intentional bone breakage includes percussion marks, pits, notches, impact flakes, and negative flake scars generated by a direct contact of the bones with the hammerstone (Espigares 2010; Espigares et al. 2013, 2019). The fossil assemblages of BL and FN3 also preserve bones with carnivore modifications, which are less frequent than those of anthropogenic origin (Espigares et al. 2019; Yravedra et al. 2021). These tooth marks are similar in morphology, dimensions, and anatomical position to those recorded at quarry VM3 of Venta Micena, an Early Pleistocene site of Orce that has been definitively interpreted as a breeding den of the giant, short-faced hyaena Pachycrocuta brevirostris (Palmqvist et al. 1996, 2011, 2022b; Arribas and Palmqvist 1998; Palmqvist and Arribas 2001; Espigares 2010). For this reason, most tooth-marked bones of BL and FN3 were probably consumed by P. brevirostris, although some were apparently gnawed by porcupines (Espigares et al. 2013, 2019). This is particularly evident in the case of the upper archaeological level of FN3, in which hyaena coprolites are abundantly preserved (Fig. 2).
Spatial distribution of bones, lithic artefacts, stones (manuports?), and coprolites recovered from Fuente Nueva 3 (FN3) in the 2001–2015 excavation seasons. This 2D scatterplot is a longitudinal section that shows the X- and Z-coordinates of the records projected on the Y-plane. The graph shows a concentration of hyaena coprolites in the Upper Archaeological Level while the Oldowan tools and stones are more abundantly represented in the Lower Archaeological Level. Scales in cm
Recently, Yravedra et al. (2021) analysed the evidence on the food procurement strategies of the hominins at FN3, focusing on the analysis of the anthropogenic traces and tooth marks identified in the bone remains of the 2017–2020 excavation seasons, thus increasing the sample size of the previous study by Espigares et al. (2019). Despite the overall similarity between the results of the two studies, Yravedra et al. (2021) concluded that the hominins had primary access in FN3 to the carcasses of a wide variety of ungulate prey. Here, we re-evaluate several issues overlooked by Yravedra et al. (2021) and reaffirm a model, originally proposed by Espigares et al. (2013, 2019), with a secondary access by hominins to the prey animals, which were abandoned by sabretooth cats H. latidens and M. whitei, as well as other predators such as jaguar Panthera gombaszoegensis and wild dog L. lycaonoides.
Primary or secondary access by the hominins to ungulate carcasses at FN3?
Yravedra et al. (2021) favoured a model of primary access to ungulate carcasses by the hominins of FN3, arguing that this would only be possible in either of the following three scenarios: (i) if the hominins hunted the prey on their own; (ii) if they engaged in confrontational scavenging, kleptoparasitizing the prey of other carnivores; or (iii) if they located carcasses of animals, which died from other natural causes before other scavengers. Although no direct method allows us to test if early Homo had primary access to ungulate carcasses by hunting or by early scavenging (Potts 1991), Yravedra et al. (2021) chose the first scenario based on a supposedly emerging consensus that the Oldowan hominins had primary access through hunting of animals of different sizes. Despite this, they acknowledged that such scenario “entails a series of important behavioural capacities among early hominins, since hunting involves some degree of planning, cooperation, and the ability to kill prey” (Yravedra et al. 2021: p. 2). However, the limited technological skills of the FN3 hominins make it difficult to argue that they could have hunted medium-to-large and very large-sized prey on a regular basis. According to Yravedra et al. (2021), the two predominant knapping techniques documented at FN3 (for details, see Barsky et al. 2010, 2015) are “bipolar knapping on anvil, as well as direct percussion knapping, used to produce small-sized flakes and choppers” (Yravedra et al. 2021: p. 5). In fact, it is the small size of the flakes unearthed from the site, few centimetres in most cases (Turq et al. 1996; Martínez-Navarro et al. 1997; Toro-Moyano et al. 2011; Espigares et al. 2013, 2019), which suggests the lack of an effective weaponry for killing large ungulate prey. For this reason, it is difficult to conceive how the hominin population of FN3 could had have a direct impact on megafauna (for a review on the ecology of Oldowan hominin carnivorous activity, see Blumenshine and Pobiner 2007). However, it must be noted that the use of organic weapons (e.g., wooden spears) for hunting cannot be discarded, although the Orce sites do not preserve evidence on them.
Endurance running (i.e., the ability to run many kilometres at relatively low speed over extended time periods using aerobic metabolism) could have been an adaptation of hominins for chasing to exhaustion small-to-medium sized ungulates to exhaustion (Liebenberg 2006). Moreover, there are predators relatively small such as the dhole (Cuon alpinus) that are capable of effective communal hunting. Humans are comparatively poor sprinters, but they perform well at running long distances due to several anatomical and physiological adaptations (Bramble and Lieberman 2004; Ruxton and Wilkinson 2012). These include: (i) a log tibia, which results in a long stride length combined with the presence of long spring-like tendons connected to short muscle fascicles (i.e., a longer Achillus tendon of the gastronemius muscle, which is where the elastic energy is stored), which can economically generate substantial force; (ii) a long plantar arch and enlarged articular surface areas in most of the joints of the lower body, which dissipate the high impact loads generated in running; (iii) derived features that enhance trunk stabilization, such as an expanded area on the sacrum for attachment of an enlarged gluteus maximus muscle; and (iv) thermoregulatory adaptations for heat dissipation that help to maintain stable body temperature, including a multiplication of eccrine sweat glands for evapotranspiration, reduced body hair, mouth breathing, and an elaborated cranial venous circulation for brain cooling (Bramble and Lieberman 2004; Ruxton and Wilkinson 2012).
Endurance running seems to have evolved early in the genus Homo. The Dmanisi hominins show a number of archaic features (e.g., a small body size, a low encephalization quotient, a more medial orientation of the foot, and absence of humeral torsion) that are largely comparable to the earliest members of Homo (i.e., H. habilis/rudolfensis). However, they also show some derived locomotor traits apparent in African H. erectus and later hominins, including modern human-like body proportions and lower limb morphology, which are indicative of the ability for long-distance traveling (Lordkipanidze et al. 2007).
Persistence hunting would take place during the hottest time of the day and would involve chasing an animal until it is run to exhaustion (Liebenberg 2006). Two critical factors for persistence running are that humans can keep their body cool by sweating while running and can track down an animal, as shown by modern hunter-gatherers. As a result, persistence hunting may have been one of the most efficient forms of hunting before the domestication of dogs and can therefore have been crucial in the evolution of humans (Liebenberg 2006).
The second scenario, confrontational scavenging, would be dangerous for the hominins (Treves and Palmqvist 2007). For this reason, Yravedra et al. (2021) also considered it unlikely. However, aggressive scavenging could always be possible with rudimentary weapons, as shows the kleptoparasitism of the prey carcasses of lions and leopards by modern African agropastoralists using simple stones or long sticks (Blumenschine and Pobiner 2007;Treves and Naughton-Treeves 1999). The studies of Lordkipanidze (2015) and Salopek (2015) are of particular interest here: they suggested that stone-throwing by the hominins could be an adaptive strategy for driving away carnivores from their kills and stealing their prey. This hypothesis would explain the huge number of allochthonous cobbles found in the Early Pleistocene site of Dmanisi (Coil et al. 2020). Although this is certainly a highly speculative scenario, it is worth noting that the bone remains preserved at FN3 are also intermingled with abundant cobbles of dolomitic limestones from the Jurassic surroundings of the basin (Espigares et al. 2013), particularly in the lower archaeological level of FN3 (Fig. 2). Interestingly, in this level, hyaena coprolites are comparatively very scarce. The small size of the clastic components of the sediment at FN3 (clays and fine-grained sands) rules out the possibility that these stones were dispersed by water currents. This opens the possibility that the hominins transported the cobbles to the site and used them as weapons for forcing predators like sabretooth cats (or other scavengers like the hyaenas, jackals, and vultures) to surrender the prey carcass, as suggested by Espigares et al. (2013).
The third possibility, primary access to intact carcasses of animals that died through natural causes or to prey leftovers left by the primary predators such as sabretooth cats, seems the most reasonable given the anatomy of early Homo as well as their apparently limited technological skills (Espigares et al. 2019). Apart from vultures, the large hyaena P. brevirostris (Palmqvist et al. 2011; Iannucci et al. 2021) and the jackal-sized dog Canis mosbachensis (Martínez-Navarro et al. 2010, 2021) would indeed be worthy competitors of the FN3 hominins in the access to carrion. Modern hyaenas and jackals often rely upon visual clues such as circling vultures to identify scavengeable resources, and then run long distances to secure the carcass (Bramble and Lieberman 2004). Compared with these carnivores, which have more twilight and nocturnal habits, the hominins were likely predominantly diurnal. This means that the information provided by the aerial scavengers was probably more helpful for the early members of Homo than for other terrestrial scavengers. Given that hominins and vultures occupied the same habitats and scavenged the same medium-to-large and very large mammals, they likely competed for access to carcasses alongside other terrestrial scavengers (Morelli et al. 2015). Vultures are such efficient scavengers that in some areas of Africa they consume more meat than all other carnivores combined (Ogada et al. 2012), which explains their high diversity. For this reason, it has been suggested that vultures used as beacons for meat were particularly important to the hominins that dispersed out of Africa, facilitating their occupation of new landscapes (see review in Morelli et al. 2015).
Endurance running may have been the key to hominin success in the access to large ungulate carcasses before other terrestrial scavengers such as the giant hyena P. brevirostris: the human adaptations discussed above for persistence hunting are equally useful for scavenging in open habitats during the day, particularly in the dry season, when other terrestrial scavengers like hyaenas and jackals avoid running long distances due to thermoregulatory constraints (Bramble and Lieberman 2004; Liebenberg 2006; Lieberman et al. 2007; Ruxton and Wilkinson 2012).
In the case of FN3, endurance running may have allowed early Homo to reach and exploit carcasses of medium-to-large sized ungulates before eventually surrendering them on the arrival of potentially dangerous hyaenas. This scenario is suggested by the finding of a partial skeleton of elephant Mammuthus meridionalis in the upper archaeological level of FN3 [equivalent to levels 9-10 of Turq et al. 1996 and Martínez-Navarro et al. 1997], which is surrounded by seventeen flint flakes and thirty-four hyaena coprolites (Espigares et al. 2013). This partial elephant carcass is composed of the mandible, the vertebral column, the ribs, and the pelvis, while the cranium and limbs are missing. Based on the shape of the mandibular symphysis and the pelvis morphology, this individual was a female, while the advanced degree of wear of the third lower molar tooth indicates an old age at death, around 60 years. Yravedra et al. (2021: p. 17) indicate that “no tooth marks or anthropogenic marks were observed on the elephantid bones, thus not conclusively proving the interaction of humans or carnivores with the proboscidean carcass” (Yravedra et al. 2021: p. 17). For this reason, they suggest that “this spatial association of lithic finds and coprolites with these elephant carcasses may have been fortuitous, resulting from independent episodes that coalesced into a palimpsest through complex site formation processes” (Yravedra et al. 2021: p. 17). However, it can be argued that more than 95% of the bones unearthed from FN3 preserve intact their cortical surface and show weathering stage 0 (Yravedra et al. 2021: table 5). This means that they were exposed before burial during a short interval, less than 1 year (Behrensmeyer 1978), which would refute the interpretation of the site as a palimpsest and argues for a primary access of the hominins to this elephant carcass.
At first sight, the low degree of bone weathering in the FN3 assemblage might indicate that the reason behind the absence of cut marks and tooth marks from the bones of the elephant skeleton is that neither hominins nor hyaenas fed on this carcass, as suggested by Yravedra et al. (2021). However, we must consider here two issues. First, the periosteum on elephant long bones is 1–3 mm thick, which accounts for the rarity of cut marks on cortical bone surfaces at megafaunal butchery sites (Espigares et al. 2013; Haynes and Krasinski 2021; Toth and Schick 2019). Moreover, the absence of tooth marks and anthropogenic marks on the elephant bones could evidence a rapid burial of these remains, as suggested by the skeleton being found in articulation. In other circumstances, the remains would be scattered, as happens when the scavengers dismember a carcass and disperse the bones (Leslie 2016; White and Diedrich 2012). Second, the excavation of this elephant skeleton was spread over 3 years, from 2001 to 2003, which means that the remains were exposed to weather conditions in a region that nowadays has an alternation of very cold winters and very hot summers. This resulted in the secondary weathering of these bones, which probably erased any cut mark or tooth mark on their surface.
Although the absence of cut marks and tooth marks precludes making precise inferences on the sequence of access of hominins and hyaenas to this carcass (Espigares et al. 2013), there are other taphonomic clues that provide relevant information. For example, spatial data analysis showed that the flint flakes and the coprolites were deposited separately in the area surrounding the partial elephant skeleton: 11 out of 27 tools are closer to another tool than to a coprolite (expected frequency for a random distribution: 5.7) and 31 out of 34 coprolites are closer to another coprolite than to a tool (expected frequency: 22.7). This indicates a non-random distribution of the tools and coprolites around the elephant carcass (Espigares et al. 2013). Moreover, the flakes are absent from the places where the limbs would have been found before being dismembered from the body. In contrast, coprolites are abundantly preserved in these areas. This suggests that hominins arrived first, dismembered the limbs from the skeleton, and transported them to another place distant from the FN3 site (Espigares et al. 2013). Later, the hyaenas, which would be able to consume meat at a more advanced stage of decomposition than the hominins due to their lower gastric pH value (Beasley et al. 2015), exploited the rest of the carcass, which at that time would be basically composed of the resources linked to the axial skeleton (Espigares et al. 2013).
Compared to other coprolites unearthed from the excavation quarry of FN3, the darker colour of those that surround the elephant skeleton tentatively suggests that the hyaenas consumed a high amount of fat and flesh from this carcass (Espigares et al. 2013). The lower part of the hyaena’s intestine contains the album graecum, a green paste which mixture with the mineral component of bone (calcium hydroxylapatite) results in the typical whitish colour of the coprolites when they are in contact with the atmosphere. However, darker excrements rich in inclusions of organic matter are produced when the hyaenas eat large quantities of flesh and grease (Matthews 1939; Bearder 1977). Hyaena coprolites are well represented in the excavated surface of FN3 (Fig. 2), particularly in the upper archaeological level (Espigares et al. 2013, 2019), but at lower densities than in the surroundings of the partial skeleton of M. meridionalis. Interestingly, the coprolites that are not associated to the elephant carcass show a whitish tone, which evidences the consumption of bones by the hyaenas (Espigares et al. 2013).
Given the evidence reviewed above, the most parsimonious hypothetical scenario is that hominins where the first to exploit this elephant carcass, and that they fractured the cranium, dismembered the legs, and transported them (as well as other grease-rich tissues like the brain, the trunk, the tongue, the temporal gland, and the edible fat inside the air cavities within the cranium; Byers and Ugan 2005; Shoshani et al. 2006; Agam and Barkai 2016) to a safer place for consuming them later (Espigares et al. 2013, 2021). In the absence of food preservation techniques, such resources — weighing more than half a tonne — would only be transported if they were to be consumed in a relatively short time, which suggests that a large group of hominins collaborated in their transport (Palmqvist et al. 2022c). However, it must be noted that the gastric pH value in modern humans (1.5 on average) lies between those of obligate and facultative scavengers (1.3 ± 0.08 and 1.8 ± 0.27, respectively) and is lower than in generalist carnivores (2.2 ± 0.44), omnivores (2.9 ± 0.33), and specialist carnivores (3.6 ± 0.51) (data from Beasley et al. 2015). This high stomach acidity allows the combatting of meat-borne pathogens, which would represent an early adaptation of Homo to either: (i) scavenging prey carcasses abandoned by carnivores that had begun to rot; or (ii) protracted consumption of prey items hunted by the hominins themselves, or that died from other causes than predation but were too large to be eaten all at once (Beasley et al. 2015; Dunn et al. 2020; Ben-Dor et al. 2021). Interestingly, although chimpanzees hunt cooperative small prey, they tend to avoid carrion, even when it is relatively fresh, which is probably due to the risk of bacterial infections and zoonoses (Pobiner 2020). In contrast to humans, the stomach acidity of chimpanzees is close to neutral in pH (Dunn et al. 2020). In fact, the ranges of stomach pH values in omnivorous (2.8–5.9) and herbivorous (3.4–5.9) primates do not encompass the highly acidic value shown by modern humans (Beasley et al. 2015). This provides a possible argument for an early adaptation to scavenging in the genus Homo and/or for a protracted consumption of carcasses of megafaunal prey (although hunting of very large prey would probably be beyond the possibilities of Oldowan hominins, as previously argued).
In summary, hominin adaptations for endurance running opened the possibility that they could have had primary access to ungulate carcasses and could have exploited these resources before surrendering them after the arrival of dangerous scavenging carnivores like the giant hyaena P. brevirostris. Certainly, this may have been the case of the partial skeleton of the elephant unearthed from FN3 (Espigares et al. 2013). However, more compelling evidence on the role played by the hominins and carnivores at this site can be retrieved from the frequencies of anthropogenic marks and tooth marks in the bone assemblage.
Anthropogenic marks, tooth marks, and the role of hominins and carnivores at FN3
Yravedra et al. (2021) indicated that the low frequency of tooth-marked bones and the scarcity of digested bones suggest a limited impact of the giant hyaenas on the FN3 assemblage. This was also the conclusion of the study of Espigares et al. (2019), based on the analysis of 9041 fossils unearthed from all stratigraphic levels of FN3 during systematic excavations and fieldwork between the years 1999 and 2015. After the exclusion of isolated teeth and of those bones badly preserved that needed restoration, 3852 bone remains were searched for traces of modification on their cortical surfaces (Espigares et al. 2019). Cut marks were identified on 32 bones (0.9%) and percussion marks (i.e., pits, notches, impact flakes, and negative flake scars generated by hammerstone impact) on 74 bones (1.9%). In contrast, the study of Yravedra et al. (2021) was based on a smaller dataset, 4801 remains unearthed from FN3 during the 2017–2020 excavation seasons. Once teeth and ivory fragments (752 specimens) were excluded, as well as the bone remains that were badly preserved (1192 specimens), the sample in the search for anthropogenic marks consisted of 2857 bones (Yravedra et al. 2021: table 5). This barely represents three-quarters of the remains studied by Espigares et al. (2019). Of these remains, 25 bones showed cut marks (0.9%) and 16 preserved evidence of intentional bone fracturing by hominins (0.6%). Therefore, the frequency of cut-marked bones identified by Yravedra et al. (2021) is identical to the one previously reported by Espigares et al. (2019). For this reason, it is surprising that Yravedra et al. (2021: p. 3) suggested that “the results of Espigares et al. (2019) have been challenged by Domínguez-Rodrigo et al. (2020), who reassessed some of the evidence.” Specifically, they referred to two cut marks figured by Espigares et al. (2019) that were reinterpreted by Domínguez-Rodrigo et al. (2020) as a tooth mark and a trampling mark, respectively, using an algorithm based on artificial-intelligence computer vision through deep learning. Both marks were attributed by Domínguez-Rodrigo et al. (2020) to FN3, but the one that they reinterpreted as a tooth mark is from BL.
