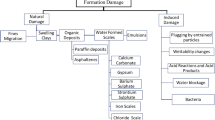
Abstract
Oil and gas recovery may cause formation damage during drilling, completion, and production phases. As a result of fundamental chemical, thermal, mechanical, and biological interactions, formation damage can occur due to impairment of permeability and porosity, causing undesirable operational and economic problem. The fluid-rock interactions resulting from oilfield chemicals injection during drilling, enhanced oil recovery (EOR) such as chemical flooding, or formation treatment could negatively impact on the formation properties such as geomechanical and geochemical, leading to alteration of the rock’s petrophysical properties. These chemical-rock interactions induce changes in both pore space geometry and rock strength. The resultant impact includes weakening of the formation bonding materials, formation damage, reduced production and consequently sand production simultaneously with reservoir fluids. It is therefore critical to evaluate these variables prior to designing any geo-sequestration, reservoir stimulation or EOR projects. Studies have shown that rock properties, especially permeability, porosity and strength, are altered or damaged during drilling, cementing, perforating, producing, stimulating, and injecting water or chemicals for EOR. Petroleum companies are likely to suffer significant financial losses due to this. This study provides a review on the influence of oilfield chemical-formation interactions on the formation rock properties both geophysical and mechanical, leading to formation damage and sand production. This study aims to provide researchers with a single document that gives insight and new perspectives on oilfield chemical-rock interactions through compilation of recent studies relating to the effect of chemical-rock interactions on rock's petrophysical properties, as well as geomechanical properties due to geochemical reactions that cause formation damage and eventually sand production. Having a solid understanding of fluid-rock interactions and how they impact petrophysical properties and cause formation damage is essential in predicting sand production and would help in minimizing economic losses, downtime and technicalities.
Similar content being viewed by others
Introduction
Petroleum has since the mid-1950s become a major source of energy to the world (UKOG 2015). As the world’s primary source of energy, it plays a major and influential role in global economy (Burclaff 2020). Petroleum, which is a naturally occurring hydrocarbon, can be found buried deep in sedimentary rocks (Priscilla 2021). To access the hydrocarbon (mainly petroleum), the sedimentary rock otherwise known as a reservoir rock has to be drilled (Alyafei 2019). However, in drilling the reservoir rock, stress is exerted on the formation, which may cause it to fail once stress exerted on the reservoir rock surpasses the reservoir rock strength (Oyeneyin et al. 2005). In addition to drilling and completion, damage to formations can result from reservoir rock-fluid interactions during reservoir stimulation or enhanced oil recovery (EOR) operations. Hence, predicting petrophysical properties of reservoir formation rocks is important for petroleum exploration, development, and production (Yang et al. 2017a, 2017b, 2017c). It is therefore obvious that the mechanism of reservoir rock failure can be categorized as either chemically, mechanically, or operationally induced (Abass et al. 2002; Zhou and Sun 2016).
Figure 1 shows a graphical process for fluid injection into an injection well for the purpose of enhancing oil production. Fluid injected into the reservoir interacts with the formation rock and impacts on the geochemical and geomehcanical properties of the rock resulting in the weakening of grain particles and consequently the production of sand while producing reservoir fluids.
This review focuses on formation damage caused by oilfield chemical-rock interaction and their impacts on formation properties and the potential for sanding. Fluid-rock interactions resulting from carbon dioxide (CO2) geo-sequestration, EOR fluids, and reservoir stimulation projects are impacted by the nature of formation rocks, the fluid type and properties, and the evolution of petrophysical properties of the formation under in situ conditions. The chemicals used for these oilfield operations include biocides (Wuyep et al. 2018), surfactants, scale inhibitors (Tomson et al. 2008; Oluyemi 2014a, 2014b), scavengers, demulsifiers, and defoamers.
The mobility and distribution of these chemicals in the reservoir depend on relative permeability and the porosity of the formation rock. Mineral dissolution and precipitation processes, as well as fine particle migration caused by fluid-rock interactions, are known to cause rock porosity, permeability, and strength alterations. Furthermore, oil production from a reservoir is strongly influenced by the permeability near the wellbore; therefore, formation damage caused by fluid-rock interactions may reduce productivity significantly (Faegestad 2016). Reservoirs formation experiences numerous fluid-rock interactions; thus, it is crucial to identify the specific geochemical reactions and processes governing the evolution of porosity and permeability (Yang et al. 2017a, 2017b, 2017c). Investigations have shown that various chemicals would have different properties, which would have varying influences on the strength of the reservoir formation when they are injected (Wuyep et al. 2020). Oluyemi 2014a, 2014b) experimentally investigated the sanding potential of using scale inhibitors (PTEMP) on a clashach reservoir rock under dynamic condition. It was discovered that detached sand grains were found in the flowing stream. This suggests that fluid-rock interactions can potentially weaken formation bond resulting in sand production with the reservoir fluids. More recently, Wuyep et al. (2018) studied the physical and chemical impacts of oilfield chemical and rock interface on reservoir rock strength using biocide to control and treat microorganisms such as sulfate reducing bacteria (SRB). The results of the work showed that chemical dissolution/precipitation reaction occurred, and rock fine particles were transported resulting in the weakening of the formation strength. SRB is widely dispersed in the oilfield waters and their activities is the cause of reservoir souring. The activities of microorganisms in oilfield reservoirs and sulfide accumulation due to bacterial sulfate reduction can cause serious damage such as H2S production and formation plugging (Larsen 2002). Not only does H2S cause damage to the reservoir, it also presents life-threatening danger to oil industry workers when exposed it. Its corrosiveness also has a major negative effect on subsea infrastructures, thereby increasing maintenance cost and increasing environmental disaster in relation to oil leakages and spills (Hubert 2017). The injection of biocides for oilwell treatment as used by Wuyep et al. (2018) is the most common method for reservoir treatment (Gieg et al. 2011). But these chemicals are unsafe to both the environment and oilfield workers. However, another approach that is used in controlling the actions of sulfate reducing bacteria is the application of nitrates, which is able to display diverse influence on the formation (Davidova et al. 2001). Most research on nitrate usage has focused on oil recovery (Hitzman and Sperl 1994; Sandbeck and Hitzman 1995; Giangiacomo and Dennis 1997; Zhao et al. 2016) and reservoir souring control and treatment (Reinsel et al. 1996; Telang et al. 1998; Sunde et al. 2004; Greene et al. 2006; Grigoryan et al. 2008; Hubert 2010; Voordou et al. 2011; Tabari et al. 2011).
