Abstract
Background
Off-label and unlicensed prescriptions pose a severe safety concern among the pediatric population. We aimed to summarize the up-to-date evidence on the extent, reasons, and consequences of off-label and unlicensed drugs in hospitalized pediatric patients.
Methods
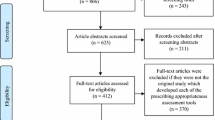
We systematically searched PubMed, EMBASE, SCOPUS, Web of Science and Google Scholar between 1990 and 2020 in which the last search was conducted on 12 February 2021. We included studies with the following inclusion criteria: (1) observational studies in design; (2) target population was hospitalized pediatric patients whether admitted in the intensive care unit or in the general ward; (3) study reporting the prevalence of off-label, unlicensed prescriptions or both; and (4) published in English.
Results
A total of 47 studies were eligible for inclusion. The proportion of off-label and unlicensed prescriptions ranged from 7.4% to 99.5% and 0.1% to 74.4%, respectively. The most frequent category of off-label prescriptions was prescription outside the age range, with the most commonly reported reason for off-label prescriptions being the lack of information specifically for pediatrics on the drug information leaflets. The consequences of off-label and unlicensed prescriptions ranged from minor and bearable skin reactions to debilitating renal failure, risking deaths.
Conclusions
Off-label and unlicensed prescriptions are extensive and require progressively meditative interventions. However, the pediatric population is currently a “therapeutic orphan”. Unless adequate pediatric clinical trials and licensed drugs become available, off-label and unlicensed drug prescription should not entirely be banned but rather promoted in an organized manner.



Similar content being viewed by others
References
Leandro JA. “Risk-free rest and sleep:” Jornal do Médico (Portugal) and the thalidomide disaster, 1960–1962. Hist Cienc Saude Manguinhos. 2020;27:15–32 (in Portuguese, English).
Botting J. The history of thalidomide. Drug News Perspect. 2002;15:604–11.
Chen J, Luo X, Qiu H, Mackey V, Sun L, Ouyang X. Drug discovery and drug marketing with the critical roles of modern administration. Am J Transl Res. 2018;10:4302–12.
Michelet R, Bocxlaer JV, Vermeulen A. PBPK in preterm and term neonates: a review. Curr Pharm Des. 2017;23:5943–54.
Thompson G, Barker CI, Folgori L, Bielicki JA, Bradley JS, Lutsar I, et al. Global shortage of neonatal and paediatric antibiotic trials: rapid review. BMJ Open. 2017;7:e016293.
Tsukamoto K, Carroll KA, Onishi T, Matsumaru N, Brasseur D, Nakamura H. Improvement of pediatric drug development: regulatory and practical frameworks. Clin Ther. 2016;38:574–81.
Coppini R, Simons SHP, Mugelli A, Allegaert K. Clinical research in neonates and infants: challenges and perspectives. Pharmacol Res. 2016;108:80–7.
Oni L, Hawcutt DB, Turner MA, Beresford MW, McWilliam S, Barton C, et al. Optimising the use of medicines to reduce acute kidney injury in children and babies. Pharmacol Ther. 2017;174:55–62.
Dornelles AD, Calegari LH, de Souza L, Ebone P, Tonelli TS, Carvalho CG. The unlicensed and off-label prescription of medications in general paediatric ward: an observational study. Curr Pediatr Rev. 2019;15:62–6.
Kouti L, Aletayeb M, Aletayeb SMH, Hardani AK, Eslami K. Pattern and extent of off-label and unlicensed drug use in neonatal intensive care units in Iran. BMC Pediatr. 2019;19:3.
Landwehr C, Richardson J, Bint L, Parsons R, Sunderland B, Czarniak P. Cross-sectional survey of off-label and unlicensed prescribing for inpatients at a paediatric teaching hospital in Western Australia. PLoS One. 2019;14:e0210237.
Gore R, Chugh PK, Tripathi CD, Lhamo Y, Gautam S. Pediatric off-label and unlicensed drug use and its implications. Curr Clin Pharmacol. 2017;12:18–25.
Aronson JK, Ferner RE. Unlicensed and off-label uses of medicines: definitions and clarification of terminology. Br J Clin Pharmacol. 2017;83:2615–25.