Given that Espigares et al. (2019) and Yravedra et al. (2021) reported on the same frequency of cut marks in the bone assemblage of FN3, it is difficult to understand the claim of Yravedra et al. (2021) that a significant fraction of the cut marks identified by Espigares et al. (2019) were not true cut marks: if the frequency of cut-marked bones is identical in both studies, they should have equally assumed that neither were a similar fraction of theirs. Otherwise, the frequency of cut marks reported in Espigares et al. (2019) should be higher than the one reported by Yravedra et al. (2021) if it was only the former that included trampling/tooth marks misidentified as cut marks. In any case, the low frequencies of cut-marked and percussion-marked bones reported by Espigares et al. (2019) and Yravedra et al. (2021) for different subsets of the bone assemblage from FN3 are close to those recorded at Pirro Nord, a late Early Pleistocene site in which 14 out of 1285 vertebrate fossil remains (1.09%) show cut marks and eight (0.6%) evidence of intentional bone breakage (Cheheb et al. 2019). Finally, Espigares (2010), Espigares et al. (2013, 2019), and Yravedra et al. (2021) all also report on a low frequency of carnivore tooth marks (e.g., scores, pits, furrowing, and crenulated edges) in the bone remains of FN3. Therefore, the study of Yravedra et al. (2021) does not add relevant information to that previously available in Espigares et al. (2019).
To return to the cut marks: Yravedra et al. (2021: p. 3) also argued that “it is unexpected for Espigares et al. (2019) to claim that hominins had secondary access to carcasses when they are documenting evisceration cut marks on the ventral side of the ribs and vertebrae as well as defleshing cut marks on long bone diaphyses.” First, Yravedra et al. (2021) did not realize that only one of the four evisceration marks described by Espigares et al. (2019), the one found on a rib ventral shaft, is from FN3. The other three (one in the ventral side of a vertebral body and two in plastron fragments of chelonians) are from BL. The study of Yravedra et al. (2021) also described one evisceration mark in their sample (in this case, on a pelvis of a very large mammal). This means that only two evisceration marks have been reported in FN3, one by Espigares et al. (2019) and the other by Yravedra et al. (2021). In any case, the low frequency of evisceration marks does not necessarily imply that the hominins had early access on a regular basis to ungulate carcasses obtained though hunting, as suggested by Yravedra et al. (2021): evisceration marks could also record the opportunistic (and occasional) access to carcasses of animals that died from other causes than predation, as in the case of the elephant carcass discussed above.
Concerning the couple of anthropogenic marks from BL and FN3 figured by Espigares et al. (2019) that Domínguez-Rodrigo et al. (2020) reinterpreted as a tooth mark and a trampling mark, respectively, it should be noted that the photographs analysed should have been taken under specific conditions, including: (i) an illumination with the same intensity from the focal centre of the image to the periphery, with the mark positioned in a plane orthogonal to the camera lens; and (ii) a correct contrast of the colour channels (i.e., the image should be undistorted, not blurred, and with the absence of brightness and reflections). This was not the case of the images published by Espigares et al. (2019), especially in the case of the one from FN3, because these images were not taken for that purpose. For this reason, they are not suitable to be analysed with the methodology of Domínguez-Rodrigo et al. (2020). As they indicated, “images from BSM from these sites should be properly taken following the same protocols as in the experimental sample used here” (Domínguez-Rodrigo et al. 2020: p. 6). Moreover, the experimental results obtained by Fernández-Jalvo and Andrews (2016) using lithic tools of different mineral compositions must also be considered, because some features of the cut marks made with limestone flakes (a frequent raw material in FN3) were not considered by Domínguez-Rodrigo et al. (2020) when they reclassified one cut mark of FN3 as a trampling mark. Specifically, limestone is a porous raw material, which means that the tool edge soon becomes blunted and needs to be frequently cleaned. The cut marks inflicted with limestone tools are wider and shallower than any other cut mark made with quartzite or flint. This makes the appearance of these cut marks very similar to the trampling marks produced on those bones that were resting on a limestone substrate (Andrews and Cook 1985). For this reason, “location of marks on the anatomical element related to muscle or ligament attachment or filleting are the main criteria distinguishing butchering and the use of stone tools from trampling marks, locations of which are not related to anatomical traits” (Fernández-Jalvo and Andrews 2016: p. 44). This criterion was followed by Espigares et al. (2019) when they described the cut marks of FN3. Finally, it is striking that Yravedra et al. (2021) assumed as correct the re-interpretations by Domínguez-Rodrigo et al. (2020) of two marks described by Espigares et al. (2019), even though very few of the images published by Yravedra et al. (2021) would pass the artificial intelligence filter used in the study of Domínguez-Rodrigo et al. (2020).
The low frequencies of tooth marks in the bone assemblage of FN3 reported by Espigares et al. (2019) and Yravedra et al. (2021) suggest a relatively low intensity of carnivore ravaging. However, there is a high frequency of coprolites attributed to P. brevirostris in the upper archaeological level of FN3 (N > 200), while the record of coprolites is very low in the lower one (N = 14) (Fig. 2). This allows the inference of a regular presence of hyaenas in the upper level, despite the low frequency of tooth-marked bones. In this level, there are also abundant allochthonous cobbles, which suggests competition between hominins and hyaenas for the consumption of carcasses, as proposed by Espigares et al. (2013) for FN3 and Coil et al. (2020) for Dmanisi.
Insights about the carnivores responsible for the tooth marks preserved in the bones of FN3 can be derived from the analysis of their size and anatomical position. Moreover, the density and thickness of the bones on which they are found also provide useful information (Espigares et al. 2019). Given that most tooth marks appear on diaphyseal bone shafts with a dense cortical surface, this suggests a large-sized carnivore like the hyaena P. brevirostris as the main biting agent (Espigares et al. 2019). This is also suggested by the presence of notches on the bone edges resulting from bone crushing, a task not performed by living felids (Palmqvist et al. 2022b), as well as by the resemblance between the tooth marks of FN3 and the hyaena den of VM3. However, the low frequency of tooth marks in FN3 (<3% of the remains in the upper archaeological level; Yravedra et al. 2021) compared with VM3, where one-third of the remains are tooth-marked (Espigares 2010), suggests that carnivore activity at FN3 was limited compared to hominin activity (Espigares et al. 2019).
It must be noted that the permanent premolar teeth of adult hyaenas are progressively blunted by bone cracking, which results in very wide tooth marks (Espigares 2010: fig. 7.117) or even in inconspicuous tooth marks if the hyaenas are very old and their premolars are heavily flattened (Arribas and Palmqvist 1998; Palmqvist et al. 2022b). Moreover, the deciduous teeth of juvenile hyaenas are sharper, more feloid in shape than those of adults, so that their nibbling of bones results in abundant tooth marks, which can be unrecognized or misidentified with those left by small carnivores like jackals. Many of the bones found at modern hyaena dens are tooth-marked (e.g., 29.0–53.5% in spotted hyaena dens, 22.1–100% in brown hyaena dens, and 6.0–56.2% in striped hyaena dens; Kuhn et al. 2010). In the assemblage of VM3, tooth marks are found in 29.4% (1555/5288) of the remains (Palmqvist et al. 2022b) and the original proportion of tooth-marked bones was in all probability higher because many limb bones that do not preserve tooth marks show fracture patterns that evidence their breakage in the fresh state by adult hyaenas for accessing their marrow contents (these anatomical portions were probably tooth-marked before being fractured and consumed). Moreover, although a significant portion of the tooth marks preserved in VM3 were most probably produced by the juvenile hyaenas, a minor implication of some small or medium-sized carnivore like the jackal-sized wolf Canis orcensis cannot be discarded (Martínez-Navarro et al. 2021). However, FN3 was clearly not a denning area of P. brevirostris (Espigares et al. 2013, 2019). Given that the juvenile hyaenas do not forage with the adults searching for scavengeable carcasses (Arribas and Palmqvist 1998), this justifies their absence from FN3 and thus a lower frequency of tooth-marked bones in the site compared to VM3.
Additional insights on the role played by hominins and scavenging carnivores at FN3 can be retrieved from the analysis of the patterns of bone survival and their comparison with the hyaena den of VM3. Figure 3A and C show a direct relationship between the survival of skeletal remains and their mineral densities in FN3 (data from Espigares et al. 2019) and VM3 (data from Palmqvist et al. 2022b), respectively. While in FN3, there is considerable scatter around the regression line, which renders the relationship statistically non-significant (p = 0.137), in the case of VM3 there is a high level of statistical significance (p = 0.003). Figure 3B and D show an inverse relationship between the abundance of major limb bone epiphyses and their marrow yields in FN3 and VM3, respectively. In this case, the least-squares regression is statistically significant in FN3 (p = 0.03) but to a lesser degree than in VM3 (p = 0.001).
Comparison of bone preservation frequencies in the fossil assemblages from two late Early Pleistocene sites of Orce (Guadix-Baza Depression, SE Spain), Fuente Nueva 3 (FN3) and quarry 3 of Venta Micena (VM3) (data from Table 1). A, C Least-squares regression of the abundance of limb bone epiphyses and other skeletal elements against their estimated mineral densities (log-transformed values; means for modern horse, plain zebra, gnu, and reindeer estimated from data in Lam et al. 1999) in FN3 and VM3, respectively. B, D Least-squares regression between the survival of major limb bone epiphyses and their marrow yields (log-transformed values; means for modern horse and bison calculated from data in Outram and Rowley-Conwy 1988; Brink 1997) in FN3 and VM3, respectively. Bone frequencies in FN3 and VM3 from Espigares et al. (2019) and updated from Espigares (2010), respectively. Striped lines show the 95% limits above and below the regression lines. Abbreviations: Hum, humerus; Rad, radius; Uln, ulna; Fem, femur; Tib, tibia; Astr, astragalus; Calca, calcaneum; Met, metapodial; Phal, phalanx; Vert, vertebrae; Scap, scapula; Pel-ace, pelvis acetabulum; Pel-isil, pelvis ischium/ilium; p, proximal epiphysis; di, diaphysis; d, distal epiphysis
These results tentatively suggest that exploitation by the hominins of bone marrow after hammerstone breakage was a usual activity during carcass processing at FN3 (Espigares et al. 2019). As a result, few bones retained their marrow contents after the exploitation of marrow by hominins, which explains the low frequencies of tooth-marked bones in the FN3 assemblage. Compared to VM3, where the bone-cracking agency was the hyaena P. brevirostris, the lower selectivity in bone fracturing at FN3 probably results from the fact that hominins fractured the bones assisted by their stone tools. This suggests that the mineral density of the skeletal elements was not as much of a problem for breaking them in the case of hominins as it was in the case of hyaenas, which performed this task using their robust jaws and enlarged premolar teeth (Arribas and Palmqvist 1998; Palmqvist et al., 2011, 2022b). This forced the hyaenas to be more selective than the hominins when choosing which skeletal elements to fracture, in order to avoid breaking their teeth or dislocating their jaws when fracturing the densest bones.
Two ratios that measure skeletal completeness have been proposed for estimating the intensity of carnivore ravaging in an assemblage (Domínguez-Rodrigo and Organista 2007). The first is the proportion of axial bones to appendicular bones, which would range between 4.25 for a carcass transported complete or from an animal that died in a setting devoid of competition among carnivores and 0 for a completely ravaged or transported skeleton. The second is the ratio between the frequencies of proximal humeri plus distal radii and the frequencies of distal humeri plus proximal radii. This ratio measures the relative abundance of the least dense bone portions (i.e., those in the numerator), which are preferentially consumed by the scavengers. Consequently, it takes a value comprised between 1 in an undisturbed carcass and 0 in the situation of highest ravaging intensity. In FN3, the axial/limb ratio has a value of 0.44 (99/227) while the (hp+rd)/(hd+rp) ratio is 0.30 (7/23) (frequencies updated from Espigares 2010). Both values indicate a high intensity of bone ravaging (Domínguez-Rodrigo and Organista 2007). The values of these ratios for VM3 are 0.13 (502/3721) and 0.32 (89/274), respectively (frequencies from Palmqvist et al. 2022b). The first ratio is much lower in VM3 than in FN3 and this difference is statistically highly significant (χ2 = 150.369, p < 0.0001). The second ratio, however, shows similar values in VM3 and FN3 (χ2 = 0.041, p = 0.8405). Given that both hominins and carnivores preferentially remove the least dense bone portions from a skeleton, the similarity between FN3 and VM3 in the (hp+rd)/(hd+rp) ratio suggests a similar degree of bone consumption at both sites. However, the axial/limb ratio is greater at FN3 than at VM3. Living hyaenas do not cooperate in the transportation of large portions of a carcass, which limits the individuals in what they can move (Cruz-Uribe 1991). The analysis of skeletal representation for ungulate taxa in VM3 has shown that hyaenas selectively transported herbivore carcasses and body parts to their maternity den as a function of the mass of the ungulates scavenged (Palmqvist and Arribas 2001; Palmqvist et al. 2011): small-to-medium sized ungulates were transported as whole carcasses to their denning area while large-sized species were dismembered and the hyaenas preferentially transported the limbs that provided higher marrow yields. This resulted in a low ratio of axial elements to limb bones at VM3. In contrast, the higher value of this ratio at FN3 suggests that the carcasses of the ungulates scavenged were less biased by selective transport.
There is a positive correlation in FN3 between bone survival and mineral density, as well as a negative one between limb abundance and marrow contents. These correlations are less significant than in the denning site of VM3, which suggests that hominins were the main agent involved in the exploitation of ungulate carcasses at FN3. This is also suggested by a higher ratio of axial elements to limb bones in FN3 than in VM3, which indicates a lower intensity of bone ravaging than the one expected for a site in which the bone accumulation was generated by a truly scavenging carnivore like the bone-destroying hyaena P. brevirostris. Therefore, the current evidence points to the involvement of hominins as the main accumulating and modifying agency of bones at FN3 and a similar conclusion can also be drawn for BL (Espigares et al. 2019; Palmqvist et al. 2022c).
In summary, there is compelling evidence of a regular access of hominins to meat and marrow resources at FN3, including the presence of cut marks associated to butchery of ungulate carcasses and the finding of percussion marks left during bone fragmentation for marrow exploitation. However, the evidence available points to a secondary access to these resources by these hominins, as argued by Espigares et al. (2013, 2019), instead of a primary one, as suggested by Yravedra et al. (2021). Given that most bones with anthropogenic marks are of medium-to-large and large-to-very large sized animals, Espigares et al. (2019) proposed that the hominins of FN3 had secondary access to the prey leftovers of sabretooth cats like H. latidens and M. whitei. Based on Martínez-Navarro and Palmqvist (1995, 1996), Arribas and Palmqvist (1999), and Palmqvist et al. (2007, 2011), the reasoning of Espigares et al. (2019) was that these primary predators exploited the carcasses of their prey to a lesser extent than do modern felids do, which would result in greater amounts of flesh and all the internal bone nutrients being available to scavengers such as the Oldowan hominins and the giant hyaenas. However, Yravedra et al. (2021) criticized this proposal, arguing that sabretooth cats exploited the prey carcass to a similar extent than pantherine felids, which in their opinion would invalidate the model of Espigares et al. (2019). For this reason, in the two sections that follow, we review in depth (i) the specialized craniodental and postcranial anatomy of sabretooth cats; (ii) the inferences on their feeding habits; and (iii) the scavenging opportunities that these hypercarnivores provided to scavengers, including the hominins.
Dietary ecology of sabretooth cats and scavenging opportunities for the hominins
Sabretooth cats have no living analogues and they dominated the mammalian carnivore guild during most of the Neogene, filling the niche now occupied by large pantherine felids (Van Valkenburgh 2001, 2007). In their paper, Yravedra et al. (2021) argued that some biomechanical studies of the dentition of sabretooth cats (e.g., Bryant et al. 1995; Harstone-Rose 2008, 2011; Desantis et al. 2012) suggested that “these carnivores would have had no issue making contact with bone surfaces when they were feeding on animal carcasses” (Yravedra et al. 2021: p. 16). For this reason, they considered that sabretooth cats could deflesh the carcasses of their prey to the same extent as modern lions, leopards, and jaguars, which inflict considerable damage to the bones of their prey and leave abundant tooth marks on them. This interpretation challenges the proposal of Martínez-Navarro and Palmqvist (1995, 1996) that M. whitei, an African immigrant in Europe when the first hominin dispersal out of Africa took place, provided hominins and other scavengers access to ungulate carcasses with significant amounts of flesh and all within-bone nutrients intact.
A detailed inspection of the references cited by Yravedra et al. (2021) does not allow to reach the conclusions they drew. In their review of nimravid and felid sabretooths, Bryant et al. (1995) explicitly acknowledged that the carnassials of these predators were: (i) placed more posteriorly than those of other carnivorans, which maximized the available leverage of their mandibles (see discussion below); and (ii) mesiodistally rotated to compensate for the restricted mediolateral movement of the jaw posed by the enlarged canines, which resulted in heavy attritional (i.e., tooth-to-tooth) wear. Such wear could be misleading, as it could be confused with the tooth abrasion caused by consuming bones, which may be behind the erroneous assertion of Yravedra et al. (2021). Moreover, Bryant et al. (1995) cited the study of Van Valkenburgh et al. (1990) of the North American sabre tooth Smilodon fatalis. This study (reviewed below) provided conclusive evidence that S. fatalis actively avoided contact with bone, possibly to avoid potential breakage of the sabres, which were vulnerable to fracture about their mesiodistal axis (Van Valkenburgh and Ruff 1987; Palmqvist et al. 2022a, 2022b).