There are three kinds of chemical damage mechanisms: fluid-rock interactions, fluid-fluid interactions, and wettability alteration (Faegestad 2016). Therefore, another major concern regarding formation damage is wettability alteration. In addition to determining the capillary pressure, wettability also determines whether the pressure is a barrier to fluid flow or a driving force for production (Deglint 2018). Hydrocarbon recovery from rock matrix by imbibition has been greatly facilitated with the use of surfactants as wettability modifiers (Yang et al. 2021). Consequently, surfactants are frequently used in carbonate reservoirs to convert oil-wetted to water-wetted wettability to enhance spontaneous imbibition. In this scenario, the pore geometry and wettability-related fluid−formation matrix interactions, as a result of mechanisms for wettability alteration, can induce changes in porosity, pore connectivity, permeability, and flow. Pal et al. (2018) published a detailed review on surfactant-based chemical EOR techniques applied in carbonate formations. Although chemical-EOR is a typical chemical-rock interaction, the focus of the review was on surfactant-driven oil recovery mechanisms, like altering wettability, reducing interfacial tension, understanding microemulsion phase behavior, analyzing surfactant adsorption and mitigation, and using foams. It is important to note that oilfield chemicals injected into the formation is absorbed and upon interaction with the rock, poses a weakening influence formation rock strength (Wuyep et al. 2019). Injecting oilfield chemicals into reservoir rocks is to improve oil and gas production, clean formations that are blocked, re-engineer formation damage, improve oil flow assurance, and prevent scale formation and corrosion can be harmful to the rocks petrophysical and geomechanical properties. Investigation by Oluyemi (2014a, 2014b) revealed that the geomechanical and geophysical impact of oilfield chemicals on the rock properties could lead to sand production, which has negative cost implication in the petroleum industry. In the petroleum industry, sanding negatively impacts flow lines and separators as a result of rock and fines plugging perforated zones (Abass et al. 2002; Willson et al. 2002). This undesirable effect also increase cost of operations (Rahmati et al. 2012; Wang et al. 2016), credited to pipeline corrosion and other surface facilities, a decrease in production rate and expensive intervention measures (Tronvoll and Fjaer 1994; Rahmati et al. 2012 ). The corrosion of well integrity and well equipment’s have an increasing and negative implications which includes wellbore failure and a rise in disposal costs and downtime (Penberthy and Jr Shaughnessy 1992; Eshiet 2012 ). The implication of producing sand is also unfavorable to the environment. For instance, the tear and wear of surface and downhole equipment, as a result of the extremely corrosive nature of fluids carrying sand, may be sufficiently adverse; it could lead to total collapse of surface and downhole facilities, resulting to severe health, safety, and environmental problems (Penberthy and Jr Shaughnessy 1992).
Geo-sequestration of CO2, injected fluid for EOR, reservoir acidizing, and reservoir stimulation projects demand an analytical assessment of rock properties, fluid properties, and the evolution of rock petrophysical properties as a result of fluid-formation interactions. For instance, the injected and in situ–produced solutes will interact chemically and physically with the storage formation rock during many underground carbon dioxide (CO2) geologic carbon sequestration. The extent of the chemical-formation interactions depends on the concentration of the propensity minerals such as carbonates and clays in the pores of the formation rock (Saeedi et al. 2016). It has been found that the injection of CO2 into a brine saturated reservoir resulted in carbonic acid formation, which in turn, react with the minerals constituting the rock’s porous framework, resulting in mineral dissolution and/or precipitation (Saeedi et al. 2016; Zhao and Yin 2021). These chemical reactions change the petrophysical properties of the formation rock. Several studies have been conducted on the influence of fluid and rock interfaces on reservoir formation properties. Yang et al. (2017a, 2017b, 2017c) investigated the transformation of reservoir formation properties of sandstone reservoir core samples, and they discovered that the alterations of the properties of the formation was due to the dissolution of feldspar, calcite, and other cement bonding minerals assembly present within the mineral content of the sandstone when reacting with brine and ethanoic acid. In a series of studies reported by Kan et al. (2005), Tomson et al. (2008), Bybee (2010), Egermann and Vizika (2011), Mohamed and Nasr-El-Din (2013), Oluyemi (2014a, 2014b), Wilson (2016), and Wuyep et al. (2019), they found that the application of oilfield chemicals resulted in changes in the formation rocks geomechanical properties and geophysical properties, respectively. On the other hand, high-temperature operations, such as steam injection and in situ combustion to mobilize oil for production, can lead to the development of thermal damage mechanisms. Minerals can also dissolve and transform at elevated temperatures when they are catalyzed and transformed from nonreactive clays to reactive products, which can swell, merge, and reduce permeability (Faegestad 2016). Also, the introduction of bacteria and nutrients into the formation can induce biological damage. Thermal gradient and pH influences dissolution/precipitation of minerals which modify the rock’s porosity and permeability (Fritz et al. 2010). The mineralogy, pore geometry, thermal maturation, and wettability of the formation are factors that are thought to control fluid flow in unconventional reservoirs (Wang et al. 2021). One of the established methods for studying fluid-rock interactions is core flooding, which is a powerful approach for elucidating fluid-formation interactions. Thereafter, imaging techniques such as scanning electron microscopy (SEM) and X-ray diffraction can be used to quantify fluid-rock interaction and characterize petrophysical properties due to dissolution/precipitation, fracturing, and fine particles migration (Deglint 2018). One of the major challenges is the scale-dependence of petrophysical and geomechanical properties of rocks. The imaging of the micro- and nano-scale should be the way forward in order to detect fluid-rock interactions at the micro- and nano-pore level, so that the distribution and flow of multi-phase fluids can be quantified at this scale. This is particularly important since the dominant pore and pore throat sizes in reservoir rocks are in the meso- and micropore range. However, the prediction of the possible evolution of petrophysics of rocks would involve geochemical modeling of fluid-rock interactions resulting in dissolution and precipitation of minerals, which would influence the mineralogy and petrography of the rocks (Fritz et al. 2010). Another approach would be a combination of experimental and analytical techniques to evaluate impacts of fluid-rock interactions on formation petrophysical properties.