Moulis F, Durrieu G, Lapeyre-Mestre M. Off-label and unlicensed drug use in children population. Therapie. 2018;73:135–49.
Tukayo BLA, Sunderland B, Parsons R, Czarniak P. High prevalence of off-label and unlicensed paediatric prescribing in a hospital in Indonesia during the period Aug.-Oct. 2014. PLoS One. 2020;15:e0227687.
‘t Jong GW, van der Linden PD, Bakker EM, van der Lely N, Eland IA, Stricker BHC, et al. Unlicensed and off-label drug use in a paediatric ward of a general hospital in the Netherlands. Eur J Clin Pharmacol. 2002;58:293–7.
Bavdekar SB, Sadawarte PA, Gogtay NJ, Jain SS, Jadhav S. Off-label drug use in a pediatric intensive care unit. Indian J Pediatr. 2009;76:1113–8.
Sucasas Alonso A, Avila-Alvarez A, Combarro Eiriz M, Martínez Roca C, Yáñez Gómez P, Codias López A, et al. Use of off-label drugs in neonatal intensive care. An Pediatr (Barc). 2019;91:237–43 (in Spanish).
Costa HTML, Costa TX, Martins RR, Oliveira AG. Use of off-label and unlicensed medicines in neonatal intensive care. PLoS One. 2018;13:e0204427.
Lee JH, Byon HJ, Choi S, Jang YE, Kim EH, Kim JT, et al. Safety and efficacy of off-label and unlicensed medicines in children. J Korean Med Sci. 2018;33:e227.
Aamir M, Khan JA, Shakeel F, Shareef R, Shah N. Drug utilization in neonatal setting of Pakistan: focus on unlicensed and off label drug prescribing. BMC Pediatr. 2018;18:242.
Nir-Neuman H, Abu-Kishk I, Toledano M, Heyman E, Ziv-Baran T, Berkovitch M. Unlicensed and off-label medication use in pediatric and neonatal intensive care units: no change over a decade. Adv Ther. 2018;35:1122–32.
Mazhar F, Akram S, Haider N, Hadi MA, Sultana J. Off-label and unlicensed drug use in hospitalized newborns in a Saudi tertiary care hospital: a cohort study. Int J Clin Pharm. 2018;40:700–3.
Zhu XQ, Hu JQ, Sun B, Deng SH, Wen YG, Chen WJ, et al. Comparison of unlicensed and off-label use of antipsychotics prescribed to child and adolescent psychiatric outpatients for treatment of mental and behavioral disorders with different guidelines: the China food and drug administration versus the FDA. J Child Adolesc Psychopharmacol. 2018;28:216–24.
Di Paolo ER, Stoetter H, Cotting J, Frey P, Gehri M, Beck-Popovic M, et al. Unlicensed and off-label drug use in a Swiss paediatric university hospital. Swiss Med Wkly. 2006;136:218–22.
Aamir M, Khan JA, Shakeel F, Asim SM. Unlicensed and off-label use of drugs in pediatric surgical units at tertiary care hospitals of Pakistan. Int J Clin Pharm. 2017;39:860–6.
‘t Jong GW, Vulto AG, de Hoog M, Schimmel KJ, Tibboel D, van den Anker JN. A survey of the use of off-label and unlicensed drugs in a Dutch children’s hospital. Pediatrics. 2001;108:1089–93.
Arocas Casañ V, Cabezuelo Escribano B, Garrido-Corro B, De la Cruz MP, Blázquez Álvarez MJ, De la Rubia Nieto MA. Off-label and unlicensed drug use in a Spanish neonatal intensive care unit. Farm Hosp. 2017;41:371–81.
Teigen A, Wang S, Truong BT, Bjerknes K. Off-label and unlicensed medicines to hospitalised children in Norway. J Pharm Pharmacol. 2017;69:432–8.
Santos DB, Clavenna A, Bonati M, Coelho HL. Off-label and unlicensed drug utilization in hospitalized children in Fortaleza. Brazil Eur J Clin Pharmacol. 2008;64:1111–8.