Harstone-Rose (2008, 2011) indicated that while the patterns of occlusal sharpness and intercuspid notches of the postcanine dentition of sabretooth cats suggest that they were not more hypercarnivorous than other felids, these predators display no dental morphology indicative of durophagy. He also agreed that sabretooth cats were almost certainly hypercarnivores, as indicated by their elongated upper carnassial blades with reduced protocones. The upper carnassial tooth (fourth premolar) of sabretooth cats was extremely specialized for slicing, which allowed these predators to deflesh their prey rapidly (Marean 1989). In Megantereon, the protocone (lingual lobe) of the upper carnassial is reduced and lowered away from the occlusal surface, thus removing it from its role as a hammer for bone crushing (Arribas and Palmqvist 1999). Among the extant carnivores, this condition is only found in the cheetah (Acinonyx jubatus), the most meat-only specialist living felid. According to Pobiner (2007), sabretooth cats likely took large prey and consumed relatively less flesh and bone from those prey than modern lions, an ecological niche that is now partially filled in the modern ecosystems by the cheetah, although the latter predator takes smaller prey than sabretooth cats. This means that the cheetah probably provided potential scavenging opportunities to early hominins (Pobiner 2007), as argued for the giant cheetah Acinonyx pardinensis in Dmanisi by Hemmer et al. (2011).
Compared to Megantereon, the upper carnassials of Homotherium represent a further step towards hypercarnivory: the protocone is vestigial, or more commonly absent, and an accessory cusp has been added anteriorly (Marean 1989). As a result, this tooth forms a long thin blade ideal for slicing through tough skin and flesh, although its resistance to stress — and particularly to the medio-lateral forces that result from bone chewing — is reduced compared to living felids (Arribas and Palmqvist 1999). Moreover, Harstone-Rose (2008) indicated that the reduction of the sabretooth premolars (which resulted from the biomechanical need to accommodate the maxillary canine through an elongation of the diastema and reduction of teeth that would encroach on this functional space) reduced the premolar sectorial function seen in other hypercarnivores. Therefore, given the extreme reduction of the premolar teeth in sabretooth cats, which is particularly evident in the case of M. whitei (see below), the assumption of their full functional equivalence with the premolars of living felids must be taken with caution.
DeSantis et al. (2012) used dental microwear texture analysis (DMTA) for differentiating between the diet of S. fatalis and the North American giant lion Panthera atrox. This study showed no evidence of bone crushing by P. atrox, whose tooth-wearing attributes are like those of the cheetah, the species that shows the lowest degree of bone consumption among the living African carnivores (Schubert et al. 2010). In contrast, their microwear data suggested that S. fatalis did not avoid bone to the extent previously considered, despite having a relatively weak bite force (approximately one-third of the force delivered with the temporal muscles by a modern lion), because the range of DMTA complexity values of this species overlapped with the lowest values obtained for modern lions (DeSantis et al. 2012: Fig. 2). However, it should be noted here that other analyses of microwear textures of the canines and carnassials of sabretooth cats indicate a lower ability of these predators to process the prey carcass compared to living felids. For example, Anyonge (1996a) analysed the number of pits and scratches in the upper canines of S. fatalis and several living carnivores with disparate feeding and hunting repertoires, including the bone-cracking spotted hyaena, the lion, the cheetah, and the wild dog. In this study, the canines of S. fatalis exhibited the least number of pits of all the canines included in the sample, which demonstrated that these teeth avoided contact with bone during prey killing and feeding (Anyonge 1996a).
Carnassial microwear provides a more compelling source of evidence on the feeding ecology of sabretooth cats: while the canines are directly involved in prey killing, the carnassial teeth (upper fourth premolar and lower first molar) are used exclusively in food processing. Van Valkenburgh et al. (1990) performed scanning electron micrographs of the wear facets of the carnassials to estimate the average density, size, shape, and orientation of microwear features in a wide sample of living carnivores that ranged in durophagy from the spotted hyaena to the cheetah. The results showed that while the carnassials of hyaenas exhibit relatively few long scratches combined with a high proportion of pits, those of the cheetah are characterized by a predominance of narrow scratches with very few pits. Strikingly, the microwear pattern of the carnassials of S. fatalis differed from the extant carnivores sampled in showing relatively narrow and long scratches combined with an extremely low frequency of pits, even lower than in the hypercarnivorous cheetah (Van Valkenburgh et al. 1990). At a kill, the cheetah opens the carcass, discards the intestines, and consumes first the liver, kidneys, heart, and lungs. Once the soft body parts are eaten, the cheetah concentrates on the musculature of the back, hind limbs, forelimbs, and neck. The skin that covers the trunk and upper limbs is usually consumed with the underlying musculature. Finally, the cheetah only occasionally consumes bones of small prey such as fawns of Thomson’s gazelle (Schaller 1968; Brain 1981; O’Connell and Hawkes 1988; Skinner and Smithers 1990; Phillips 1993). No study on the microwear features of the canines and carnassials of M. whitei is available, but it is reasonable to think that this highly specialized dirk-toothed predator would have been at least as constrained as S. fatalis to feed on soft tissues.
Following their arguments that sabretooth cats could inflict considerable bone damage to the prey carcass, Yravedra et al. (2021: p. 16) emphasized that “high frequencies of bone damage, particularly in relation to those documented at FN3, are noteworthy” in the bone assemblage of Friesenhahn Cave, a Late Pleistocene site interpreted as a denning refuge of the North American scimitar cat Homotherium serum (Marean and Eckhardt 1995). However, it must be noted that although the Friesenhahn Cave assemblage preserves skeletal remains of 20 species of large mammals, a diversity roughly equivalent to that recorded at FN3, it shows strikingly low evenness: juvenile mammoths represent one-third of the remains at Friesenhahn Cave, most of them from animals 2–4 years of age with an estimated mass of 500–800 kg, while another third are remains of H. serum, mostly juveniles and senile individuals (Marean and Eckhardt 1995). This is in contrast with the more balanced distribution of species abundances in the assemblage of FN3 (Espigares et al. 2019; Yravedra et al. 2021) and argues against H. latidens, remains of which have been identified in FN3, as the bone collecting agency at this site. Concerning the remains of juvenile mammoths preserved at Friesenhahn Cave, limb bones are over-represented, which suggests that the scimitar cats selectively transported these parts of the skeleton away from the kill site to their denning refuge (Marean and Eckhardt 1995). Moreover, the frequency of tooth-marked bones in this assemblage is very high (>60%), a figure like those recorded at leopard dens (Brain 1981), while the frequency of tooth-marked bones at FN3 (<3%) is remarkably lower (Espigares et al. 2019; Yravedra et al. 2021). According to Marean and Eckhardt (1995), tooth marks are particularly abundant on the footbones of juvenile elephants from Friesenhahn Cave and most of them derive from the incisors of the scimitar cats contacting the bone during flesh removal, a task performed by the living felids with the assistance of their shorter and more robust canines (see below). Of interest here, a recent analysis of dental microwear texture and anisotropy of the carnassial teeth of H. serum from Friesenhahn Cave showed that this predator consumed soft and tough foods, like the tough flesh of juvenile mammoths, and actively avoided bone (DeSantis et al. 2021: Fig. 1). In VM3, a site that preserves remains of the prey of sabretooth cats that were scavenged by the giant hyaenas (Palmqvist et al. 1996, 2011, 2022b; Arribas and Palmqvist 1998; Palmqvist and Arribas 2001), the predator-prey relationships were inferred from carbon and nitrogen stable isotope signals in the bone collagen of the large mammals (Palmqvist et al. 2003, 2008a, 2008b) and the use of the dual-isotope, three-source linear mixing (Phillips 2001). This approach showed that juveniles of elephant M. meridionalis also represented a significant proportion (∼10%) of the diet of the scimitar cat H. latidens, while bison Bison sp. (52%) and horse E. altidens (38%) comprised the bulk of its diet.
Yravedra et al. (2021) indicated that some studies have shown that the extant felids have the ability of inflicting considerable bone damage. Panthera gombaszoegensis, the European jaguar, is the only pantherine felid known from the Orce sites. This species is recorded in VM3, but not in FN3. Although no taphonomic field study is available for the extant jaguar (Panthera onca), an experimental feeding program designed for assessing the nature and extent of alterations that captive jaguars can produce on horse bones showed that they make tooth marks on long limb bone diaphyses and destroy epiphyses (Rodríguez-Alba et al. 2019). However, the breakage of major limb bones of medium-to-large ungulates resulting in green fractures, as those abundantly recorded in the Orce sites (Arribas and Palmqvist 1998; Espigares 2010; Palmqvist et al. 2011, 2022b; Espigares et al. 2019; Luzón et al. 2021), is clearly beyond the capacity of a jaguar.
The bone modifying abilities of lions are difficult to estimate, because these felids consume most of their prey near the kill site, where the skeletal remains are subsequently scavenged by the hyaenas, jackals, and vultures. A monospecific assemblage of wildebeest bones, presumably accumulated by lions within Olduvai Gorge in the short-grassland ecological unit of the Serengeti, provides information on the ability of lions for inflicting bone damage (Arriaza et al. 2016). Although these remains were probably secondarily ravaged by spotted hyaenas, the assemblage shows many complete carcasses; there are tooth-marked skeletal elements and the frequency of fractured bones (mostly small elements) is very low. This pattern of bone completeness argues against hyaenas as the main bone modifying agent, because most long bones found at spotted hyaena dens are broken and bone cylinders (i.e., isolated diaphyses) and diaphyseal bone shafts are common. Bone diaphyses are also abundant in the hyaena den of VM3 (Arribas and Palmqvist 1998; Espigares 2010; Palmqvist et al. 2011, 2022b), VM4 (Luzón et al. 2021; Palmqvist et al. 2022b) and, to a lesser extent, also in FN3 (Espigares 2010; Espigares et al. 2019), while they are missing in the Olduvai assemblage (Arriaza et al. 2016).
Leopards are an effective bone-accumulating agent in caves (but not in open air sites like FN3) and their assemblages are dominated by small skeletal elements like vertebrae, ribs, and phalanges, which represent up to 60% of the remains (Brain 19801981; Arribas and Palmqvist 1998). According to Espigares et al. (2019: table S4) and Yravedra et al. (2021: table 3), small bones represent 11.2% (535/4,764) and 24.8% (256/1,032) of the number of identifiable specimens unearthed from FN3, respectively, figures well below those reported at leopard dens.
The predatory guild of the Early Pleistocene sites of Orce was dominated by H. latidens and M. whitei (Martínez-Navarro and Palmqvist 1995, 1996; Arribas and Palmqvist 1999). Megantereon was a stoutly built predator with an estimated body mass of 100–110 kg, like a small lioness or a large male jaguar (Palmqvist et al. 2007). The upper canines of this sabretooth cat are extremely long, sharp, and laterally compressed, and its forelimbs are powerful with a large dewclaw (Christiansen and Adolfssen 2007; Palmqvist et al. 2007). The brachial index (i.e., the ratio of radius length to humerus length) is low (∼80%) and the metapodials are short, suggesting an ambush predator that hunted in mixed or closed habitats (Palmqvist et al. 2003). This is also suggested by isotopic analyses at VM3, which indicate that browsing deer represented the bulk of its prey (Palmqvist et al. 2003, 2008a, 2008b).
Homotherium is similar in size to a modern lion (145–220 kg; Anyonge 1993) and has more gracile and elongated forelimbs (brachial index of ∼90%) than Megantereon, which indicates increased cursoriality in open habitats (Anyonge 1996b; Martin et al. 2000; Palmqvist et al. 2003). The reduced asymmetry of the middle phalanges in Homotherium (Antón et al. 2005) and the small attachment scars on the metacarpals of digits II–V for the muscles used by felids to retract the claws indicate the presence of partially retractable claws (Rawn-Schatzinger 1992), a condition only found in the cheetah among extant felids. This indicates an adaptive trade-off between improved traction for pursuing prey and reduced prey-grappling ability (Antón 2013). Moreover, the greater tuberosity of the humerus is higher relative to the humeral head in Homotherium than in the pantherine felids, while the distal radius is narrower. Both features suggest adaptations for cursoriality, although it must be noted that the lumbar spine is shorter and stiffer than in the cheetah, which would provide strength rather than speed, and the tail is very short (Rawn-Schatzinger 1992; Antón et al. 2005). The presence of a short tail is also suggested in Megantereon by the strong posterior tapering of the sacrum (Christiansen and Adolfssen 2007).
The cheetah has long hindlimbs relative to its forelimbs, but this proportion is reversed in Homotherium, which results in a sloping back, like in a hyaena (Rawn-Schatzinger 1992). These features, well studied in the North American H. serum, suggest that while the cheetah can accelerate quickly to develop short bursts of maximum speed, Homotherium was better equipped to run at more moderate speeds but over longer distances, similar to hyaenas (Antón et al. 2005; DeSantis et al. 2021).
Homotherium had a comparatively large brain with an enlargement of the optic centre, like in the diurnal and cursorial cheetah (Rawn-Schatzinger 1992). In contrast, Megantereon had a smaller brain with well-developed olfactory lobes, as to be expected in an ambush predator (Arribas and Palmqvist 1998).
Compared with the dirk-toothed machairodonts like Megantereon and Smilodon, the loss of retractable claws in Homotherium limited grappling efficiency in the forepaws for subduing and immobilizing its prey. In addition, the skull of this scimitar cat is poorly equipped for delivering a unique, fatal stabbing bite to its prey (Figueirido et al. 2018). Both features suggest that Homotherium may have required the action of a group for bringing down large prey, which would make it a unique cursorial, flesh-specialized predator of open habitat, and potentially social (De Santis et al. 2021). Interestingly, a comparative genomic analysis found evidence of positive selection in several genes of H. latidens involved in vision, cognitive function, and energy consumption, which is putatively consistent with diurnal activity, well-developed social behaviour, and cursorial hunting (Barnett et al. 2020).
There is broad consensus that the elongated and laterally compressed upper canines of sabretooth cats represented an adaptation for killing large prey quickly and efficiently with deep wounds to the throat rather than using the prolonged suffocating throat or muzzle bite typical of extant felines (Gonyea 1976; Akersten 1985; Anyonge 1996b; Antón et al. 2004; McHenry et al. 2007; Palmqvist et al. 2007, 2022b; Christiansen 2008; Salesa et al. 2010; Meachen-Samuels and Van Valkenburgh 2010; Andersson et al. 2011; Meachen-Samuels 2012; Martín-Serra et al. 2017; Figueirido et al. 2018; Lautenschlager et al. 2020). However, canine hypertrophy resulted in the need for increasing mandibular gape for clearing the tips of the canines when delivering the killing bite. This in turn involved the reorganization of the jaw-adducting muscles to avoid the over-stretching of temporalis muscle fibres during wide gaping while retaining substantial bite force at the carnassial. This led to several changes in the craniodental anatomy of sabretooth cats compared to the pantherine felids (Fig. 4), including: (i) a lowered glenoid fossa, which resulted in a ventral displacement of the jaw joint; (ii) an upward rotation of the palate relative to the braincase, which led to an upward rotation of the facial portion of the skull; (iii) a shortening of the coronoid process in the mandible, coupled with an antero-posteriorly narrower temporal fossa and a more vertical occiput in the cranium, all of which resulted in a narrowing of the temporalis fibres and their more perpendicular orientation to the tooth row; and (iv) a laterally-shifted angular process in the mandible and a smaller, less laterally-projected postglenoid process in the cranium, which both helped the mandible to avoid contact with the postglenoid when abducting the lower jaw (Emerson and Radinsky 1980; Akersten 1985; Martin et al. 2000; Palmqvist et al. 2007; Slater and Van Valkenburgh 2008; Figueirido et al. 2011).
Comparison of the skulls of a leopard, Panthera pardus (left images), and a sabretooth cat, Megantereon nihowahensis (right images), showing different angles of jaw opening. A With the jaw closed and compared to the leopard skull, Megantereon shows a shortening of the coronoid process of the mandible, an upward rotation of the palate relative to the braincase, a shorter and narrower temporal fossa in the cranium, and a more vertical occiput (see discussion in text); these changes result in a narrowing of the temporalis fibres (the line X measures their length with the mouth closed from the sagittal crest to the tip of the coronoid) and their more perpendicular orientation to the tooth row. Such skull reorganization helps to avoid muscle over-stretching during wide gaping while retaining bite force at the carnassial. B With a jaw gape of 45°, the stretching of the temporalis muscles (measured by the ratio between X’ and X) is similar in the leopard (~30%) and Megantereon (~40%). C With a jaw gape of 120°, muscle stretching in the skull of Megantereon (~80%) is similar to that in the leopard with a jaw gape of 60° (~85%)
The narrowing and more perpendicular orientation of the temporalis muscle allowed sabretooth cats to increase their jaw gape up to 180° while retaining a similar degree of muscle stretch to pantherine felids (Fig. 4). However, this also posed an unavoidable biomechanical constraint on the point of maximum bite force exerted at the carnassial, which was positioned backward, and this led to a reduction of the post-canine dentition not related to the slicing function of the carnassial (Martínez-Navarro and Palmqvist 1995, 1996; Palmqvist et al. 2007). Moreover, the size of masseter muscle, which exerts its maximum force at smaller gapes, was also reduced. The relationship between the enlargement of the upper canines and the reduction of the post-canine teeth is particularly evident in the case of the African sabretooth cat M. whitei, in which the third lower premolar is reduced to a vestigial peg or even lost, as in the specimen from South Turkwel, Kenya (Palmqvist 2002), and this results in the appearance of a diastema between this tooth and the fourth premolar (Martínez-Navarro and Palmqvist 1995, 1996; Palmqvist et al. 2007). To a lesser degree, this also occurs in the fourth lower premolar and even in the paraconid (i.e., the cusp more anteriorly positioned) of the lower carnassial, which are both shortened in relation to their dimensions in the less advanced M. cultridens, the species replaced in Europe by M. whitei (Palmqvist et al. 2007). For this reason, although the elongated canines of sabretooth cats provided greater killing efficiency compared to pantherine felids, which allowed them to prey upon larger ungulates, the marked reduction of the postcanine dentition resulted in the lesser ability to process the prey carcass and to consume bones, particularly in the case of M. whitei (Martínez-Navarro and Palmqvist 1995, 1996; Palmqvist et al. 2007). In this way, the avoidance by sabretooth cats of hard tissues in the prey carcass would result in a significant quantity of resources left over to the scavengers, including the Oldowan hominins and the giant hyaenas. This provides the ecological connection between the dispersal of M. whitei out of Africa and the first human arrival in Europe, recorded at the Orce sites of BL and FN3. On this continent, the survival of hominin populations during the cold season, when plant resources are lowered, would depend on the regular scavenging of ungulate carcasses (Turner 1992; Martínez-Navarro and Palmqvist 1995, 1996; Arribas and Palmqvist 1999; Palmqvist et al. 2007, 2022a, 2022b; Espigares et al. 2013, 2019; Martínez-Navarro et al. 2014).