Therefore, evaluating the thermos-chemo-mechanical processes resulting from fluid-formation interactions and their impact on the petrophysical properties of the rock during production is of critical importance (Faegestad 2016). This reactive transport through oil-formation causes rock microstructural properties evolution, such as porosity and permeability as a result of the flow and chemical reactions. This information can be used to formulate chemical and simulation studies, rock evolution models, and formation failure predictions. It is crucial to understand the evolution of formation petrophysical properties because the changes affect reservoir quality. An integrated model combining kinetic rate laws for minerals dissolution/precipitation, particle migration, and fluid flow is needed to predict the geochemical evolution of the fluid interactions and also sanding. It is therefore necessary to fully understand the performance of the reservoir formation and the tendency to produce sand or rock failure upon interaction with oilfield chemicals, in order to avoid or minimize losses resulting from negative impacts of oilfield chemicals on the petrophysical and geomechanical properties of the formation. Therefore, this paper reviews previous studies on oilfield chemicals and reservoir rocks interaction and their impact on reservoir petrophysical and geomechanical properties, analyzing and evaluating findings relevant to developing new perspectives for further investigation. In this review, we examine the effects of chemical injection on petrophysical and geomechanical properties of rock, geochemical reactions such as mineral dissolution and precipitation, and interactions between chemicals and rock, which result in formation damage and sanding. It is the aim of this review to provide insight into oilfield chemical-rock interactions, and to offer new perspectives on its impact on petrophysical and geomechanical properties of rocks by critical evaluating recent studies on the subject in order to guide future research.
Effects of oilfield chemical on petrophysical properties
It is well known that injected fluids interact with rock minerals and/or formation fluids and generate fine particles in addition to dissolution/precipitation which can cause damage to the formation, either from fluids in the reservoir or generated in situ as result of fluid-rock interactions. These processes induce properties changes to the rock permeability, porosity, and strength, which are considered to be very important factors for reservoir rock petrophysical properties (Ahmad et al. 2018), indicating that it can be adversely affected by geochemical and geomechanical phenomenon. Crawford (2011) developed a model which relates petrophysical and petrographic properties to rock strength. This suggests it is plausible to predict and estimate mechanical properties from geophysical properties of the rocks due to fluid-rock interactions. As a consequence, formation damage and onset of sanding can be predicted also. Using rock properties to calculate onset collapse of a pore can be helpful approximation to predicting onset collapse of the pore and changes in compressibility of the reservoir formation with the reduction in pressure of the fluid at uniaxial strain boundary settings. A review of selected approaches and models that have been developed for sanding prediction has been published (Rahmati et al. 2013). The majority of these models are built on a continuum assumption, but a few have recently been developed on the basis of discrete element models. The analytical models are commonly used for estimation of the onset of sanding, while numerical models are suitable for predicting sanding rate. Overall, a realistic sanding model should include all failure mechanisms (shear, tensile, compression), as well as fluid flow effects. Discrete element method (DEM) is another valuable tool to simulate sand production specially to understand the mechanism of sanding, but it cannot be used for large-scale problems because of large computational time required and difficulty in the calibration of model (Rahmati et al. 2013). In the investigation as described by Hill et al. (2018), data on the formations geomechanical and petrophysical properties like the rock mineralogy and grain size distribution were combined to explain the impact of fluid-rock interaction, the formation stability and the prospect of sand failure and its production. The pressure gradient developed in the pore channels due to fluid flow also facilitates the detachment of sand grains. Additionally, the flow of fluid leads to the transport and production of detached sand aggregates, as well as disaggregated sand grains. Therefore, sand management and modeling are always cost-effective if they are implemented early before a well is drilled. This complex phenomenon depends on various factors such as the stress distribution, the properties of the rock, and fluid-rock interactions in the reservoir (Rahmati et al. 2013). The fluid flow is mostly modeled using the Navier-Stokes equations solved by a finite volume method. However, it can be challenging to account for all these factors and mechanisms in the numerical models, resulting in limitations on their applicability.