García-López I, Fuentes-Ríos JE, Manrique-Rodríguez S, Fernández-Llamazares CM. Off-label and unlicensed drug use: results from a pilot study in a pediatric intensive care unit. An Pediatr (Barc). 2017;86:28–36 (in Spanish).
de Souza AS Jr, Dos Santos DB, Rey LC, Medeiros MG, Vieira MG, Coelho HLL. Off-label use and harmful potential of drugs in a NICU in Brazil: a descriptive study. BMC Pediatr. 2016;16:13.
Cuzzolin L, Agostino R. Off-label and unlicensed drug treatments in neonatal intensive care units: an Italian multicentre study. Eur J Clin Pharmacol. 2016;72:117–23.
Ellul IC, Grech V, Attard-Montalto S. Paediatric off-label and unlicensed prescribing in primary care in Malta: prospective observational drug utilisation study. Int J Risk Saf Med. 2015;27:123–34.
Jobanputra N, Save SU, Bavdekar SB. Off-label and unlicensed drug use in children admitted to pediatric intensive care units (PICU). Int J Risk Saf Med. 2015;27:113–21.
Bajcetic M, Jelisavcic M, Mitrovic J, Divac N, Simeunovic S, Samardzic R, et al. Off label and unlicensed drugs use in paediatric cardiology. Eur J Clin Pharmacol. 2005;61:775–9.
Joret-Descout P, Prot-Labarthe S, Brion F, Bataille J, Hartmann JF, Bourdon O. Off-label and unlicensed utilisation of medicines in a French paediatric hospital. Int J Clin Pharm. 2015;37:1222–7.
Schweigertova J, Durisova A, Dolnikova D, Ondriasova E, Balazova M, Slezakova V, et al. Off-label and unlicensed use of medicinal products in the neonatal setting in the Slovak Republic. Pediatr Int. 2016;58:126–31.
Czarniak P, Bint L, Favié L, Parsons R, Hughes J, Sunderland B. Clinical setting influences off-label and unlicensed prescribing in a paediatric teaching hospital. PLoS One. 2015;10:e0120630.
Riou S, Plaisant F, Maucort Boulch D, Kassai B, Claris O, Nguyen KA. Unlicensed and off-label drug use: a prospective study in French NICU. Acta Paediatr. 2015;104:e228–31.
Palmaro A, Bissuel R, Renaud N, Durrieu G, Escourrou B, Oustric S, et al. Off-label prescribing in pediatric outpatients. Pediatrics. 2015;135:49–58.
Mohamad NF, Mhd Ali A, Mohamed SN. Respiratory drugs prescribed off-label among children in the outpatient clinics of a hospital in Malaysia. Int J Clin Pharm. 2015;37:127–32.
Langerová P, Vrtal J, Urbánek K. Incidence of unlicensed and off-label prescription in children. Ital J Pediatr. 2014;40:12.
Kieran EA, O’Callaghan N, O’Donnell CPF. Unlicensed and off-label drug use in an Irish neonatal intensive care unit: a prospective cohort study. Acta Paediatr. 2014;103:e139–42.
Lindell-Osuagwu L, Hakkarainen M, Sepponen K, Vainio K, Naaranlahti T, Kokki H. Prescribing for off-label use and unauthorized medicines in three paediatric wards in Finland, the status before and after the European Union paediatric regulation. J Clin Pharm Ther. 2014;39:144–53.
Lee JL, Redzuan AM, Shah NM. Unlicensed and off-label use of medicines in children admitted to the intensive care units of a hospital in Malaysia. Int J Clin Pharm. 2013;35:1025–9.
Laforgia N, Nuccio MM, Schettini F, Dell’Aera M, Gasbarro AR, Dell’Erba A, et al. Off-label and unlicensed drug use among neonatal intensive care units in Southern Italy. Pediatr Int. 2014;56:57–9.
Kimland E, Nydert P, Odlind V, Böttiger Y, Lindemalm S. Paediatric drug use with focus on off-label prescriptions at Swedish hospitals-a nationwide study. Acta Paediatr. 2012;101:772–8.