As noted before, the elongated and laterally compressed canines of sabretooth cats were optimal for killing large prey. However, they were more vulnerable to fracture than the shorter, conically shaped canines of living felids due to the unpredictable, non-directed loads generated in prey stabilization during the killing bite (Van Valkenburgh and Hertel 1993; Van Valkenburgh 2009; Palmqvist et al. 2022a). For this reason, the heavily muscled forelimbs of sabretooth cats were imperative for pulling down and immobilizing prey before positioning the bite over the prey throat or belly, where bite depth is essential to generate strikes reaching major blood vessels (Gonyea 1976; Akersten 1985; Anyonge 1996b; Antón et al. 2004; Christiansen 2008; Salesa et al. 2010; Andersson et al. 2011; Meachen-Samuels 2012; Martín-Serra et al. 2017; Figueirido et al. 2018; Janis et al. 2020). Such functional need is reflected in the postcranial anatomy of Smilodon and Megantereon: their short and robust forelimb bones are reinforced by cortical thickening to a greater degree than in extant pantherine cats, which allowed them to minimize prey struggling, helping to position the killing bite carefully to avoid contact with bone such as the vertebral column of the prey (Van Valkenburgh and Ruff 1987; Meachen-Samuels and Van Valkenburgh 2010; Meachen-Samuels 2012; Martín-Serra et al. 2017).
A pioneer study based on finite element analysis (FEA) of the skulls of the iconic S. fatalis and the living lion analysed their biomechanical response to extrinsic forces, which allowed the simulation of a prey struggling scenario during the killing bite (McHenry et al. 2007). This showed that the skull of S. fatalis was less equipped than the skull of the lion to resist extrinsic forces, which pointed to rapid slashing bites by this sabretooth cat during prey killing. In contrast, living big cats use their stouter, conical-shaped canines to hold a suffocating bite in the snout of large prey such as buffalo. For this reason, pantherine felids do not rely on their forelimbs for subduing prey to the same extent than did sabretooth cats (McHenry et al. 2007; Salesa et al. 2010; Meachen-Samuels 2012; Martín-Serra et al. 2017). Moreover, combined microanatomical analyses with FEA for simulating different prey-killing scenarios in S. fatalis, H. serum and a sample of living carnivores showed that while the rostrum of S. fatalis was almost entirely composed of cortical bone, the skull of the lion has a substantial amount of trabecular bone (Figueirido et al. 2018). Given that cortical bone supports better directed loads while trabecular bone can resist unpredicted and multidirectional forces, this suggests that the skull was better equipped in S. fatalis than in the living lion to deliver a quick killing-shear bite (Figueirido et al. 2018). In contrast, the forces generated when feeding on hard objects like bones are unpredictable, which implies that the large amount of cortical bone in the skull of S. fatalis (and presumably in other dirk-toothed cats like Megantereon) would be an impediment to feeding on bones.
Other morphological features of the skull of sabretooth cats relate to the need of avoiding canine breakage when feeding on prey carcass. For example, all sabretooth cats possess a protruding incisor arcade and exhibit a marked diastema between the third incisor and the upper canine (the only exception is the North American Xenosmylus hodsonae). Incisor protrusion would make the function of the incisor teeth independent from the function of the hypertrophied upper canines, and the incisors would be employed by sabretooth cats to tear chunks of flesh from the prey carcass (a task performed in modern felids with the assistance of their stout, conically shaped canines); in contrast, the canines in sabretooths would be used exclusively to deliver deep wounds during prey dispatch (Biknevicius et al. 1996). As noted above, there is taphonomic evidence from Friesenhahn Cave on the use by H. serum of incisor teeth for defleshing juvenile mammoths (Marean and Ehrhardt 1995).
Prevention of canine breakage during prey killing and feeding encounters seems to have been a strong selective force in sabretooth cats and limited them to a non-scavenging behaviour, except perhaps if they came across a complete carcass (this opportunistic behaviour is common among modern hypercarnivores). This contradicts the suggestion of Yravedra et al. (2021) that sabretooth predators exploited the prey carcass as thoroughly as do living felids.
In summary, the derived craniodental and postcranial anatomy of sabretooth cats suggests that, compared to living pantherine felids, they: (i) were able to hunt larger ungulate prey relative to their body size, exerting a higher predation pressure on the juveniles of the megafauna; and (ii) exploited the prey carcass to a lesser extent, which would result in larger amounts of flesh and all internal bone nutrients left over to scavengers like the hyaena P. brevirostris in VM3 (Arribas and Palmqvist 1998; Palmqvist et al. 2011, 2022b) and the Oldowan hominins in the case of FN3 (Espigares et al. 2013, 2019). However, although Yravedra et al. (2021) acknowledged that the sabretooth cats M. whitei and H. altidens would have had primary access to ungulate carcasses, they criticize the opinion of Martínez-Navarro and Palmqvist (1995, 1996), Arribas and Palmqvist (1999), Palmqvist et al. (2007), and Espigares et al. (2013, 2019) that these predators only consumed part of the muscles and viscera of the prey carcass, thus providing the hominins the opportunity to scavenge sizeable chunks of meat and within-bone nutrients before the arrival of hyaenas. The evidence discussed above argues against the interpretation of Yravedra et al. (2021).
Hyaenid activity at FN3
A biomechanical study of the mandible of the giant hyaena P. brevirostris showed that it had higher moment arms for the masseter and temporalis muscles than any of the three living durophagous hyaenas, which indicated a substantial strength for bone fracturing with its well-developed premolar teeth (Palmqvist et al. 2011). Similarly, using an approach that models the mandibles of hyaenas as beams, mandibular force profiles were estimated showing a greater resistance of the jaw of P. brevirostris against dorsoventral loads than in living bone-cracking hyaenas. This was particularly evident in the jaw symphysis, which is extremely buttressed dorsoventrally and thus better equipped for fracturing bones than those of the three extant ossifragous hyaenas (Palmqvist et al. 2011). Moreover, an ecomorphological study showed that P. brevirostris was specialized in non-dragging carcass transport over long distances (Pérez-Claros and Coca-Ortega 2020). In this species, the lower canines are comparatively more developed than in the spotted hyaena (Crocuta Crocuta), which behaves both as a predator and a scavenger. In contrast, the two truly scavenging hyaenas, the striped hyaena (Hyaena hyaena) and the brown hyaena (Parahyaena brunnea), show enlarged lower canines, like in P. brevirostris. This represents an adaptation to carry heavy loads to the denning site (Pérez-Claros and Coca-Ortega 2020), as reflected in the mean distance of transport of the remains: 6.50 ± 2.53 km in P. brunnea (Kuhn et al. 2008) and 0.56 ± 0.08 km in C. crocuta (Skinner et al. 1986). Although transport distance is shorter in the spotted hyaenas, it is important to note that they transport the remains over greater distances than leopards, 0.18 ± 0.03 km (Brain 1981).
Compared to living hyaenas, P. brevirostris also shows a shortening of the distal limb segments, which represents an adaptation for long-distance transport of ungulate carcasses (Turner and Antón 1996; Palmqvist et al. 2011): the brachial index (radius length/humerus length) and the crural index (tibia length/femur length) have lower values in P. brevirostris (0.91 and 0.74, respectively) than in C. crocuta (1.08 and 0.82, respectively), H. hyaena (1.07 and 0.88, respectively), and P. brunnea (1.00 and 0.82, respectively). Given the inverse relationship in modern carnivores of the brachial and crural indexes with body mass (Anyonge 1996b), such distal limb shortening may be explained in part by the enormous size of this extinct hyaena (~110 kg; Palmqvist et al. 2011). However, the tibia of P. brevirostris seems short in relation to the femur (Turner and Antón 1996), a reduction that has been envisioned as an adaptation of hyaenas to carry large pieces of carcasses without dragging them (Spoor 1985). Living hyaenas have a comparatively long radius, which raises the forequarters and therefore the head of the animal. Although the radius is also shortened in P. brevirostris compared to other hyaenas, the ratio between the lengths of the forelimb and the hindlimb in P. brevirostris (1.06) is similar to that of P. brunnea (1.06), which is achieved by an extreme shortening of the tibia in P. brevirostris, and higher than in H. hyaena (1.04) and C. crocuta (1.0) (Palmqvist et al. 2011: table 5). This indicates a similar emphasis on the forequarters in P. brevirostris to that seen in the brown hyaena (Turner and Antón 1996). All these features suggest a less cursorial lifestyle for the giant, short-faced hyaena than in the spotted hyaena, which would have limited the predatory abilities of this species, although the shortening of the limbs provided greater power and more stability to dismember and carry large pieces of the carcasses scavenged (Turner and Antón 1996; Palmqvist and Arribas 2001; Palmqvist et al. 2011).
Carnivore and hominin involvement at FN3
Yravedra et al. (2021) criticized our model of carcass acquisition and processing at FN3, which they called the “Megantereon–hominins–Pachycrocuta model,” but they did not offer a convincing alternative explanation. For example, they indicated that “the very low frequencies of carnivore tooth marks documented at FN3 are not consistent with the percentage ranges generated by carnivore-hominin models, with the exception of cheetah or leopard accumulations of size 1–2 animals” (Yravedra et al. 2021: p. 11). However, they acknowledged that “the FN3 assemblage has a considerable representation of animals >150 kg, a size range that neither cheetahs nor leopards target, which suggests that carnivores were not the main accumulation agents at FN3” (Yravedra et al. 2021: p. 11). They used the frequencies of tooth-marked bones in the upper (femur and humerus) and intermediate (radius and tibia) bones of the appendicular skeleton as a line of argumentation for discarding a major role for carnivores in the site formation process at FN3. In their opinion, these frequencies “are considerably lower than those that would be expected in episodes of primary access by carnivores […], a pattern inconsistent with the observations of carnivore feeding when they have access to the most nutritious portions of the carcasses” (Yravedra et al. 2021: p. 11). They also indicated that the low frequency of tooth-marked limb bone diaphyses agrees with the one expected from a secondary access of carnivores. This frequency is “well below the outcomes of actualistic observations of carnivore-first models” and “fits much better the patterns left by carnivores when they engage with carcasses after human intervention, including those instances when carcasses were initially modified by vultures” (Yravedra et al. 2021: p. 11).
Once discarded a primary access by carnivores, Yravedra et al. (2021) then focused on the frequencies of anthropogenic traces for evaluating hominin involvement at the site. However, they acknowledged that the very low percentages of bones with cut marks and percussion marks have led them to a somewhat inconclusive result, because these frequencies more closely resemble the profiles that result from secondary hominin access to carcasses previously consumed by carnivores (Yravedra et al. 2021). In fact, the low percentages of cut-marked and percussion-marked bones agree with the expectations of our Megantereon–hominins–Pachycrocuta model (Espigares et al. 2019).
At this point, Yravedra et al. (2021) seem to be at a dead-end, as they discard both carnivores and hominins as the primary agents at the site. In their own words: “the taphonomic evidence currently available does not allow us to determine with certainty the temporality of hominin access to animal carcasses at FN3” (Yravedra et al. 2021: p. 12). However, it is worth noting that their analysis of the distribution and inferred functionality of the cut marks showed their presence on the diaphyses of long bones such as the femur or tibia, as well as on axial elements such as ribs or the pelvis, which was also noted by Espigares et al. (2019). This pattern would be consistent with defleshing and evisceration activities on carcass elements with high nutritional values, which may indicate early access to at least some of the carcasses, rather than secondary scavenging (Yravedra et al. 2021). For all these reasons, and despite their lack of conclusive evidence, they concluded that “there is little evidence to support previous models of hominin acquisition of animal resources at Orce consisting in the secondary exploitation of carcasses left by large felids, and prior to the intervention of the giant hyaena” (Yravedra et al. 2021: p. 14-16).
In summary, several lines of evidence suggest a regular access to meat and marrow resources by hominins at FN3, including a high degree of bone fragmentation (Fig. 3), the presence of cut marks associated with butchery activities (disarticulation, defleshing, and, to a lesser extent, evisceration) and percussion marks that indicate the exploitation of marrow (Espigares et al. 2013, 2019; Yravedra et al. 2021). Therefore, it is surprising that although the low frequency of bones bearing anthropogenic traces agrees with the expectations from actualistic and experimental data compiled by Yravedra et al. (2021) for a secondary access of the hominins to the carcasses at FN3, they discarded this interpretation. The reason is that they considered that primary access by the carnivores to the carcasses, followed by secondary access by the hominins, would imply a higher frequency of tooth-marked bones than those recorded at FN3, as expected from their data on actualistic frameworks of reference. However, we must remember here that the latter assumption would only apply if sabretooth cats, the predators that dominated the Orce ecosystems, were truly functional analogues as prey consumers of living pantherine felids as prey consumers. The biomechanical studies reviewed above indicate that this was not the case, because the highly specialized craniodental anatomy of sabretooth cats limited them to exploiting the prey carcass to a lesser extent than lions and leopards. In fact, the only species among extant felids with an analogous feeding mode to that of sabretooth cats is the cheetah, whose prey was proposed by Pobiner (2007) as a model for flesh availability resulting from the kills of sabretooth cats. Cheetahs never completely deflesh bones, even in the absence of interspecific competition or any other limitation on consumption time, and the consumed carcasses are abandoned with large strips of flesh on both axial and appendicular elements (95% of the bones of the carcasses consumed by cheetahs preserved flesh scraps; Pobiner 2007). As a result, the frequency of bones tooth marked by cheetahs (13%) is lower than the one for lions (35%) (see data in Pobiner 2007).
Given the arguments discussed so far, there is only a single interpretation that accounts for both the scarcity of tooth marks and anthropogenic traces in the bone assemblage of FN3: the involvement of sabretooth cats as primary predators, followed by the scavenging of the carcasses of their prey by hominins and later by hyaenas. This interpretation of carcass acquisition and processing, which is referred to by Yravedra et al. (2021) as our “Megantereon–hominins–Pachycrocuta model,” is the one that better explains the taphonomic features of the bone assemblage preserved at FN3 (Espigares et al. 2019).
Tooth mark frequencies in the bone assemblage of FN3
Yravedra et al. (2021) based their discussion on the role played by the hominins and carnivores as bone accumulating and modifying agents at FN3 on several graphical comparisons between the frequencies of tooth-marked, cut-marked, and percussion-marked skeletal elements in the site and those recorded in actualistic frameworks of reference. Specifically, they classified these frameworks within three groups: (i) tooth mark frequencies in bones scavenged by carnivores during secondary access after human abandonment of the carcasses; (ii) tooth mark frequencies when vultures accessed first the carcasses, followed by humans and then carnivores; and (iii) tooth marks in primary accumulations made by carnivores.
The graphical depiction of the results is a standard scientific procedure that helps to provide a synthetic view. However, this may lead to misinterpretations if the data depicted do not accurately reflect the results obtained. This is the case of figure 3 of Yravedra et al. (2021), which shows a comparison between the frequencies of tooth-marked appendicular bones in levels 3–5 of FN3 and the three actualistic frameworks of carnivore feeding behaviours cited above. According to the scale of this figure, the frequencies of tooth-marked remains in FN3 are ~11.8% for size classes 1–2, ~5.8% for size class 3, and ~1.9% for size classes 4–5. However, the data provided by Yravedra et al. (2021) in the Excel sheet of their supplementary information show tooth mark frequencies for these size classes of 18.2% (4/22), 9.5% (7/74), and 8.3% (1/12), respectively. This means that the frequencies depicted by Yravedra et al. (2021) in their figure 3 are systematically lower (between one-half and one-quarter) than those calculated with the actual data from which they were supposedly derived. Moreover, Yravedra et al. (2021) did not provide the confidence intervals for these frequencies in their figure 3, although they did include them for many of the actualistic frameworks used in their comparison.
The incorrect data represented by Yravedra et al. (2021) in their figure 3 led them to conclude that the very low frequencies of tooth-marked appendicular bones in levels 3–5 of FN3 are only consistent with those found in the carnivore-hominin models derived from cheetah or leopard accumulations of size 1–2 animals. As noted before, animals weighing >150 kg (a prey size range that neither cheetahs nor leopards target) are well represented in the FN3 assemblage. For this reason, Yravedra et al. (2021: p. 11) concluded that “carnivores were not the main accumulation agents at FN3.” However, the 95% confidence intervals (CI) calculated with a binomial approach for the frequencies of tooth-marked remains in levels 3–5 of FN3 (5.2–40.2% for size classes 1–2; 3.9–18.5 for size class 3; 0.2–38.5 for size classes 4–5) overlap with most of the ranges depicted by Yravedra et al. (2021) in their figure 3 for tooth mark frequencies resulting from secondary scavenging by carnivores after human abandonment of the carcasses, including cases in which vultures accessed first the carcasses. For example, the frequency of tooth-marked bones for size 1–2 animals in FN3 (18.2%, CI: 5.2–40.2%) is very close to those found in experimental models for carnivore scavenging of hammerstone-broken, demarrowed bones by Blumenshine (1995: table 3), Capaldo (1997: table 9), and Pante (2013: table 8): 21.9% (CI: 5.1–38.7%), 21.7% (CI: 18.5–24.9%), and 19.1% (CI: 14.9–23.7%), respectively. Moreover, the CIs for the frequencies of tooth-marked bones distributed among size classes in FN3 also overlap with several examples of accumulations produced by primary carnivores like lions, wolves, and bears compiled by Yravedra et al. (2021: see the ranges of values depicted in their Fig. 3).
In summary, the data compiled by Yravedra et al. (2021) on “actualistic frameworks of reference” for evaluating the involvement of carnivores and hominins as bone accumulating agents at FN3 do not provide conclusive evidence for their proposed sequence of access to ungulate carcasses. Moreover, their comparative analyses are biased by the depiction of incorrect data for the bone assemblage from this site, which casts serious doubts on the reliability of their study.