Pore volume, porosity, and permeability
Petroleum reservoirs which are made of sedimentary rocks usually have porosity with ranges of 10–40% in sandstone formations and between 5 and 25% in carbonate formations. Sand production is a common occurrence in weakly consolidated sandstone reservoirs, which host a substantial portion of the world’s oil and gas reserves (Rahmati et al. 2013). The strength-weakening effect of fluid-rock interactions may gradually lead to sandstone reservoir formation degradation. Additionally, rock porosity and permeability evolution influence chemical distribution and mobility in the reservoir. Porosity, as shown in Fig. 2, determines the rocks storing capacity which is described as the ratio of its void spaces, generally termed pore volume, to bulk volume and is described either as a percentage or a fraction (Coneybeare 1967; Keelan 1982).
A graphic view of a porous media (Liou 2005)
The study of fluid-rock interaction requires an understanding of transport and geochemical kinetics. Presently, reactive transport is faced with the most challenging problem of how to treat cases where the petrophysical properties of the porous reservoir formation change due to geochemical reactions (Steefel and Mäher 2009). The most frequently identified evolution in petrophysical properties are porosity and permeability which are induced by chemical reactions such as dissolution or precipitation. The modeling approach involves coupled flow and chemical reaction. In reactive transport simulations, porosity is typically the first-order parameter predicted, as it is directly related to the total volume of minerals precipitated or dissolved (Steefel and Mäher 2009). Since the detailed pore geometry is required, it is more difficult to predict transport parameters, in particular permeability and diffusivity. It is possible to circumvent this problem by combining modeling and microscopic characterization of the porous reservoir formation as mineral dissolution or precipitation occurs. The governing advection-dispersion-reaction model is shown in the equation below (equation 1).
The aqueous (homogeneous) reactions, Rr, and the rates of the Nm solid-phase (mineral) reactions, Rm, u is the fluid velocity, D is diffusivity, and stoichiometry given in the coefficients νir and νim.
Currently, reactive transport in porous media is described by three types of models: (1) continuum models, (2) pore-scale models, and (3) multiple continuum or hybrid models involving a combination of scales (Steefel and Mäher 2009). Among the transport processes relevant for the study of chemical-rock interaction are advection, which occurs most commonly due to fluid flow through porous media, molecular diffusion, which is modified by electrochemical migration in the presence of charged species, and hydrodynamic dispersion.
The phenomena of oilfield chemical-rock interaction can actually cause alterations to the formation’s porosity and permeability rock (Xu et al. 2018; Lamy-Chappuis et al. 2014). Therefore, this modifies the rock permeability and porosity characteristics. Also, a scientific model that describes the evolution mechanism of porosity and permeability triggered by several chemical and physical interactions among reservoir rocks and fluids has been investigated by Chang and Civan (1991). There is however not one fit all relationship among permeability and porosity applied to all porous medium. This can be credited to the added complication introduced by precipitation and the release of fine grains which is likely to plug pore throats, swelling of clay and improved compaction (Chang and Civan 1991). Thus, the link between permeability, porosity, and rock strength has been a focus of wide study, soon to be completely understood. Conversely, sedimentary rocks such as carbonate reservoirs are known from studies for their huge commercial hydrocarbon accumulation around the world with altered petrophysical properties, mainly due to precipitation and dissolution of minerals leading to increased porosity. The dissolution/precipitation of chemical specie that leads to changes in porosity depends on parameters like mineral composition, shape of grain, grain size distribution, and pore size (Wuyep et al. 2018). Additionally, Egermann et al. (2010) studied the petrophysical properties as a result of fluid (CO2 injection) and rock interface on the capacity to hold and store carbon dioxide. The rock-CO2 interaction was observed to alter the petrophysical properties such as permeability of the carbonate rock. This occurred as a result of the pore network framework imbalance and the degree of mineral dissolution in the rock. In light of this, many permeability and porosity experimental equations have been reported in literatures. Porosity is primarily associated with tortuosity and a large number of small pores, so dissolution of small sand particles or distinct calcite may have only a small impact on the connectivity of pores, as well as a decrease in tortuosity and/or dissolution at pore throats. As a result, the maximum strength of siltstones, sandstones, and a few limestones will reduce with increased porosity (Friedman 1976; Dyke and Dobereiner 1991; Chang et al. 2006; Vasarhelyi and Van 2006; Atapour and Mortazavi 2018; Zhao et al. 2018; Amiri and Moomivand 2019).
To study rock’s petrophysical properties, the Kozeny-Carman (K-C) equation is usually fitted with experimental data to define the relation between porosity (ϕ) and permeability (κ) in a fluid-rock interface system (Walsh and Brace 1994; Lamy-Chappuis et al. 2014). This advocates power-law relationships among the appropriate pore geometry, such as tortuosity and surface area, hydraulic radius, porosity, and permeability. But Lamy-Chappuis and co-workers (Lamy-Chappuis et al. 2014) validated their experimental data with the use of equation (1), whereas equation 3 as established by Chang and Civan (1991) can be used to evaluate permeability alteration due to formation damage. Hence, equation 4 is the general form of equations (1) and (3).
In the equations, ϕ denotes porosity, k permeability (ϕo and κo represent initial porosity and permeability respectively), n ranges between 3 to 7, the efficiency factor f stated as the unplugged pore throat area fraction (determined stochastically) and the experimental matching parameters are represented as a, b, and c.