Ferreira Lde A, Ibiapina Cda C, Machado MG, Fagundes ED. High prevalence of off-label and unlicensed drug prescribing in a Brazilian intensive care unit. Rev Assoc Med Bras. 1992;2012:82–7.
Palčevski G, Skočibušić N, Vlahović-Palčevski V. Unlicensed and off-label drug use in hospitalized children in Croatia: a cross-sectional survey. Eur J Clin Pharmacol. 2012;68:1073–7.
Oguz SS, Kanmaz HG, Dilmen U. Off-label and unlicensed drug use in neonatal intensive care units in Turkey: the old-inn study. Int J Clin Pharm. 2012;34:136–41.
Dos Santos L, Heineck I. Drug utilization study in pediatric prescriptions of a university hospital in southern Brazil: off-label, unlicensed and high-alert medications. Farm Hosp. 2012;36:180–6.
Lass J, Käär R, Jõgi K, Varendi H, Metsvaht T, Lutsar I. Drug utilisation pattern and off-label use of medicines in Estonian neonatal units. Eur J Clin Pharmacol. 2011;67:1263–71.
Khdour MR, Hallak HO, Alayasa KS, AlShahed QN, Hawwa AF, McElnay JC. Extent and nature of unlicensed and off-label medicine use in hospitalised children in Palestine. Int J Clin Pharm. 2011;33:650–5.
Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. European network for drug investigation in children. BMJ. 2000;320:79–82.
van den Berg H, Tak N. Licensing and labelling of drugs in a paediatric oncology ward. Br J Clin Pharmacol. 2011;72:474–81.
Lass J, Irs A, Pisarev H, Leinemann T, Lutsar I. Off label use of prescription medicines in children in outpatient setting in Estonia is common. Pharmacoepidemiol Drug Saf. 2011;20:474–81.
Nguyen KA, Claris O, Kassai B. Unlicensed and off-label drug use in a neonatal unit in France. Acta Paediatr. 2011;100:615–7.
WHO. The anatomical therapeutic chemical classification system with defined daily doses (ATC/DDD). https://www.who.int/classifications/atcddd/en/. Accessed 24 Apr 2020.
van Riet-Nales DA, Schobben AFAM, Egberts TCG, Rademaker CMA. Effects of the pharmaceutical technologic aspects of oral pediatric drugs on patient-related outcomes: a systematic literature review. Clin Ther. 2010;32:924–38.
WHO. Medicines regulatory suppor. https://www.who.int/medicines/areas/quality_safety/regulation_legislation/en/. Accessed 31 Jul 2020.
Magalhães J, Rodrigues AT, Roque F, Figueiras A, Falcão A, Herdeiro MT. Use of off-label and unlicenced drugs in hospitalised paediatric patients: a systematic review. Eur J Clin Pharmacol. 2015;71:1–13.
Balan S, Hassali MAA, Mak VSL. Two decades of off-label prescribing in children: a literature review. World J Pediatr. 2018;14:528–40.
Carvalho CG, Ribeiro MR, Bonilha MM, Fernandes M Jr, Procianoy RS, Silveira RC. Use of off-label and unlicensed drugs in the neonatal intensive care unit and its association with severity scores. J Pediatr (Rio J). 2012;88:465–70.
Wilson JT. An update on the therapeutic orphan. Pediatrics. 1999;104:585–90.
Acknowledgements
We acknowledge assistance in the PubMed search, from Dr. Joel Swai (ORCID: 0000-0001-5363-3977); Department of Nephrology and Rheumatology, The Third Xiangya Hospital of Central South University, Changsha, China.
Funding
None.
Author information
Authors and Affiliations
Contributions
SW contributed to study designing, data search, data extraction, and manuscript drafting. WXY contributed to data search, data extraction, and manuscript drafting. XF contributed to data extraction and manuscript drafting. All authors revised the intellectual content of the manuscript critically, and read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not needed.
Conflict of interest
The authors have no conflict of interest to declare.
Data availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shuib, W., Wu, XY. & Xiao, F. Extent, reasons and consequences of off-labeled and unlicensed drug prescription in hospitalized children: a narrative review. World J Pediatr 17, 341–354 (2021). https://doi.org/10.1007/s12519-021-00430-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-021-00430-3