Meat consumption, competition intensity with other carnivores, and subsistence strategies of the hominins at FN3
In this section, we discuss the results obtained with a mathematical model that allows the estimation of resource availability and competition intensity among carnivores, as this approach provides inferences on the roles played by primary predators and secondary scavengers at the archaeological site of FN3. Specifically, we briefly describe the model used by Rodríguez-Gómez et al. (2016a) and expand on several issues not covered in that study.
In both the title and abstract of their paper, Yravedra et al. (2021) explicitly mentioned their interest on the consumption of meat by the hominins of FN3, as well as on the intensity of their competition with other large carnivores in the access to this resource. However, they overlooked the results of Rodríguez-Gómez et al. (2016a), who: (i) quantified the availability of meat for the large carnivores (including the hominins) of the FN3 palaeoecosystem; (ii) estimated the intensity of the competition for these resources within the carnivore guild; and (iii) tested which subsistence strategy would be optimal for the hominins at FN3. The absence of any comment by Yravedra et al. (2021) on the study of Rodríguez-Gómez et al. (2016a) is surprising, as they cited it five times in relation to the hypothesis that they attempted to reject on the role of sabretooth cats as meat suppliers for the hominins, also making also reference to this study when they discussed on the level of competition between the hominins and the carnivores.
Resource availability was essential for the survival of the earliest hominin populations in Europe (Hublin and Richards 2009; Roebroeks 2001). Rodríguez-Gómez et al. (2013, 2014a, 2014b, 2016b) developed a model that allows the estimation of the meat resources accessible to these populations in their ecosystems. On the one hand, the model calculates values of sustainable density for the hominins and other carnivores, on the one hand, and measures the intensity of the competition for these resources among the members of the carnivore guild, on the other. Although the model was inspired by previous studies (e.g., Bermúdez de Castro et al. 1995; Fariña 1996; Palmqvist et al. 2003; Vizcaíno et al. 2004, 2010), it introduced several innovations such as the calculation of mortality profiles for the ungulate species. This allowed the estimation of the resources that could be sustainably exploited in the long term by the secondary consumers of the palaeocommunity (i.e., carnivores and scavengers, including hominins) without causing the collapse of the prey populations. The model uses the faunal list and the body mass estimates available for the primary consumers identified at the site in order to compute: (i) the age structure that makes each ungulate population stable and stationary; (ii) the mortality profiles of the ungulate species, inferred from fertility parameters (e.g., adult and neonate body mass, litter size, breeding interval, age at reproductive maturity, growth rate, and lifespan) deduced from their living analogues, which provides the biomass of ungulates that can be extracted yearly by the secondary consumers; (iii) the distribution of dead individuals among body mass classes, as prey size is the most relevant factor in the selection of prey by predators (Palmqvist et al. 1996); (iv) a “wastage factor,” which refers to the percentage of biomass discarded by the secondary consumers (e.g., skin, horns, and bones); (v) the dietary requirements of the secondary consumers when they reach their maximum population densities; and (vi) the distribution of prey biomass among the carnivores and scavengers, which allows the estimation of their sustainable densities (Rodríguez-Gómez et al. 2013, 2014a, 2014b, 2022; Martín-González et al. 2019). The model computes the TAB (total available biomass) as the estimate of yearly available biomass (both parameters are calculated in kg/km2 and kcal/km2) for the secondary consumers (Fig. 5). Given that multiple mortality profiles are obtained for each species of ungulate prey, the extreme values that correspond to the maximum and minimum mortality of juveniles are selected for each species. In this way, TAB-m is the available biomass that corresponds to a maximum mortality of juveniles (and minimum of adults) while TAB-M accounts for the opposite situation (Fig. 5).
Carrying capacity (A) and minimum and maximum total available biomass (B) of the Fuente Nueva 3 (FN3) palaeoecosystem, distributed among body mass categories and prey species. Both graphs are depicted in kcal/km2 per year (left scales) and kg/km2 per year (right scales). Based on data from Rodríguez-Gómez et al. (2016a)
In the Orce sites, dietary inferences retrieved from isotopic studies of the bone collagen preserved in the remains of large mammals from VM3 (Palmqvist et al. 2003, 2008a, 2008b) were used by Rodríguez-Gómez et al. (2016a) for defining the prey preferences and consumption patterns of the secondary consumers identified at FN3 and BL. As noted above, the model distributes the meat offered yearly among the members of the carnivore guild (predators, scavengers, and hominins) based on their dietary demands (which is called in the model as the Total Demanded Biomass, TDB). This distribution of TAB considers the preferences of each carnivore species for each prey mass category and the intensity of consumption of each prey category is weighted according to the requirements of other secondary consumers (see Rodríguez-Gómez et al. 2013, 2014b, 2016a). This means that the distribution of TAB is dynamic, being modified as the requirements of each carnivore species are satisfied. The distribution of TAB ends when: (i) all meat is completely distributed; (ii) all species have covered their energetic requirements; or (iii) there are no species that have access to the body mass categories in which there remains unconsumed meat. As a result, the distribution of TAB between carnivores and hominins translates into the populations densities that keep the palaeoecosystem out of danger of collapse.
The model also provides three indexes that estimate the level of competition for meat among the secondary consumers. In doing so, these indexes compare the sustainable conditions inferred by the model with the consumption of meat under optimal conditions (Rodríguez-Gómez et al. 2014b, 2016a, 2016b). At the species level, the species competition index (SCI) measures the degree to which the members of the carnivore guild reach their maximum population densities. At a community level, the model provides two indexes, GCI (global competition index) and GCIB (global competition index biomass), which estimate the degree to which the expected density and the expected biomass of the whole carnivore guild for optimal conditions are reached, respectively. Each of the three indexes shows two values, one for conditions of maximum juvenile mortality (TAB-m) and other for minimum mortality (TAB-M) (for details, see Rodríguez-Gómez et al. 2016a, 2016b).
Rodríguez-Gómez et al. (2016a) provided estimates on the amount of meat available, the requirements of the secondary consumers, their sustainable densities, and the competition indexes for FN3 and BL (given that both sites have the same faunal list, these estimates are identical for them). In terms of meat supply, the model provided a value of 410,904 kcal/km2 per year [≈273.94 kg/(km2*year−1)] for TAB-m and 575,454 kcal/km2 per year [≈383.64 kg/(km2*year−1)] for TAB-M. The demands of meat estimated for the secondary consumers under optimal conditions were substantially higher than the availability of meat, 1,051,606 kcal/km2 per year [≈701.07 kg/(km2*year−1)]. In terms of ecological densities per 100 km2 for the members of the carnivore guild, the model provided an interval between TAB-m and TAM-M of 9–14 for Homo sp., 17–25 individuals for C. mosbachensis, 9–10 for L. lycaonoides, 4–6 for P. brevirostris, 9–16 for L. pardinus, 4–5 for M. whitei, 3–4 for H. latidens, and 2–3 for U. etruscus (Rodríguez-Gómez et al. 2016a: table 6). As well as from these estimates, Rodríguez-Gómez et al. (2016a) tested which of the following subsistence strategies was optimal for the hominin population of FN3 and BL: (i) to perform only as hunters; (ii) to adopt a strict scavenging behaviour; (iii) or to behave as both hunters and scavengers. The results obtained showed that the optimal strategy would be the second, as this would give the hominins access to a greater number of resources, which would in turn result in a higher population density, 10–14 individuals per 100 km2. In contrast, the least adaptive strategy would be the first, which would lead to 9–10 individuals per 100 km2. However, it must be noted that the differences in population density between the three strategies are slight.
In a study performed at a continental level, Rodríguez-Gómez et al. (2017b) used the model described above for analysing the faunal assemblages of Europe in the time interval of 1.1–0.2 Ma. The aim was to determine if an increase in competition intensity took place at the beginning of the Middle Pleistocene, which records a decrease in the evidence of hominin presence in Europe compared to the Early Pleistocene. However, the results obtained suggested that competition intensity was lower in the early Middle Pleistocene than in the late Early Pleistocene. Given that the decrease in the number of hominin settlements in Europe coincided with more favourable conditions in the availability of meat resources, Rodríguez-Gómez et al. (2017b) suggested that the apparent reduction in hominin presence at the beginning of the Middle Pleistocene was caused by the climatic changes that resulted from changes in orbital periodicity from 41 ka cycles to 100 ka cycles, which led to the Mid-Pleistocene Revolution (MPR) (Maslin and Ridgwell 2005). During the MPR, a faunal replacement took place in Europe with the appearance of new carnivores (see below), an increase in herbivore richness and a change in the structure of the mammalian communities (Turner 1992; Palombo 2010, 2014; Rodríguez et al. 2012).
Arribas and Palmqvist (1999) and Turner et al. (2008) argued that the sabretooth cats M. whitei and H. latidens were replaced during the Middle Pleistocene by the modern pantherine felids, which exploited their prey in more depth, and that this resulted in the loss of a regular source of scavengeable carcasses for both the hominins and the giant hyaenas (however, note that Homotherium survived in NW Europe until the Late Pleistocene; Reumer et al. 2003). Under these new ecological circumstances, the trophic niche that hominins exploited vanished, and this forced them towards behavioural improvements that resulted in the development of the more effective Acheulean handaxes, which replaced the technologically less elaborated Oldowan flakes of the earlier populations. In the case of P. brevirostris, being constrained by its enormous size and by a highly specialized anatomy derived from its strict scavenging behaviour, the inevitable destiny of this species during this period of change was extinction (Arribas and Palmqvist,1999; Palmqvist et al. 2011; Iannucci et al. 2021). Therefore, the results obtained by Rodríguez-Gómez et al. (2017b) on the changes in meat availability and competition intensity at the beginning of the Middle Pleistocene also support a scavenging role for hominins during the late Early Pleistocene in Europe.
Another aspect of interest related to meat availability, not addressed by Rodríguez-Gómez et al. (2016a), is the carrying capacity of the palaeoecosystem of FN3 and BL (as indicated above, FN3 and BL were not studied separately with the model because the same faunal list is assumed for both sites). Following the third definition of Sayre (2008), carrying capacity can be defined as the maximum population sizes that an ecosystem can sustain in the long term. In the study of Rodríguez-Gómez et al. (2022), average population weights were used for estimating the carrying capacity of different palaeoecosystems of the Orce and Atapuerca sites. These estimates were obtained with the survival profiles provided by the Weibull model (Martín-González et al. 2019) and the population densities derived from the allometric equations of Damuth (1981). The value obtained by Rodríguez-Gómez et al. (2022) for FN3 and BL was 3,307.63 kg/km2 (≈4,961,445 kcal/km2) (see also Palmqvist et al. 2022d). However, given that Rodríguez-Gómez et al. (2016a) did not consider the porcupine Hystrix refossa as a prey species, we have modified here these estimates with the exclusion of this taxon, which provides a carrying capacity of 3,169.64 kg/km2 (≈4,754,004 kcal/km2). The latter estimate indicates that the availability of meat at FN3 and BL (see the TAB values provided before) would represent between 8.6% (TAB-m) and 12.1% (TAB-M) of the carrying capacity of the ecosystem. Therefore, an increase in the consumption of meat above these percentages would drive the community to collapse. Figure 5 shows the carrying capacity of the FN3 and BL palaeoecosystem (Fig. 5A) and the values of TAB-m and TAB-M distributed among the body mass categories considered for the primary consumers (Fig. 5B). The patterns of carrying capacity and meat availability differ because the former has more biomass concentrated in the higher body mass categories, which do not translate into biomass available for the secondary consumers. This results from differences in reproductive rates among mammals: the larger a species is, the lower are its fertility rate and population turnover (see Jones et al. 2009; Magalhães and Costa 2009; Myers et al. 2020). It is worth noting that the prey size categories with the highest TAB values are those that correspond to the preferred prey of the main three predators of the Orce ecosystems (Fig. 3B): the wild dog L. lycaonoides (45–90 kg) and the sabretooth cats M. whitei (180–360 kg) and H. latidens (360–1000 kg) (see Rodríguez-Gómez et al. 2016a). This relates to the proposal of Rodríguez-Gómez et al. (2017b) on the changes in the composition of the large carnivore guild between VM3 and FN3/BL due to top-down forces, following Ripple and Van Valkenburgh (2010). The only large carnivore of VM3 that is absent from FN3/BL is Panthera cf. gombaszoegensis, the European jaguar, whose preferred body mass category was 90-180 kg (see Rodríguez-Gómez et al. 2017a). This prey category is represented in VM3 by Sorgelia minor and the juveniles of Hemibos aff. gracilis and Praeovibos sp., species which are all absent from FN3 and BL (although some of them are erroneously cited at FN3 by Yravedra et al. 2021).
To further explore the role of top-down forces in the palaeocommunity of FN3, we analyse here whether the pattern of prey abundances in this site is closer to the pattern of carrying capacity or to the pattern of resource availability (for a similar study in VM3, see Rodríguez-Gómez et al. 2017a). In so doing, we compare the frequencies of each species in the assemblages of FN3 and BL deduced from MNI values (Espigares et al. 2019) with their proportions according to the estimates of carrying capacity and TAB (Fig. 6). Taking into account that there are slight differences between the patterns of carrying capacity (CC) and those of TAB values, the following inferences can be drawn: (i) compared to the expectations from the CC and TAB frequencies (Fig. 6), there is a higher proportion of the cervids Metacervocerus rhenanus and P. verticornis at level D of BL, and also a lower proportion of the sheep Ammotragus europaeus and the elephant M. meridionalis; (ii) there is an overrepresentation of the horse E. altidens in the lower archaeological level of FN3, where this species is recorded by a frequency well above any of the reference patterns; and (iii) the upper archaeological level of FN3 shows a pattern of prey abundance that is closer to the one expected from CC, but with a slight increase in the biomass of Bison sp. and M. meridionalis, together with a decrease for A. europaeus and the large horse E. suessenbornensis (Fig. 6). These differences probably result from the taphonomic biases involved in the site formation processes (see Palmqvist and Arribas 2001; Espigares 2010).
Prey abundances at Barranco León (BL) and Fuente Nueva 3 (FN3). The upper row of graphs shows the relative abundances of herbivore species calculated with the model of Rodríguez-Gómez et al. (2016a) in the estimation of carrying capacity (CC), TAB-m (minimum total available biomass), and TAB-M (maximum total available biomass) values for BL/FN3. The lower row of graphs shows herbivore abundances calculated from MNI values estimated for level D of Barranco León (BL-D) and the Lower and Upper Archaeological levels of FN3 (FN3-Lower and FN3-Upper, respectively). MNI data from Espigares et al. (2019)
In summary, Rodríguez-Gómez et al. (2016a, 2017a) provided estimates on meat availability for the ecosystems inhabited by the earliest European hominins in Orce, as well as on the intensity of the competition with other carnivores in the access to these resources. Given that Yravedra et al. (2021) focused their paper on the use of meat resources in the late Early Pleistocene assemblage of FN3, they should have cited and discussed these studies. The estimates of Rodríguez-Gómez et al. (2016a) unequivocally indicate that the optimal subsistence strategy of the FN3 hominins was a strict scavenging behaviour, and that they possibly relied on sabretooth cats to gain access to meat resources. After the extinction of these predators, such relationship of trophic dependency was circumvented with the inception of the Acheulean technology, which allowed the hominins to hunt by themselves large ungulates (Arribas and Palmqvist 1999; Palmqvist et al. 2011; Rodríguez-Gómez et al. 2017b). Moreover, the distribution of the meat available in the FN3 ecosystem coincides with the prey size categories preferred by the three main hypercarnivores identified in the assemblage, the wild dog L. lycaonoides and the sabretooth cats M. whitei and H. latidens, which suggests that Homo sp. played a secondary role in FN3 behaving more as a scavenger than as a hunter. However, the hunting by the hominins of small-sized prey should not be discarded, as the level of competition for these prey with other carnivores was less intense (Rodríguez-Gómez et al. 2016a).
Faunal turnovers and hominin ecology in the Early Pleistocene
The archaeological sites of FN3 and BL preserve huge tool assemblages of Oldowan tradition and a rich fossil record of carnivore remains. This scenario changes when the archaeological assemblages become dominated by the presence of developed Acheulian tools: many carnivores disappear, and the small diversity of surviving species are recorded by only a few remains. This happens in East Africa before 1.7 Ma, as recorded in the Ethiopian sites of Melka Wakena, 1.6–0.7 (Hovers et al. 2021), and Asbole, ~0.6 Ma (Geraads et al. 2004), or the Eritrean sites of Engel Ela-Ramud and Buia, both dated to ~1.0 Ma (Martinez-Navarro et al. 2004, 2016). In North Africa, a similar situation is depicted at Wadi Sarrat in Tunisia, dated to ~0.7 Ma (Martínez-Navarro et al. 2014). The same scenario is found outside of Africa when the dispersal of the developed Acheulian tools takes place in the Early-Middle Pleistocene transition, as recorded in Gesher Benot Ya’akob (GBY) in Israel, dated to 0.8–0.7 Ma (Martínez-Navarro and Rabinovich 2011), and also in several Middle Pleistocene Acheulian assemblages of Europe, including Venosa Notarchirico in Italy, ~0.7 Ma (Piperno 2000; Moncel et al. 2019, 2020), and la Solana del Zamborino in Spain, dated to ~0.4 Ma (Jiménez-Arenas et al. 2011; Álvarez-Posada et al. 2017). Although carnivores are well represented in some Acheulian localities like Tighenif in Algeria, dated to ~1.0 Ma (Geraads 2016), this is the exception rather than the rule.
These data show that carnivores were abundantly represented and had a high diversity in the archaeological sites of Oldowan assemblages, but the situation is reversed in the sites that record Acheulian tools. How should these data be interpreted? The most parsimonious explanation, suggested by Martinez-Navarro (2018) and Bartolini-Lucenti et al. (2022), is that this relates to a change in hominin behaviour from scavenging to active hunting. The hominins that developed the Oldowan tools probably did not dominate the ecological scenario, because they were merely passive scavengers that needed to fight for the carrion with other scavenging carnivores, especially the large hyaenas, represented in Eurasia by P. brevirostris. Therefore, Homo was just another member of the carnivore guild, not the dominant one. For this reason, other carnivores used to be abundantly represented in the Early Pleistocene assemblages of Eurasia, as in the case of FN3. Hominin behaviour changed radically with the emergence of the Acheulian complex and its expansion through Africa and Eurasia, most probably because our ancestors acquired the abilities to hunt large prey and, above all, to secure the prey carcass from other carnivores and to expunge them from their living areas. Following this reasoning, humans became the dominant predator in the new ecological scenario, which explains why other large carnivores are rare in, or even absent from, the Acheulian sites. Both FN3 and BL are typical Oldowan sites, with an excellent record of large and small carnivores.