Furthermore, Yang et al. (2017a, 2017b, 2017c) investigated sandstone rock and brine interface, examine rock properties during diagenesis, and the results revealed that minerals such as calcite dissolution leads to porosity increase. Using empirical, numerical simulations, petrographic observations, and the ability to restructure the diagenetic process, the authors concluded that reservoir formation petrophysical properties can be predicted. Additionally, Liu et al. (2019) examined the effect shale and water interface on the sensitivity of stress on shale rock rich in organic materials. Shale reservoirs were found to have natural fractures and low permeability based on the study results. However, when the fluid used is water, the chemistry between water and rock is rather different from that between oilfield chemicals and the rock interface, which is the focus of this study. On the other hand, the interaction between iron carbonate and corrosion inhibitors showed that imidazoline can bind to, adsorb to, and develop a film on non-conductive materials such as iron carbonate (FeCO3), resulting in a reduction in porosity and delaying the transportation of reactants to the corrosion surfaces has reported in the literature. This suggests that porosity is a function of the materials strength. In addition, Wuyep et al. (2019) found that a decrease in permeability and porosity suggests a decrease in pore space due to new minerals formation in the carbonate rock when treated with Betaine and Aminotri methylphosphonic acid (ATMP). It is therefore obvious that there is a strong connection between uniaxial strength and the porosity of formation materials, which is a function of the composition and internal structures (Li and Aubertin (2003). In other words, porosity is an important parameter when dealing with uniaxial strength. In a study by Bertaux and Lemanczyk (1987), the behavior of sandstone reservoir rock treated with potassium hydroxide and sodium hydroxide (KOH and NaOH) was examined. It was discovered that the interaction between the sandstone rock and alkali fluid at 90°C and 150°C, produced a new mineral crystalline zeolite and precipitated amorphous at 200°C. The amount of precipitated and dissolved minerals was found to be responsible for changes in permeability and clay reactivity with alkali chemical.
A typical example where the impact of oilfield chemical-rock interaction is experience is in chemical flooding techniques, such as alkali-surfactant-polymer (ASP) flooding for EOR (Pal et al. 2018). A study on the incremental oil recovery factor and degree of permeability damage in heterogeneous sandstone reservoirs subjected to strong-base and weak-base ASP flooding processes were also evaluated and compared (Zhong et al. 2020). It was found that the permeability-damage ratio can be reduced by approximately 15% with the weak-base ASP flooding compared to the strong-base flooding, and the reservoir flow assurance issues related to chemical loss can be addressed. This shows that the formulation of oilfield chemicals and their concentration. As reservoir rock minerals dissolve, the grains are carried away by fluid flow, which settle on the surface of the pore walls. The induced porosity change during fluid flow and rock-fluid interface is as a result of the precipitation of insoluble salts, formation swelling, clay particles deposition, mineral dissolution, and surface solid entrainment (Chang and Civan 1991). Shebl and Surdam (1995) empirically studied the possibility of oil-water-rock reacting. The authors utilized oxidized sandstone rock which originally contains anhydrite and intergranular clay cements with 6–15% range porosity and about 10–25% carbonate. After the experiment, it was found that there was a major possibility for a redox reaction to occur between an oxidized mineral and crude, and this reaction impacted permeability and porosity (increasing it from a range of 12–20%) of an elastic reservoir formation. Based on the results from literature, it can be concluded that chemical and rock interfaces cause mineral precipitation/dissolution, changing the porosity and permeability of the formation. But, changes in the permeability and porosity of the rock depends on the rock’s mineralogy, particle size distribution, shape of the grain, and pore size (Lamy-Chappuis et al. 2014; Wuyep et al. 2019).
Effect of chemical-rock interaction on particle size distribution
When a reactive fluid flows through a geologic porous medium, such as a reservoir rock, it is possible for minerals embedded in the reservoir formation to dissolve, releasing fine particles. While the dissolved mineral species could be in ionic form, the precipitated counterparts and the sand grains comprise a range of particle size distribution. More also, the minerals (ions) may as well precipitate from other ions dissolved in the fluid or they may also be conveyed with the dissolved minerals to form precipitates when the concentration of the ions is greater than the solubility of the precipitates (Chang and Civan 1991). The solid particle size distribution in solution (see Fig. 3) may influence the ionic exchange between ions in the solution and rock surface and the possibility to precipitate. This is because several materials make up the reservoir rock, which differ in their porosity, micro-voids, and boundary structure, and thus are characterized by a fragile structure (Jongpradist et al. 2011). Therefore, the redistribution of grains and their arrangement in the reservoir formation is closely related to the characteristics of the rock (Kwan and Surip 2020). It can increase the deformation of rocks, affect their porosity and permeability, and lead to structural uncertainty (Alonso 2010).