In summary, the most parsimonious interpretation of the data and arguments discussed in this study is that: (i) the hominins who inhabited the Guadix-Baza Depression during late Early Pleistocene times were scavengers with a secondary access to the meat and fat provided by the prey carcasses of sabretooth cats, the dominant primary predators of these ecosystems; and (ii) they competed for carrion with the large hyaenas as well as with other opportunistic carnivores in the access to these resources, as suggested by Espigares et al. (2019). This interpretation, described by Yravedra et al. (2021) as the “Megantereon–hominins–Pachycrocuta model,” is the one that better explains the taphonomic evidence available at FN3.
References
Agam A, Barkai R (2016) Not the brain alone: the nutritional potential of elephant heads in Paleolithic sites. Quat Int 406:218–226
Akersten WA (1985) Canine function in Smilodon (Mammalia: Felidae: Machairodontidae). Contrib Sci Nat Hist Mus Los Ang Co 356:1–22
Álvarez C, Parés JM, Granger D, Duval M, Sala R, Toro I (2015) New magnetostratigraphic and numerical age of the Fuente Nueva-3 site (Guadix-Baza basin, Spain). Quat Int 389:224–234
Álvarez-Posada C, Parés JM, Sala R, Viseras C, Pla-Pueyo S (2017) New magnetostratigraphic evidence for the age of Acheulean tools at the archaeopalaeontological site “Solana del Zamborino”. Sci Rep 7:13495. https://doi.org/10.1038/s41598-017-14024-5
Anadón P, De Deckker P, Juliá R (1986) The Pleistocene lake deposits of the NE of Baza basin (Spain): salinity variation and ostracod succession. Hydrobiologia 143:199–208
Anadón P, Oms O, Riera V, Julià R (2015) The geochemistry of biogenic carbonates as a paleoenvironmental tool for the Lower Pleistocene Barranco León sequence (BL-5D, Baza Basin, Spain). Quat Int 389:70–83
Andersson K, Norman D, Werdelin L (2011) Sabretoothed carnivores and the killing of large prey. PLoS One 6(10):e24971. https://doi.org/10.1371/journal.pone.0024971
Andrews P, Cook J (1985) Natural modifications to bones in a temperate setting. Man 20:675–691
Antón M (2013) Sabertooth (Life of the Past). Indiana University Press, Bloomington
Antón M, Salesa MJ, Pastor JF, Sánchez IM, Fraile S, Morales J (2004) Implications of the mastoid anatomy of larger extant felids for the evolution and predatory behaviour of sabretoothed cats (Mammalia, Carnivora, Felidae). Zool J Linnean Soc 140:207–221
Antón M, Galobart A, Turner A (2005) Co-existence of scimitar-toothed cats, lions and hominins in the European Pleistocene. Implications of the post-cranial anatomy of Homotherium latidens (Owen) for comparative palaeoecology. Quat Sci Rev 24:1287–1301
Anyonge W (1993) Body mass in large extant and extinct carnivores. J Zool 231:339–350
Anyonge W (1996a) Microwear on canines and killing behavior in large carnivores: saber function in Smilodon fatalis. J Mammal 77:1059–1067
Anyonge W (1996b) Locomotor behaviour in Plio-Pleistocene sabre-tooth cats: a biomechanical analysis. J Zool 238:395–413
Arriaza MC, Domínguez-Rodrigo M, Yravedra J, Baquedano E (2016) Lions as bone accumulators? Paleontological and ecological implications of a modern bone assemblage from Olduvai Gorge. PLoS One 11(5):e0153797. https://doi.org/10.1371/journal.pone.0153797
Arribas A, Palmqvist P (1998) Taphonomy and paleoecology of an assemblage of large mammals: hyaenid activity in the lower Pleistocene site at Venta Micena (Orce, Guadix-Baza Basin, Granada, Spain). Geobios 31(Suppl):3–47
Arribas A, Palmqvist P (1999) On the ecological connection between sabre-tooths and hominids: faunal dispersal events in the lower Pleistocene and a review of the evidence for the first human arrival in Europe. J Archaeol Sci 26:571–585
Arribas A, Palmqvist P (2002) The first human dispersal to Europe: remarks on the archaeological and palaeoanthropological record from Orce (Guadix-Baza basin, southeastern Spain). Hum Evol 17:55–78
Ballesio R (1986) Les Carnivores du gisement Pléistocéne d’Oubéidiyeh. In: Tchernov E (ed) Les Mammiféres du Pléistocène Inférieur, de la Vallée du Jourdain è Oubéidiyeh. Association Paléorient Edition, Paris, pp 63–92
Barnett R, Westbury MV, Sandoval-Velasco M, Vieira FG, Jeon S, Zazula G, Martin MD, Ho SYW, Mather N, Gopalakrishnan S, Ramos-Madrigal J, de Manuel M, Zepeda-Mendoza ML, Antunes A, Carmona Baez A, De Cahsan B, Larson G, O’Brien SJ, Eizirik E (2020) Genomic adaptations and evolutionary history of the extinct scimitar-toothed cat, Homotherium latidens. Curr Biol 30:5018–5025
Barsky D, Celiberti V, Cauche D, Grégire S, Lebègue F, Lumley H, Toro I (2010) Raw material discernment and technological aspects of the Barranco León and Fuente Nueva 3 stone assemblages (Orce southern Spain). Quat Int 223-224:201–219
Barsky D, Sala R, Menéndez L, Toro-Moyano I (2015) Use and re-use: re-knapped flakes from the Mode 1 site of Fuente Nueva 3 (Orce, Andalucía, Spain). Quat Int 361:21–33
Barsky D, Vergès JM, Sala R, Menéndez L, Toro I (2016) Limestone percussion tools from the late Early Pleistocene sites of Barranco León and Fuente Nueva 3 (Orce, Spain). Philos Trans R Soc B 370:20140352. https://doi.org/10.1098/rstb.2014.0352
Bartolini-Lucenti S, Madurell-Malapeira J, Martínez-Navarro B, Cirilli O, Pandolfi L, Rook L, Bushkhianidze M, Lordkipanidze D (2022) A comparative study of the Early Pleistocene carnivore guild from Dmanisi (Georgia). J Hum Evol 162:103108. https://doi.org/10.1016/j.jhevol.2021.103108
Bar-Yosef O, Goren-Inbar N (1993) The lithic assemblages of the site of Ubeidiya, Jordan valley. Qedem 34:1–266
Bearder SK (1977) Feeding habits of spotted hyaenas in woodland habitat. E Afr Wildl J 15:263–280
Beasley DE, Koltz AM, Lambert JE, Fierer N, Dunn RR (2015) The evolution of stomach acidity and its relevance to the human microbiome. PLoS One 10(7):e0134116. https://doi.org/10.1371/journal.pone.0134116
Behrensmeyer AK (1978) Taphonomic and ecologic information from bone weathering. Paleobiology 4:150–162
Ben-Dor M, Sirtoli R, Barkai R (2021) The evolution of the human trophic level during the Pleistocene. Yearb Phys Anthropol 175:27–56. https://doi.org/10.1002/ajpa.24247
Bermúdez de Castro JM, Díez Fernández-Lomana JC, Mosquera Martínez M, Nicolás Checa ME, Pérez Pérez A, Rodríguez Méndez J, Sánchez MA (1995) El nicho ecológico de los homínidos del Pleistoceno Medio de Atapuerca. Complutum 6:9–56
Biknevicius AR, Van Valkenburgh B, Walker J (1996) Incisor size and shape: implications for feeding behavior in sabertoothed “cats”. J Vertebr Paleontol 16:510–521
Blumenschine RJ (1995) Percussion marks, tooth marks, and experimental determination of the timing of hominid and carnivore access to long bones at FLK Zinjanthropus, Olduvai Gorge, Tanzania. J Hum Evol 29:21–51
Blumenschine RJ, Pobiner BL (2007) Zooarchaeology and the ecology of Oldowan hominin carnivory. In: Ungar P (ed) Evolution of the human diet. Oxford University Press, Oxford, pp 167–190
Boscaini A, Madurell-Malapeira J, Llenas M, Martínez-Navarro B (2015) The origin of the critically endangered Iberian lynx: speciation, diet and adaptive changes. Quat Sci Rev 123:247–253
Braga JC, Martín JM, Quesada C (2003) Patterns and average rates of late Neogene-Recent uplift of the Betic Cordillera, SE Spain. Geomorphology 50:3–26
Brain CK (1980) Some criteria for the recognition of bone-collecting agencies in African caves. In: Behrensmeyer AK, Hill AP (eds) Fossils in the making. The University of Chicago Press, Chicago, pp 108–130
Brain CK (1981) The hunters or the hunted: an introduction to African Cave Taphonomy. The University of Chicago Press, Chicago, p 375
Bramble D, Lieberman D (2004) Endurance running and the evolution of Homo. Nature 432:345–352
Brink JW (1997) Fat content in leg bones of Bison bison, and applications to archaeoloy. J Archaeol Sci 24:259–274
Bryant HN, Russell AP, Thomason JJ (1995) Carnassial functioning in nimravid and felid sabertooths: theoretical basis and robustness of inferences. In: Thomason JJ (ed) Functional morphology in vertebrate paleontology. Cambridge University Press, Cambridge, pp 116–135
Byers DA, Ugan A (2005) Should we expect large game specialization in the late Pleistocene? An optimal foraging perspective on early Paleoindian prey choice. J Archaeol Sci 32:1624–1640
Capaldo SD (1997) Experimental determinations of carcass processing by Plio-Pleistocene hominids and carnivores at FLK 22 (Zinjanthropus), Olduvai Gorge, Tanzania. J Hum Evol 33:555–597
Carbonell E, Bermúdez de Castro JM, Parés JM, Pérez-González A, Cuenca-Bescós G, Ollé A, Mosquera M, Huguet R, van der Made J, Rosas A, Sala R, Valverdú J, García N, Granger DE, Martinón-Torres M, Rodríguez XP, Stock GM, Vergès JM, Allué E et al (2008) The first hominin of Europe. Nature 452:465–470
Cheheb RC, Arzarello M, Arnaud J, Berto C, Cáceres I, Caracausi S, Colopi S, Daffara S, Canini GM, Huguet R, Karambatsou T, Sala B, Zambaldi M, Berruti GLF (2019) Human behavior and Homo-mammal interactions at the first European peopling: new evidence from the Pirro Nord site (Apricena, Southern Italy). Sci Nat 106:16. https://doi.org/10.1007/s00114-019-1610-4
Cherin M, Sorbelli L, Crotti M, Iurino DA, Sardella SA (2018) New material of Sus strozzii (Suidae, Mammalia) from the Early Pleistocene of Italy and a phylogenetic analysis of suines. Quat Sci Rev 194:94–115
Cherin M, Alba DM, Crotti M, Menconero S, Moullé P-E, Sorbelli L, Madurell-Malapeira J (2020) The post-Jaramillo persistence of Sus strozzii (Suidae, Mammalia) in Europe: new evidence from the Vallparadís Section (NE Iberian Peninsula) and other coeval sites. Quat Sci Rev 233:106234. https://doi.org/10.1016/j.quascirev.2020.106234
Christiansen P (2008) Evolution of skull and mandible shape in cats (Carnivora: Felidae). PLoS One 3(7):e2807. https://doi.org/10.1371/journal.pone.0002807
Christiansen P, Adolfssen JS (2007) Osteology and ecology of Megantereon cultridens SE311 (Mammalia; Felidae; Machairodontinae), a sabrecat from the Late Pliocene – Early Pleistocene of Senéze. France Zool J Linn Soc 151:833–884
Coil R, Tappen M, Ferring R, Bukhsianidze M, Nioradze M, Lordkipanidze D (2020) Spatial patterning of the archaeological and paleontological assemblage at Dmanisi, Georgia: an analysis of site formation and carnivore-hominin interaction in Block 2. J Hum Evol 143:102773. https://doi.org/10.1016/j.jhevol.2020.102773
Cruz-Uribe K (1991) Distinguishing hyena from hominid bone accumulations. J Field Archael 18:467–486
Damuth J (1981) Population density and body size in mammals. Nature 290:699–700
DeSantis LRG, Schubert BW, Scott JR, Ungar PS (2012) Implications of diet for the extinction of saber-toothed cats and American lions. PLoS One 7:e52453. https://doi.org/10.1371/journal.pone.0052453
DeSantis LRG, Feranec RS, Antón M, Lundelius EL (2021) Dietary ecology of the scimitar-toothed cat Homotherium serum. Curr Biol 31:2674–2681
Domínguez-Rodrigo M, Organista E (2007) Natural background bone assemblages and their ravaging stages in Olduvai Bed I. In: Domínguez-Rodrigo M, Barba R, Egeland CP (eds) Deconstructing Olduvai: a taphonomic study of the Bed I Sites. Springer, Dordrecht, pp 201–215
Domínguez-Rodrigo M, Cifuentes G, Jiménez B, Abellán N, Pizarro M, Organista E, Baquedano E (2020) Artificial intelligence provides greater accuracy in the classification of modern and ancient bone surface modifications. Sci Rep 10:18862. https://doi.org/10.1038/s41598-020-75994-7
Dunn RR, Amato KR, Archie EA, Arandjelovic M, Crittenden AN, Nichols LM (2020) The internal, external and extended microbiomes of hominins. Front Ecol Evol 8:25. https://doi.org/10.3389/fevo.2020.00025
Duval M, Falguères C, Bahain JJ, Grün R, Shao Q, Aubert M, Dolo JM, Agustí J, Martínez-Navarro B, Palmqvist P, Toro-Moyano I (2012) On the limits of using combined U-series/ESR method to date fossil teeth from two Early Pleistocene archaeological sites of the Orce area (Guadix-Baza basin, Spain). Quat Res 77:481–482
Emerson SB, Radinsky L (1980) Functional analysis of sabertooth cranial morphology. Paleobiology 6:295–312
Espigares MP (2010) Análisis y modelización del contexto sedimentario y los atributos tafonómicos de los yacimientos pleistocénicos del borde nororiental de la cuenca de Guadix-Baza. PhD Thesis Dissertation. University of Granada, Spain, p 533. https://digibug.ugr.es/handle/10481/4949?locale-attribute=en
Espigares MP, Martínez-Navarro B, Palmqvist P, Ros-Montoya S, Toro I, Agustí J, Sala R (2013) Homo vs. Pachycrocuta: earliest evidence of competition for an elephant carcass between scavengers at Fuente Nueva-3 (Orce, Spain). Quat Int 295:113–125
Espigares MP, Palmqvist P, Guerra-Merchán A, Ros-Montoya S, García-Aguilar JM, Rodríguez-Gómez G, Serrano FJ, Martínez-Navarro B (2019) The earliest cut marks of Europe: a discussion on hominin subsistence patterns in the Orce sites (Baza basin, SE Spain). Sci Rep 9:15408. https://doi.org/10.1038/s41598-019-51957-5
Espigares MP, Martínez-Navarro B, Ros-Montoya S, García-Aguilar JM, Guerra-Merchán A, Rodríguez-Gómez G, Palmqvist P (2021) Hominins, mammoths, saber-tooths and giant hyenas in the Early Pleistocene of the Baza Basin (SE Spain). In: Konidaris GE, Barkai R, Tourloukis V, Harvati K (eds) Human-elephant interactions: from past to present. Tuebingen Paleoanthropoogy Book Series-Contributions in Paleonthropology 1. Tübingen University Press, pp 47–66
Fariña RA (1996) Trophic relationships among Lujanian mammals. Evol Theory 11:125–134
Fernández-Jalvo Y, Andrews P (2016) Atlas of taphonomic identification. Springer, p 359
Figueirido B, MacLeod N, Krieger J, de Renzi M, Pérez-Claros JA, Palmqvist P (2011) Constraint and adaptation in the evolution of carnivoran skull shape. Paleobiology 37:490–518
Figueirido B, Lautenschlager S, Pérez-Ramos A, Van Valkenburgh B (2018) Distinct predatory behaviors in scimitar- and dirk-toothed sabertooth cats. Curr Biol 28:3260–3266
García-Aguilar JM, Palmqvist P (2011) A model of lacustrine sedimentation for the Lower Pleistocene deposits of Guadix-Baza basin (southeast Spain). Quat Int 243:3–15
García-Aguilar JM, Guerra-Merchán A, Serrano F, Palmqvist P, Flores-Moya A, Martínez-Navarro B (2014) Hydrothermal activity and its paleoecological implications in the latest Miocene to Middle Pleistocene lacustrine environments of the Baza Basin (Betic Cordillera, SE Spain). Quat Sci Rev 96:204–221
García-Aguilar JM, Guerra-Merchán A, Serrano F, Flores-Moya A, Delgado-Huertas A, Espigares MP, Ros-Montoya S, Martínez-Navarro B, Palmqvist P (2015) A reassessment of the evidence for hydrothermal activity in the Neogene-Quaternary lacustrine environments of the Baza basin (Betic Cordillera, SE Spain) and its paleoecological implications. Quat Sci Rev 112:226–235
Geraads D (2016) Pleistocene carnivora (Mammalia) from Tighennif (Ternifine), Algeria. Geobios 49:445–458
Geraads D, Alemseged Z, Reed D, Wynn J, Roman DC (2004) The Pleistocene fauna (other than Primates) from Asbole, lower Awash Valley, Ethiopia, and its environmental and biochronological implications. Geobios 37:697–718
Gonyea WJ (1976) Behavioral implications of saber-toothed felid morphology. Paleobiology 2:332–342
Harstone-Rose A (2008) Evaluating the hominin scavenging niche through analysis of the carcass-processing abilities of the carnivore guild. PhD Thesis Dissertation, Department of Biological Anthropology and Anatomy in the Graduate School of Duke University. https://dukespace.lib.duke.edu/dspace/bitstream/handle/10161/704/D_Hartstone-Rose_Adam_a_200808.pdf?sequence=1&isAllowed=y
Hartstone-Rose A (2011) Reconstructing the diets of extinct South African carnivorans from premolar “intercuspid notch” morphology. J Zool 285:119–127