The reactions that occur when oilfield chemicals are introduced into a geological formation are classified as follows: (1) mineral dissolution and precipitation of the rock, (2) precipitation and dissolution of metallic ions and ionic compounds in the chemical, (3) ion exchange between the rock surface and the chemical, and (4) crystal growth and nucleation of the precipitate (Chang and Civan 1991). Dvorkin and Gutrierrez proposed that particle size distribution (PSD) is an important feature of rocks, as it directly affects the rocks geomechanical properties and geochemical characteristics. It is possible therefore for chemical-reservoir rock interaction to cause distortions in mineral configuration, causing mineral dissolution and precipitation and, in turn, decreasing or increasing rocks petrophysical properties depending on particle size distributions (Lamy-Chappuis et al. 2014). But when interparticle pores dominate fluid flow channels, such as sandstone, they are moderately consistent in their spread. Therefore, it is possible for all pores of the bonding material to be completely sealed off by its range of particle sizes distribution (He and Stephens 2011). According to Oluyemi et al. (2006), changes in the PSD of a sand producing formation during production may change the uniaxial compressive strength (UCS) of the rock. This changes UCS can weaken the rocks resulting formation damage. Consequently, Israeli and Emmanuel (2018) with the use of MATLAB simulated the effect of PSD on formation weathering. It was observed that rocks with fine grains rapidly withered more but slows down when the grain size is increased. This reveals that particle size distribution strongly influences the rate of fluid-rock interactions. Even so, the study ignored physical processes such as fluid flow, diffusion, and the impact of oilfield chemicals and rock interface, making extrapolation difficult. On the other hand, Wuyep et al. (2019) reported the impact of oilfield chemical and rock interface on the rocks mechanical strength with a combined use of uniaxial compressive and analytical test. The results showed that there is a major difference between particle size of brine and its effluent from reservoir samples. The increase in PSD observed may be caused by the dissolution/precipitation reactions, which can weaken the grain-grain bonding and result in detachment. However, results from other reported researches showed that rock strength can be linked with the composition of its minerals (Li et al. 2014; Pan et al. 2016; Sun et al. 2017; Zhang et al. 2018), grain shape and size (Sun et al. 2017; Jin et al. 2017), and its particle size distribution (Hussain et al. 2006; Shimizu et al. 2010 ). According to Jin et al. (2017 ), the shapes of rock materials and grain size contribute to the strength of its cemented soil-rock mixtures. It is found that the cement-cemented soil-rock mixtures could have localized shear- and strain-softening bands with a better strength and modulus than soil-rock mixtures without cement. In other words, the cement materials help to improved strength and modulus of formation soil-rock mixture. In general, the PSD is critical in evaluating the response of the reservoir rock formation to fluid interaction (Lade et al. 1996; Yu et al. 2016). By using DEM code to investigate the effect of PSD on the mechanical properties of the reservoir, it was found that the mechanical properties are influenced by the porosity of the formation and PSD (Shimizu et al. 2010 ). As the small grains of the rock occupies the space between the larger grains, they are restricted from dislodging by opposing grains. Furthermore, Wu et al. (2018 ) investigated the effects of grain size distribution on the structural and mechanical properties of cemented rockfills based on loading, the results obtained showed that cemented rockfill with rough particles displays a decreasing microstructure with an early failure. This is attributed to the effect of PSD composed within the rock. In essence, particle size distribution plays a very important role on rock deformation (Wang 1994; Zhang et al. 2018), as well as changes in permeability and porosity (Seely and Johns 2018).
Geomechanical properties
There is the possibility of sand production when there is not enough strength in the formation to withstand the destabilizing forces generated by reservoir fluid flow (Gharagheizi et al. 2017). Therefore, predicting sanding conditions in advance is necessary to provide technical support for sand control strategies. Therefore, a complete knowledge of rocks’ mechanical properties such as elastic properties and the inelastic properties (e.g., fracture gradient and compressive strengths) are crucial for the successful design of oil recovery and a safe oilwell drilling and stimulation of petroleum reservoirs. In order to predict distortion and failure of reservoir rock, reservoir fluid flow through porous formations and fluid-rock interfaces must be modeled. The general behavior of the rock as a result of external load or stress majorly depends on the rocks geomechanical properties. It is also dependent on other parameters which include non-hydraulic stress, temperature, pressure, and the formation failure history. The compressive strengths, Young’s modulus, and elastic modulus have been considered as important parameters for relating rocks’ mechanical behavior under stress. Makani noted a strong connection between elastic modulus and compressive strength of the rock. Similarly, it has been reported that the rocks petrophysical properties, geochemical properties, and composition exact much greater influence on the mechanical behavior. Therefore, the mechanical and physicochemical properties of the rock controls its behavior in addition to the interaction between the reactive chemical and its flow. Therefore, rock texture characterization is needed to provide insight on microstructure and morphology. Engebretson et al. investigated the effect of polyethylene oxide (PEO), dodecyltrimethyl ammonium bromide (DTAB), and aliminium chloride (AICl3) on sandstone reservoir rock strength with the aim of establishing fundamental knowledge that can be used to optimize chemically assisted fracking. This study was however limited in scope to static tests, may not adequately predict the dynamic processes occurring during reservoir stimulation or production. Jegarluei and co-workers (2010) reported the impact of cementation on the reservoir rocks mechanical properties where it was noted that the rocks mechanical properties are significant parameters for modeling wellbore stability and sand production.
In terms of understanding the in situ loads or stresses experienced in reservoir rocks, geomechanics can provide insight on the rock’s stability and strength. By using the petrophysical and mechanical properties of the formation, we can predict the stability of the rock and its ability to withstand the changes in stress as a result of fluid flow. Oluyemi (2014a, 2014b) empirically investigated a clashach reservoir core to evaluate the interaction that takes place between a clastic reservoir formation and a chemical inhibitor. The results of this study showed that the interaction between a chemical inhibitor (phosphate scale inhibitor) and clastic reservoir rock could result in a physical failure of the rock and the release of sand during reservoir fluid production. Although numerical analysis was not fully taken into account in this study, it is recommended that further empirical studies be conducted in order to confirm the physicochemical models. Oilfield chemicals have been shown to have a large impact on the formation’s geomechanical properties when injected into the formation (Wuyep et al. 2018 and 2019). In 2018, Giani et al. investigated the effects of gas production–induced compaction and subsidence analyses, coupling fluid-flow and geomechanics (i.e., stress-strain phenomena) by combining three numerical models: (1) geological model of the rock structure and its geologic features; (2) geomechanical model that predicts the rocks stress-strain behavior; and (3) fluid flow model that simulates the evolution of pressure in space and time. As part of the study, an experimental scale that represents an offshore, weakly consolidated, shallow, multilayered gas formation was constructed, utilizing already prevailing data from offshore gas bearing formations. It was found that the evolution of the stress-strain on the rock remained within its elastic limits. The changes were small, the compaction-induced porosity reduction was negligible, and the maximum permeability variation reached 12%. The reason for the minimal impact on the rock geomechanical and petrophysical properties could be ascribed to the gas fluid and the weak compaction induced by the flow field. However, mineral precipitation and dissolution processes may alter permeability, flow pathways, and therefore deform a formation by fluid-rock interaction. The flow of fluids in porous rock formation occurs either through interconnected pores or through a network of natural pre-existing or induced rock fractures. Obviously, rock permeability, porosity, and geomechanical properties are altered by reactive fluid-rock interaction. Therefore, there need to be considerations for the coupling of flow, reaction, and rock deformation in modeling the processes. Furthermore, the chemicals could change the consistency of the stressed rock by mineralizing or eroding contact surfaces, resulting in chemically motivated deformation. This is due to changes in volume triggered by processes, such as oxidation, hydration, precipitation, and dissolution, carbonation of minerals in the rock that is restricted under stress. However, geochemical and mechanical disturbances to reservoir formation can occur independently, the processes are often in combination with fluid and mineral interactions (i.e., fluid-rock interaction). Though geochemical reactions may start and promulgate crack, change fracture opening because of mineral dissolution, pore closing due to mineral precipitation, lead to volume change and rock deformation. At the same time, it could affect the geomechanical processes. Hence, models that combine chemical-mechanical process in stressed rocks are critical and valuable for predicting and monitoring dynamic changes to the state of the rock and deformation evolution. Finally, the petrophysical, geomechanical properties, and morphology of the formation develop vigorously because of mineral precipitation and dissolution caused by pressure variations, temperature, fluid motion, and composition.
The compressive strength refers to the rock’s ability to withstand stress intended to reduce its size. The compressive strength is a very significant geomechanical property of the formation mostly used to determine the stability of the structure when exposed to stress (Romana and Vásárhelyi 2007; Gholami and Fakhari 2017). The test determination approach involving core samples of rocks is destructive, costly, and time-consuming. To estimate UCS, various models have been proposed using mineralogical and petrographic characteristics, and rock’s physical properties. In light of this, the strength of a rock is exponentially determined by its porosity, and this increases with a decrease in porosity, as demonstrated by Sarda et al. (1993). Thus, it is important not only for engineering designs, but also an important element of an effective design and implementation (Aladejare 2020). A good knowledge and precise evaluation would improve the proficiency of oilfield stimulations and other oilfield operations (Negara et al. 2017). Rashidi et al. (2008) in their effort to enhance bit real-time wear with the use of an intelligent drilling advisory system was able to demonstrate that uniaxial compression strength (UCS) test may be affected by particle shape, grain size, sorting, minerology, size and shape of test sample, and rate of loading. On the other hand, Sonmez et al. (2006) noted that compressive strength which is an input in rock design is essential in the prediction of the deformation modulus. Nevertheless, Palmstrom (1996), Aladejare and Wang (2019) utilized rock mass classifications system which combines rock mass index (RMI) and rock mass rating (RMR) system in evaluating the formation strength properties and rock mass deformation connected to response to load and its design. Tixier et al. (1973) proposed a simple correlation to estimate formation strength from its mechanical property, especially UCS and rock strength;
where ρb and ∆t can be obtained through sonic and density logs.
Using a triaxial test, Yadav et al. (2016) investigated the changes in geomechanical properties of Berea shale and sandstone reservoir rock samples when they were interacting with oil and water based muds. It was shown that the oil-based-mud (OBM) preserves the shale strength better than the water-based-mud (WBM). Xu et al. (2018) empirically studied the influence of drilling fluid on tight sandstone hardness using WBM (water-based mud) and OBM (oil-based mud), and observed that after 2 h, the hardness of the rock (sandstone) decreased by 22.9% and 10.1% with WBM and OBMs respectively. It also shown that after 15 days, the sandstone hardness with WBM was observed to have decreased by 33.1% but remained unchanged with OBM. It was also noted that temperature has but only little influence on sandstone hardness with WBM but at temperatures of 50°C and above decreases the sandstone hardness with OBM. This means water exerts more impact on the geomechanical properties of the rock formation than oil. According to Wuyep et al. (2019), fluid-rock interaction resulting from mineral dissolution, precipitation, and geochemical reactions weakens reservoir rock grain fabrics of carbonate and sandstone. This decreased the UCS under atmospheric and ambient conditions of the experiment. Kitamura and Hirose (2017) investigated the impact of distillated water on the strength of several reservoir rocks (sandstone) with the use of indentation test in studying hardness of the different sandstone rock samples. The Young’s modulus and compressive strength were found to increase with a decrease in porosity of the rock when the compressive strength was correlated with the ultrasonic wave velocity. Experimentally, Al-Osta (2018) evaluated the compressive strength of rocks using a correlation developed between the compressive strength and some important parameters gotten through indirect methods on the rock such as ultrasonic pulse velocity, mass density, and point load strength index. The obtained results showed that the connection between ultrasonic pulse velocity and UCS compressive strength displayed the best match, followed by compressive strength and the mass density, and lastly point load index and compressive strength. Likewise, Muqtadir et al. (2018) explored how the mechanical properties of a sandstone formation with low permeability is affected by fluid saturation. The authors observed that the sandstone formation when saturated in brine becomes weaker compared to when it is saturated in oil. The investigation also showed a 9% reduction in the UCS and 40%d tensile strength (TS) decrease when saturated with brine. But when the formation is saturated with oil, the reduction in the TS and UCS were discovered to be 25% and 10% respectively. This suggests that oilfield chemicals exert significant impact on the geomechanical properties of the reservoir rocks. Similarly, Smorodinov et al. (1970) explored correlations between some physical characteristics of rocks and rock strength, developing two relationships to estimate UCS based on porosity and density as a direct determinant of rock strength which are exponential functions of density and porosity. This indicates the possibility of estimating geomechanical properties of the rocks from their petrophysical properties. For instance, it has been generally shown that the rock’s compressive strength is a function of mass density or porosity. Conversely, other correlations are based on the Poisson’s ratio, the rock strength, sonic wave velocity, and bulk density (Fjær et al. 1992; Moos et al. 1999).