Haynes G, Krasinski K (2021) Butchering marks on bones of Loxodonta africana (African savanna elephant): implications for interpreting marks on fossil proboscidean bones. J Archaeol Sci Rep 37:102957. https://doi.org/10.1016/j.jasrep.2021.102957
Hemmer H, Kahlke RD, Vekua AK (2011) The cheetah Acinonyx pardinensis (Croizet et Jobert, 1828) s.l. at the hominin site of Dmanisi (Georgia) — a potential prime meat supplier in Early Pleistocene ecosystems. Quat Sci Rev 30:2703–2714. https://doi.org/10.1016/j.quascirev.2011.05.024
Hovers E, Gossa T, Asrat A, Niespolo EM, Resom A, Renne PR, Ekshtain R, Herzlinger G, Ketema N, Martínez-Navarro B (2021) The expansion of the acheulian to the Southeastern Ethiopian highlands: insights from the new Early Pleistocene site-complex of Melka Wakena. Quat Sci Rev 253:106763. https://doi.org/10.1016/j.quascirev.2020.106763
Hublin J-J, Richards MP (2009) The evolution of hominin diets: integrating approach to the study of palaeolithic subsistence. Springer, Leipzig, p 264. https://link.springer.com/book/10.1007/978-1-4020-9699-0
Hüsing SK, Oms O, Agustí J, Garcés M, Kouwenhoven TJ, Krijgsman W, Zachariasse WJ (2010) On the late Miocene closure of the Mediterranean-Atlantic gateway through the Guadix basin (southern Spain). Palaeogeogr Palaeoclimatol Palaeoecol 291:167–179
Iannucci A, Mecozzi B, Sardella R, Iurino DA (2021) The extinction of the giant hyena Pachycrocuta brevirostris and a reappraisal of the Epivillafranchian and Galerian Hyaenidae in Europe: Faunal turnover during the Early–Middle Pleistocene Transition. Quat Sci Rev 272:107240. https://doi.org/10.1016/j.quascirev.2021.10724
Janis CM, Figueirido B, DeSantis L, Lautenschlager S (2020) An eye for a tooth: Thylacosmilus was not a marsupial “saber-tooth predator”. PeerJ 8:e9346. https://doi.org/10.7717/peerj.9346
Jiménez-Arenas JM, Santonja M, Botella M, Palmqvist P (2011) The oldest handaxes in Europe: fact or artefact? J Archaeol Sci 38:3340–3348
Jones KE, Bielby J, Cardillo M, Fritz SA, O’Dell J, Orme CDL, Safi K, Sechrest W, Boakes EH, Carbone C, Connolly C, Cutts MJ, Foster JK, Grenyer R, Habib M, Plaster CA, Price SA, Rigby EA, Rist J et al (2009) PanTHERIA: a species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 90:2648. https://doi.org/10.1890/08-1494.1
Kuhn BF, Wiesel I, Skinner JD (2008) Diet of brown hyaenas (Parahyaena brunnea) on the Namibian coast. Trans R Soc S Afr 63:150–159
Kuhn BF, Berger LR, Skinner JD (2010) Examining criteria for identifying and differentiating fossil faunal assemblages accumulated by hyenas and hominins using extant hyenid accumulations. Int J Osteoarchaeol 20:15–35
Lam YM, Chen X, Pearson OM (1999) Intertaxonomic variability in patterns of bone density and the differential representation of bovid, cervid, and equid elements in the archaeological record. Am Antiq 64:343–362
Lautenschlager S, Figueirido B, Cashmore DD, Bendel EM, Stubbs TL (2020) Morphological convergence obscures functional diversity in sabre-toothed carnivores. Proc R Soc B 287:20201818. https://doi.org/10.1098/rspb.2020.1818
Leslie DE (2016) A striped hyena scavenging event: implications for Oldowan hominin behavior. Field Notes J Coll Anthropol 8:122–138
Liebenberg L (2006) Persistence hunting by modern hunter-gatherers. Curr Anthropol 47:1017–1026
Lieberman DE, Bramble DM, Raichlen DA, Shea JJ (2007) The evolution of endurance running and the tyranny of ethnography: a reply to Pickering and Bunn. J Hum Evol 53:439–442
Lordkipanidze D (2015) The traces of the first humans in Eurasia. In: Sanz N (ed) Human origin sites and the world heritage convention in Eurasia, vol I. United Nations Educational, Scientific and Cultural Organization, pp 153–162
Lordkipanidze D, Jashashvili T, Vekua A, Ponce de León MS, Zollikofer CPE, Rightmire GP, Pontzer H, Ferring R, Oms O, Tappen M, Bukhsianidze M, Agusti J, Kahlke R, Kiladze G, Martinez-Navarro B, Mouskhelishvili A, Nioradze M, Rook L (2007) Postcranial evidence from early Homo from Dmanisi, Georgia. Nature 449:305–310
Lozano-Fernández I, Cuenca-Bescós G, Blain H-A, López-García JM, Agustí J (2013) Mimomys savini size evolution in the Early Pleistocene of south-western Europe and possible biochronological implications. Quat Sci Rev 76:96–101
Lozano-Fernández I, Blain H-A, López-García JM, Agustí J (2014) Biochronology of the first hominid remains in Europe using the vole Mimomys savini: Fuente Nueva 3 and Barranco León D, Guadix-Baza Basin, south-eastern Spain. Hist Biol 27:1021–1028
Luzón C, Yravedra J, Courtenay LA, Saarinen J, Blain H-A, DeMiguel D, Viranta S, Azanza B, Rodríguez-Alba JJ, Herranz-Rodrígo D, Serrano-Ramos A, Solano JA, Oms O, Agustí J, Fortelius M, Jiménez-Arenas JM (2021) Taphonomical and spatial analyses from the Early Pleistocene site of Venta Micena 4 (Orce, Guadix-Baza, Basin, southern Spain). Sci Rep 11:13977. https://doi.org/10.1038/s41598-021-93261-1
Madurell-Malapeira J, Minwer-Barakat R, Alba DM, Garcés M, Gómez M, Aurell-Garrido J, Ros-Montoya S, Moyà-Solà S, Berástegui X (2010) The Vallparadís section (Terrassa, Iberian Peninsula) and the latest Villafranchian faunas of Europe. Quat Sci Rev 29:3972–3982
Madurell-Malapeira J, Martínez-Navarro B, Ros-Montoya S, Espigares MP, Toro I, Palmqvist P (2011) The earliest European badger (Meles meles), from the Late Villafranchian site of Fuente Nueva 3 (Orce, Granada, SE Iberian Peninsula). C R Palevol 10:609–615
Madurell-Malapeira J, Ros-Montoya S, Espigares MP, Alba DM, Aurell-Garrido J (2014) Villafranchian large mammals from the Iberian Peninsula: paleogeography, paleoecology and dispersal events. J Iber Geol 40:167–178
Magalhães JP, Costa J (2009) A database of vertebrate longevity records and their relation to other life-history traits. J Evol Biol 22:1770–1774
Marean CW (1989) Sabertooth cats and their relevance for early hominid diet and evolution. J Hum Evol 18:559–582
Marean CW, Ehrhardt CL (1995) Paleoanthropological and paleoecological implications of the taphonomy of a sabertooth’s den. J Hum Evol 29:515–547
Martin RA (2014) A critique of vole clocks. Quat Sci Rev 94:1–6
Martin LD, Babiarz JP, Naples VL, Hearst J (2000) Three ways to be a saber-toothed cat. Naturwissenschaften 87:41–44
Martínez Navarro B, Karoui-Yaakoub N, Oms O, Amri L, López-García JM, Zerai K, Blain HA, Mtimet MS, Espigares MP, Ben Haj Ali N, Ros-Montoya S, Boughdiri M, Agustí J, Khayati-Ammar H, Maalaoui K, Om El Khir M, Sala R, Othmani A, Hawas R et al (2014) The early Middle Pleistocene archaeopaleontological site of Wadi Sarrat (Tunisia) and the earliest record of Bos primigenius. Quat Sci Rev 90:37–46
Martínez-Navarro B (2018) Oldowan scavengers vs. Acheulian hunters: what does the faunal record say? Glob J Archaeol Anthropol 6:555679. https://doi.org/10.19080/GJAA.2018.06.555679
Martínez-Navarro B, Palmqvist P (1995) Presence of the African machairodont Megantereon whitei (Broom, 1937) (Felidae, Carnivora, Mammalia) in the Lower Pleistocene site of Venta Micena (Orce, Granada, Spain), with some considerations on the origin, evolution and dispersal of the genus. J Archaeol Sci 22:569–582
Martínez-Navarro B, Palmqvist P (1996) Presence of the African saber-toothed felid Megantereon whitei (Broom, 1937) (Mammalia, Carnivora, Machairodontinae) in Apollonia-1 (Migdonia Basin, Macedonia, Greece). J Archaeol Sci 23:869–872
Martínez-Navarro B, Turq A, Agustí J, Oms O (1997) Fuente Nueva-3 (Orce, Granada, Spain) and the first human occupation of Europe. J Hum Evol 33:611–620
Martínez-Navarro B, Rook L, Segid A, Yosief D, Ferretti MP, Shoshani J, Tecle TM, Libsekal Y (2004) The large fossil mammals from Buya (Eritrea): systematics, biochronology and Paleoenvironments. Riv It Pal Strat 110:61–88
Martínez-Navarro B, Belmaker M, Bar-Yosef O (2009) The large carnivores from Ubeidiya (Early Pleistocene, Israel): biochronological and biogeographical implications. J Hum Evol 56:514–524
Martínez-Navarro B, Palmqvist P, Madurell J, Ros-Montoya S, Espigares MP, Torregrosa V, Perez-Claros JA (2010) La Fauna de Grandes Mamíferos de Fuente Nueva-3 y Barranco León-5: Estado de la Cuestión. In: Toro I, Martínez-Navarro B, Agustí J (eds) Ocupaciones Humanas en el Pleistoceno inferior y medio de la Cuenca de Guadix-Baza, Junta de Andalucía. Consejería de Cultura. Arqueología Monografías, pp 197–236
Martínez-Navarro B, Espigares MP, Pastó I, Ros-Montoya S, Palmqvist P (2014) Early Homo fossil records of Europe. In: Smith C (ed) Encyclopedia of Global Archaeology, pp 2561–2570
Martínez-Navarro B, Madurell-Malapeira J, Ros-Montoya S, Espigares MP, Medin T, Hortolà P, Palmqvist P (2015) The Epivillafranchian and the arrival of pigs into Europe. Quat Int 389:131–138
Martínez-Navarro B, Carbonell E, Medin T, Oms O, Rodriguez-Álvarez XP, Ros-Montoya S, Araia D, Tsehale L, Libsekal Y (2016) The Engel Ela-Ramud Basin: a new Plio-Pleistocene archaeo-paleontological site in Eritrea. Proc Eur Soc Hum Evol 5:154
Martínez-Navarro B, Bartolini Lucenti S, Palmqvist P, Ros-Montoya S, Madurell-Malapeira J, Espigares-Ortiz MP (2021) A new species of dog from the Early Pleistocene site of Venta Micena (Orce, Baza Basin, Spain). C R Palevol 20:297–314
Martín-González JA, Rodríguez-Gómez G, Palmqvist P (2019) Survival profiles from linear models versus Weibull models: estimating stable and stationary population structures for Pleistocene large mammals. J Archaeol Sci Rep 25:370–386
Martín-Serra A, Figueirido B, Palmqvist P (2017) Non-decoupled morphological evolution of the fore- and hindlimb of sabretooth predators. J Anat 231:532–542
Martínez-Navarro B, Rabinovich R (2011) The fossil Bovidae (Artiodactyla, Mammalia) from Gesher Benot Ya‘aqov, Israel: out of Africa during the Early-Middle Pleistocene transition. J Hum Evol 60:375–386
Maslin MA, Ridgwell PL (2005) Mid-Pleistocene revolution and the “eccentricity myth”. In: Head MJ, Gibbard EL (eds) Early–Middle Pleistocene transitions: the land–ocean evidence. Geological Society, London, pp 19–34
Matthews LH (1939) The bionomics of the spotted hyaena, Crocuta crocuta Erxl. Proc Zool Soc London ser A 109:43–56
McHenry C, Wroe S, Clausen PD, Moreno K, Cunningham E (2007) Supermodeled sabercat, predatory behavior in Smilodon fatalis revealed by high-resolution 3D computer simulation. Proc Natl Acad Sci U S A 104:16010–16015
Meachen-Samuels JA (2012) Morphological convergence of the prey-killing arsenal of sabertooth predators. Paleobiology 38:715–728
Meachen-Samuels JA, Van Valkenburgh B (2010) Radiographs reveal exceptional forelimb strength in the sabertooth cat, Smilodon fatalis. PLoS One 5:e11412. https://doi.org/10.1371/journal.pone.0011412
Medin T, Martínez-Navarro B, Rivals F, Madurell-Malapeira J, Ros-Montoya S, Espigares MP, Figueirido B, Rook L, Palmqvist P (2017) Late Villafranchian Ursus etruscus and other large carnivorans from the Orce sites (Guadix-Baza basin, Andalusia, Southern Spain): taxonomy, biochronology, paleobiology, and ecogeographical context. Quat Int 431:20–41
Moncel M-H, Santagata C, Pereira A, Nomade S, Bahain J-J, Voinchet P, Piperno M (2019) A biface production older than 600 ka ago at Notarchirico (Southern Italy) contribution to understanding early Acheulean cognition and skills in Europe. PLoS One 14:e0218591. https://doi.org/10.1371/journal.pone.0218591
Moncel M-H, Santagata C, Pereira A, Nomade S, Voinchet P, Bahain J-J, Daujeard C, Curci A, Lemorini C, Hardy B, Eramo G, Berto C, Raynal JP, Arzarello M, Mecozzi B, Iannucci A, Sardella R, Allegretta I, Delluniversita E et al (2020) The origin of early Acheulean expansion in Europe 700 ka ago: new findings at Notarchirico (Italy). Sci Rep 10:13802. https://doi.org/10.1038/s41598-020-68617-8
Morelli F, Kubicka AM, Tryjanowski P, Nelson E (2015) The vulture in the sky and the hominin on the land: three million years of human–vulture interaction. Anthrozoös 28:449–468
Moullé PE, Lacombat F, Echassoux A (2006) Contribution of the large mammals of Vallonnet cave (Roquebrune-Cap-Martin, Alpes-Maritimes, France) to the knowledge of biochronological frame of the second half of the Lower Pleistocene in Europe. L’anthropologie 110:837–849
Myers P, Espinosa R, Parr CS, Jones T, Hammond GS, Dewey TA (2020) The animal diversity web (online). Accessed at https://animaldiversity.org
O’Connell JF, Hawkes K (1988) Hadza hunting, butchering, and bone transport and their archaeological implications. J Anthropol Res 44:114–161
O’Regan HJ (2007) Revision of the Carnivora from Member 5, Sterkfontein, South Africa, based on a re-assessment of the published material and site stratigraphy. Ann Transv Mus 44:209–214
O’Regan HJ, Steininger CM (2017) Felidae from Cooper’s cave, South Africa (Mammalia: Carnivora). Geodiversitas 39:315–332
Ogada DL, Keesing F, Virani MZ (2012) Dropping dead: causes and con sequences of vulture population declines worldwide. Ann N Y Acad Sci 1249:57–71
Oms O, Parés JM, Martínez-Navarro B, Agustí J, Toro I, Martínez-Fernández G, Turq A (2000) Early human occupation of Western Europe: paleomagnetic dates for two Paleolithic sites in Spain. Proc Natl Acad Sci U S A 97:10666–10670
Outram A, Rowley-Conwy P (1988) Meat and marrow utility indices for horse (Equus). J Archaeol Sci 25:839–849
Palmqvist P (2002) On the presence of Megantereon whitei at the South Turkwel hominid site, northern Kenya. J Paleontol 76:928–930
Palmqvist P, Arribas A (2001) Taphonomic decoding of the paleobiological information locked in a lower Pleistocene assemblage of large mammals. Paleobiology 27:512–530
Palmqvist P, Martínez-Navarro B, Arribas A (1996) Prey selection by terrestrial carnivores in a lower Pleistocene paleocommunity. Paleobiology 22:514–534
Palmqvist P, Gröcke DR, Arribas A, Fariña R (2003) Paleoecological reconstruction of a lower Pleistocene large mammals community using biogeochemical (δ13C, δ15N, δ18O, Sr: Zn) and ecomorphological approaches. Paleobiology 29:205–229
Palmqvist P, Martínez-Navarro B, Toro I, Espigares MP, Ros-Montoya S, Torregrosa V, Pérez-Claros JA (2005) Réévaluation de la présence humaine au Pléistocène inférieur dans le Sud de l’Espagne. L’anthropologie 109:411–450
Palmqvist P, Torregrosa V, Pérez-Claros JA, Martínez-Navarro B, Turner A (2007) A re-evaluation of the diversity of Megantereon (Mammalia, Carnivora, Machairodontinae) and the problem of species identification in extinct carnivores. J Vertebr Paleontol 27:160–175
Palmqvist P, Pérez-Claros JA, Gröcke DR, Janis CM (2008a) Tracing the ecophysiology of ungulates and predator-prey relationships in an early Pleistocene large mammal community. Palaeogeogr Palaeoclimatol Palaeoecol 266:95–111
Palmqvist P, Pérez-Claros JA, Janis CM, Figueirido B, Torregrosa V, Gröcke DR (2008b) Biogeochemical and ecomorphological inferences on prey selection and resource partitioning among mammalian carnivores in an early Pleistocene community. Palaios 23:724–737
Palmqvist P, Martínez-Navarro B, Pérez-Claros JA, Torregrosa V, Figueirido B, Jiménez-Arenas JM, Espigares MP, Ros-Montoya S, de Renzi M (2011) The giant hyena Pachycrocuta brevirostris: modelling the bone-cracking behavior of an extinct carnivore. Quat Int 243:61–79
Palmqvist P, González-Donoso JM, De Renzi M (2014) Rectilinear evolution in arvicoline rodents and numerical dating of Iberian Early Pleistocene sites. Quat Sci Rev 98:100–109
Palmqvist P, Duval M, Diéguez A, Ros-Montoya S, Espigares MP (2016) On the fallacy of using orthogenetic models of rectilinear change in arvicolid teeth for estimating the age of the first human settlements in Western Europe. Hist Biol 28:734–752
Palmqvist P, Rodríguez-Gómez G, Figueirido B, García-Aguilar JM, Pérez-Claros JA (2022a) On the ecological scenario of the first hominin dispersal out of Africa. L’Anthropologie 126:102998. https://doi.org/10.1016/j.anthro.2022.102998
Palmqvist P, Espigares MP, Pérez-Claros JA, Figueirido B, Guerra-Merchán A, Ros-Montoya S, Rodríguez-Gómez G, García-Aguilar JM, Granados A, Martínez-Navarro B (2022b) Déjà vu: a reappraisal of the taphonomy of quarry VM4 of the Early Pleistocene site of Venta Micena (Baza Basin, SE Spain). Sci Rep 12:705. https://doi.org/10.1038/s41598-021-04725-3
Palmqvist P, Rodríguez-Gómez G, Bermúdez De Castro JM, García-Aguilar JM, Espigares MP, Figueirido B, Ros-Montoya S, Granados A, Serrano FJ, Martínez-Navarro B, Guerra-Merchán A (2022c) Insights on the Early Pleistocene hominin population of the Guadix-Baza Depression. Front Ecol Evol 10:881651. https://doi.org/10.3389/fevo.2022.881651
Palmqvist P, Rodríguez-Gómez G, Espigares MP (2022d) Climate and environmental conditions in the Guadix-Baza Depression during the first hominin dispersal in Western Europe: comment on Saarinen et al. (2021). Quat Sci Rev 297:107731. https://doi.org/10.1016/j.quascirev.2022.107731
Palombo MR (2010) A scenario of human dispersal in the northwestern Mediterranean throughout the Early to Middle Pleistocene. Quat Int 223–224:179–194
Palombo MR (2014) Deconstructing mammal dispersals and faunal dynamics in SW Europe during the Quaternary. Quat Sci Rev 96:50–71
Pante MC (2013) The larger mammal fossil assemblage from JK2, Bed III, Olduvai Gorge, Tanzania: implications for the feeding behavior of Homo erectus. J Hum Evol 64:68–82
Pérez-Claros JA, Coca-Ortega C (2020) Canines and carnassials as indicators of sociality in durophagous hyaenids: analyzing the past to understand the present. PeerJ 8:e10541. https://doi.org/10.7717/peerj.10541
Petrucci M, Cipullo A, Martínez-Navarro B, Rook L, Sardella R (2013) The Late Villafranchian (Early Pleistocene) carnivores (Carnivora, Mammalia) from Pirro Nord (Italy). Palaeontogr Abt A Paläozool Strat 298:113–145
Phillips JA (1993) Bone consumption by cheetahs at undisturbed kills: evidence for a lack of focal-palatine erosion. J Mammal 74:487–492
Phillips DL (2001) Mixing models in analyses of diet using multiple stable isotopes: a critique. Oecologia 127:166–170
Piperno M (2000) Notarchirico. Un Sito del Pleistocene Medio Iniziale nel Bacino di Venosa (Poliedrica). Osanna Edizioni, Venosa. https://www.jstor.org/stable/26295809
Pobiner B (2007) Hominid-carnivore interactions: evidence from modern carnivore bone modification and Early Pleistocene archaeofaunas (Koobi Fora, Kenya; Olduvai Gorge, Tanzania). Ph.D. Thesis Dissertation. Department of Anthropology, Rutgers University, New Brunswick. https://paleoanthro.org/media/dissertations/Briana%20Pobiner-Abstract.pdf
Pobiner BL (2020) The zooarchaeology and paleoecology of early hominin scavenging. Evol Anthropol 29:68–82. https://doi.org/10.1002/evan.21824
Potts R (1991) Why the Oldowan? Plio-Pleistocene toolmaking and the transport of resources. J Anthropol Res 47:153–176
Rawn-Schatzinger V (1992) The scimitar cat Homotherium serum Cope: osteology, functional morphology, and predatory behaviour. Illinois State Mus Rep Investig 47:1–80
Reumer JWF, Rook L, Van der Borg K, Post K, Mol D, De Vos J (2003) Late Pleistocene survival of the saber-toothed cat Homotherium in Northwestern Europe. J Vertebr Paleontol 23:260–262
Ripple WJ, Van Valkenburgh B (2010) Linking top-down forces to the Pleistocene megafaunal extinctions. BioScience 60:516–526
Rodríguez J, Rodríguez-Gómez G, Martín-González JA, Goikoetxea I, Mateo A (2012) Predator–prey relationships and the role of Homo in Early Pleistocene food webs in Southern Europe. Palaeogeogr Palaeoclimatol Palaeoecol 365–366:99–114
Rodríguez-Alba JJ, Linares-Matás G, Yravedra J (2019) First assessments of the taphonomic behaviour of jaguar (Panthera onca). Quat Int 517:88–96
Rodríguez-Gómez G, Rodríguez J, Martín-González JA, Goikoetxea I, Mateos A (2013) Modeling trophic resource availability for the first human settlers of Europe: the case of Atapuerca-TD6. J Hum Evol 64:645–657
Rodríguez-Gómez G, Martín-González JA, Goikoetxea I, Mateos A, Rodríguez J (2014a) A new mathematical approach to model trophic dynamics of mammalian palaeocommunities. The Case of Atapuerca-TD6. In: Pardo-Igúzquiza E, Guardiola-Albert C, Heredia J, Moreno-Merino L, Durán JJ, Vargas-Guzmán JA (eds) Mathematics of Planet Earth15th Annual Conference of the International Association for Mathematical Geosciences, Lectures Notes in Earth System Sciences. Springer, Madrid, pp 739–745
Rodríguez-Gómez G, Mateos A, Martín-González JA, Blasco R, Rosell J, Rodríguez J (2014b) Discontinuity of human presence at Atapuerca during the early Middle Pleistocene: a matter of ecological competition? PLoS One 9:e101938. https://doi.org/10.1371/journal.pone.0101938
Rodríguez-Gómez G, Palmqvist P, Rodríguez J, Mateos A, Martín-González JA, Espigares MP, Ros-Montoya S, Martínez-Navarro B (2016a) On the ecological context of the earliest human settlements in Europe: resource availability and competition intensity in the carnivore guild of Barranco León-D and Fuente Nueva-3 (Orce, Baza Basin, SE Spain). Quat Sci Rev 134:69–83
Rodríguez-Gómez G, Mateos A, Martín-González JA, Rodríguez J (2016b) Measuring intraguild competition from faunal assemblages to compare environmental conditions among paleocommunities. Quat Int 413:55–68
Rodríguez-Gómez G, Palmqvist P, Ros-Montoya S, Espigares MP, Martínez-Navarro B (2017a) Resource availability and competition intensity in the carnivore guild of the Early Pleistocene site of Venta Micena (Orce, Baza Basin, SE Spain). Quat Sci Rev 164:154–167
Rodríguez-Gómez G, Rodríguez J, Martín-González JA, Mateos A (2017b) Evaluating the impact of Homo-carnivore competition in European human settlements during the Early to Middle Pleistocene. Quat Res 88:129–151
Rodríguez-Gómez G, Palmqvist P, Martínez-Navarro B, Martín-González JA, Bermúdez de Castro JM (2022) Mean body size estimation in large mammals and the computation of biomass in past ecosystems: an application to the Pleistocene sites of Orce and Sierra de Atapuerca. C R Palevol 21:207–233. https://doi.org/10.5852/cr-palevol2022v21a10
Roebroeks W (2001) Hominid behaviour and the earliest occupation of Europe: an exploration. J Hum Evol 41:437–461
Ros-Montoya S, Martínez-Navarro B, Espigares MP, Guerra-Merchán A, García-Aguilar JM, Piñero P, Rodríguez-Rueda A, Agustí J, Oms O, Palmqvist P (2017) A new Ruscinian site in Europe: Baza-1 (Baza Basin, Andalusia, Spain). C R Palevol 16:746–761
Ros-Montoya S, Bartolini-Lucenti S, Espigares MP, Palmqvist P, Martínez-Navarro B (2021) First review of Lyncodontini material (Mustelidae, Carnivora, Mammalia) from the lower Pleistocene archaeo-palaeontological sites of Orce (southeastern Spain). Riv It Pal Strat 127:33–47
Ruxton GD, Wilkinson DM (2012) Endurance running and its relevance to scavenging by early hominins. Evolution 67:861–867
Sahnouni M, van der Made J (2009) The Oldowan in North Africa within a biochronological framework. In: Schick K, Toth N (eds) The cutting edge: new approaches to the archaeology of human origins. Stone Age Institute Press, Bloomington, pp 179–210
Salesa M, Antón M, Turner A, Morales J (2010) Functional anatomy of the forelimb in Promegantereon ogygia (Felidae, Machairodontinae, Smilodontini) from the Late Miocene of Spain and the origins of the sabre-toothed felid model. J Anat 216:381–396
Salopek P (2015) Did chucking stones make us more human? https://www.nationalgeographic.com/history/article/150819-republic-of-georgia-david-lordkipanidze-dmanisi-homo-erectus-stone-throwing-prehistory
Sayre NF (2008) The genesis, history, and limits of carrying capacity. Ann Assoc Am Geogr 98:120–134
Schaller GB (1968) Hunting behaviour of the cheetah in the Serengeti National Park, Tanzania. Afr J Ecol 6:95–100
Schubert BW, Ungar PS, DeSantis LRG (2010) Carnassial microwear and dietary behaviour in large carnivorans. J Zool 280:257–263
Shoshani J, Kupsky WJ, Marchant GH (2006) Elephant brain. Part I: gross morphology, functions, comparative anatomy, and evolution. Brain Res Bull 70:124–157
Skinner JD, Smithers RHN (1990) The Mammals of the Southern African Subregion. Cambridge University Press, Cambridge, MA, p 814
Skinner JD, Henschel JR, Van Jaarsveld AS (1986) Article bone-collecting habits of spotted hyaenas Crocuta crocuta in the Kruger National Park. Afr Zool 21:303–308
Slater GJ, Van Valkenburgh B (2008) Long in the tooth: evolution of sabertooth cat cranial shape. Paleobiology 34:403–419
Soria JM, Fernández J, Viseras C (1999) Late Miocene stratigraphy and palaeogeographic evolution of the intramontane Guadix Basin (Central Betic Cordillera, Spain): implications for an Atlantic-Mediterranean connection. Palaeogeogr Palaeoclimatol Palaeoecol 151:255–266
Spoor CF (1985) Body proportions in Hyaenidae. Anat Anz 160:215–220
Titton S, Barsky D, Bargalló A, Vergès JM, Guardiola M, García-Solano J, Jiménez Arenas JM, Toro-Moyano I, Sala-Ramos R (2018) Active percussion tools from the Oldowan site of Barranco León (Orce, Andalusia, Spain): the fundamental role of pounding activities in hominin lifeways. J Archaeol Sci 96:131–147
Titton S, Oms O, Barsky D, Bargalló A, Serrano-Ramos A, García-Solano J, Sánchez-Bandera C, Yravedra J, Blain H-A, Toro-Moyano I, Jiménez Arenas JM, Sala-Ramos R (2021) Oldowan stone knapping and percussive activities on a raw material reservoir deposit 1.4 million years ago at Barranco León (Orce, Spain). Archaeol. Anthropol Sci 13:108. https://doi.org/10.1007/s12520-021-01353-w
Tixier J, Roe D, Turq A, Gibert J, Martínez-Navarro B, Arribas A, Gibert L, Gaete R, Maillo A, Iglesias A (1995) Presence d’industries lithiques dans le Pléistocène inférieur de la région d’Orce (Grenade, Espagne): etat de la question. C R Acad Sci Ser IIa 321:71–78
Toro-Moyano I, Barsky D, Cauche D, Celiberti V, Grégoire S, Lebegue F, Moncel MH, Lumley H (2011) The archaic Stone tool industry from Barranco León and Fuente Nueva 3 (Orce, Spain): evidence of the earliest hominin presence in southern Europe. Quat Int 243:80–91
Toro-Moyano I, Martínez-Navarro B, Agustí J, Souday C, Bermúdez de Castro JM, Martinón-Torres M, Fajardo B, Duval M, Falguères C, Oms O, Parés JM, Anadón P, Julilà R, García-Aguilar JM, Moigne AM, Espigares MP, Ros-Montoya S, Palmqvist P (2013) The oldest human fossil in Europe, from Orce (Spain). J Hum Evol 65:1–9
Toth N, Schick K (2019) Why did the Acheulean happen? Experimental studies into the manufacture and function of Acheulean artifacts. L’anthropologie 123:724–768
Treves A, Naughton-Treeves L (1999) Risk and opportunity for humans coexisting with large carnivores. J Hum Evol 36:275–282
Treves A, Palmqvist P (2007) Reconstructing hominin interactions with mammalian carnivores (6.0-1.8 Ma). In: Gursky SL, KAI N (eds) Primate anti-predator strategies, Developments in Primatology Series: Progress and Prospects. Springer, New York, pp 355–381
Turner A (1992) Large carnivores and earliest European hominids: changing determinants of resource availability during the lower and middle Pleistocene. J Hum Evol 22:109–126
Turner A, Antón M (1996) The giant hyena, Pachycrocuta brevirostris (Mammalia, Carnivora, Hyaenidae). Geobios 29:455–468
Turner A, Antón AC, Werdelin L (2008) Taxonomy and evolutionary patterns in the fossil hyaenidae of Europe. Geobios 41:677–687
Turq A, Martínez-Navarro B, Palmqvist P, Arribas A, Agustí J, Rodríguez-Vidal J (1996) Le Plio-Pléistocène de la région d’Orce, province de Grenada, Espagne: Bilan et perspectivas de Recherche. Paléo Rev Archéol Préhistorique 8:161–204
Van Valkenburgh B (2001) Predation in sabre-tooth cats. In: Briggs DEG, Crowther PR (eds) Paleobiology II. Blackwell, Oxford, pp 420–423
Van Valkenburgh B (2007) Déjà vu: the evolution of feeding morphologies in the Carnivora. Integr Comp Biol 47:147–163
Van Valkenburgh B (2009) Costs of carnivory: tooth fracture in Pleistocene and Recent carnivorans. Biol J Linn Soc 96:68–81
Van Valkenburgh B, Hertel F (1993) Tough times at La Brea: tooth breakage in large carnivores of the late Pleistocene. Science 261:456–459
Van Valkenburgh B, Ruff CB (1987) Canine tooth strength and killing behavior in large carnivores. J Zool 212:379–397
Van Valkenburgh B, Teaford MF, Walker A (1990) Molar microwear and diet in large carnivores: inferences concerning diet in the sabretooth cat, Smilodon fatalis. J Zool 222:319–340
Vizcaíno SF, Fariña RA, Zárate MA, Bargo MS, Schultzd P (2004) Palaeoecological implications of the mid-Pliocene faunal turnover in the Pampean Region (Argentina). Palaeogeogr Palaeoclimatol Palaeoecol 213:101–113
Vizcaíno SF, Bargo MS, Kay RF, Fariña RA, Giacomo MD, Perry JMG, Prevosti FJ, Toledo N, Cassini GH, Fernicola JC (2010) A baseline paleoecological study for the Santa Cruz Formation (late–early Miocene) at the Atlantic coast of Patagonia, Argentina. Palaeogeogr Palaeoclimatol Palaeoecol 292(3-4):507–519
White PA, Diedrich CG (2012) Taphonomy story of a modern African elephant Loxodonta africana carcass on a lakeshore in Zambia (Africa). Quat Int 276:287–296
Yravedra J, Solano JA, Courtenay LA, Saarinen J, Linares-Matás G, Luzón C, Serrano-Ramos A, Herranz-Rodrigo D, Cámara JM, Ruiz A, Titton S, Rodríguez-Alba JJ, Mielgo C, Blain H-A, Agustí J, Sánchez-Bandera C, Montilla E, Toro-Moyano I, Fortelius M et al (2021) Use of meat resources in the Early Pleistocene assemblages from Fuente Nueva 3 (Orce, Granada, Spain). Archaeol Anthropol Sci 13:213. https://doi.org/10.1007/s12520-021-01461-7
Acknowledgements
The “Consejería de Cultura y Patrimonio Histórico” of Grenade provided the permission to study the fossil collections from Fuente Nueva 3, Venta Micena and other sites of Orce (ref. BC.03.174/19). G. Rodríguez-Gómez is granted by an “Atracción de Talento” postdoctoral contract (2019-T2/HUM-13370) from “Comunidad de Madrid/Universidad Complutense,” intended for developing the project “Estudios paleoecológicos en los yacimientos de Orce y de la sierra de Atapuerca.” A. Granados enjoys a FPI predoctoral grant from the Spanish Ministry of Science, Innovation and University. We gratefully acknowledge the insightful comments and suggestions provided by two anonymous reviewers and by Associate Editor Sireen ElZaatari. Last but not least, Prof. Christine M. Janis and Dr. Robert Walsh revised thoroughly manuscript wording and style. BMN is funded by the Generalitat de Catalunya (2021 SGR 01238).
Funding
Funding for open access publishing: Universidad Málaga/CBUA This work has been granted by projects CGL-2016-78577-P, CGL-2016-80975-P, and PID2019-111185GB-I00 of the Spanish Ministry of Science, Innovation and University, “Junta de Andalucía” (FEDER) project UMA18-FEDERJA-188, “Generalitat de Catalunya” grant GENCAT 2017SGR 859, and by Research Group RNM-146 from “Junta de Andalucía.”
Author information
Authors and Affiliations
Contributions
P.P., G.R.G., B.F., B.M.N., and J.A.P.C. wrote the paper and all co-authors made contributions. M.P.E. prepared Table 1. A.G.M., S.R.M., J.M.G.A., and A.G. prepared Fig. 1. M.P.E. and S.R.M. prepared Fig. 2. P.P. prepared Fig. 3. B.F. prepared Fig. 4. G.R.G. prepared Figs. 5 and 6.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Palmqvist, P., Rodríguez-Gómez, G., Martínez-Navarro, B. et al. Déjà vu: on the use of meat resources by sabretooth cats, hominins, and hyaenas in the Early Pleistocene site of Fuente Nueva 3 (Guadix-Baza Depression, SE Spain). Archaeol Anthropol Sci 15, 17 (2023). https://doi.org/10.1007/s12520-022-01712-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12520-022-01712-1