It is therefore expected that the UCS decrease as the rock porosity increases; hence, at a certain porosity, the rock will become weakened and merely a loose aggregate. The confining pressure (stress) below which a reservoir formation deforms is acknowledged as its confined compressive strength (CCS), at which point, a breakdown in the rock gain structure begins, leading to a compaction of the rock and a loss in porosity. The CCS of a formation is a very significant parameter in petroleum engineering for selecting drill bits and drilling process optimization, predicting the rate of penetration (ROP) and performance analysis (Shi et al. 2015). It is also general understanding that the heterogeneity at rock grain scale, such as mineral stiffness and grain geometry, as well as particle and particle boundary micro-cracks (called micro-defects) will reduce the UCS of the rock (Bahrani and Kaiser 2016). In considering rock strength, Rampersad et al. (1994) used a power function to develop a link between the CCS of the rock, strength of the rock, and confining pressure. Nonetheless, the model proposed neglected the impact of porosity on the strength of the rock, which is a critical petrophysical property. Shi et al. (2015) considered the effect of porosity and nonlinear properties with increasing confining pressure for selecting bits, predict rate of penetration (ROP), drilling optimization, and developed a compressive strength model. The authors’ model when in combination with equation for mechanical specific energy was used to optimize drilling parameters. Figure 4 shows the relationship between confining pressure and rock strength for different types of rock. It can be observed that the model can recognize unproductive drilling circumstances and is appropriate for both overbalanced drilling (OBD) and underbalanced drilling (UBD). However, the study was carried out on a dry rock sample and therefore makes the result difficult for extrapolation in accounting for the rock behavior interacting with fluids.
Curves for confining pressure and strength for different types of rock (Shi et al. 2015)
The rock porosity is a function of peak stress at varying confining pressure as presented in Fig. 5. It is clear the rock porosity plays a significant role in both the elastic parameters of the rock and the strength of the rock. As a result, the load-bearing capability of the formation changes with change in the rock porosity, of which it has been reported that oilfield chemicals interaction with reservoir formation induces significant alterations to petrophysical properties of the rock, and by extension to the geomechanical properties, such as rock strength and UCS.
Peak stress and porosity at varying confining pressure. (Shi et al. 2015)
Chemical-rock interaction mechanisms
The main mechanisms when fluid and rock interact are mineral phase precipitation and dissolution (William et al. 2016). It is therefore important to understand the mechanisms occurring at the chemical-rock interfaces in order to determine what impact they have on the formation strength. To develop the governing equations, momentum and mass conservation, continuity, reaction rate laws, and other constitutive relationships will be considered. Thus, the formation-damaged model for the rock during chemical-rock interaction should be the combination of fine particles migration, chemical reactions (dissolution and precipitation), and fluid flow models.
Wuyep et al. (2019) evaluated biocides, scale, and corrosion inhibitors effects on carbonate and sandstone rocks. It was found that ionic exchange, mineral precipitation, and mineral dissolution contributed to the weakening of the rock grain fabrics, leading to rock failure. Guodong et al. (2017) examined the influence of CO2-water interface on permeability and porosity. It was observed that increasing CO2 injection without proportionate pressure build-up influences the permeability-porosity relationship. It was however resolved that an increase in CO2 injectivity was accountable for the increased rock mineral dissolution (dolomite). Bertaux and Lemanczy reported the interaction mechanism between potassium hydroxide (KOH), sodium hydroxide (NaOH), and the minerals of a sandstone reservoir formation. The results from these investigations confirmed that the primary mechanisms due to fluid-rock interactions are precipitation and dissolution.
Horsrud et al. (1998) carried out a study to predict borehole instability by exposing shale sample rich in smectite to KCL. It was discovered that the shrinkage in the shale sample was as a result of cation exchange. The effect of the shrinkage was simulated, and it was observed that increasing the concentration of KCL resulted in a decreased compressive stress. This suggests that the chemical interaction exerted a significant effect on the rock’s strength due to ion precipitation. In another investigation by El-Hajj et al. (2013), a carbonate reservoir sample was flooded with carbon dioxide at reservoir condition and assessed the CO2-carbonate formation interaction with an X-ray fluorescence (XRF) analyzer, scanning electron microscope (SEM), and an atomic force microscope (AFM). The authors noted that at high-pressure aging, CO2 dissolved some of the rock minerals and also precipitated some of the dissolved carbonate. Equally, Shiraki and Dunn (2000) empirically studied the effect of brine and saturated CO2 on dolomite-anhydrite-cemented sandstone rock. A week after, the authors discovered that the dissolution of dolomite and K-feldspar led to the formation of kaolinite in the pore throat and consequently reduced the permeability. Muller et al. (2009) hydrochemicaly experimented on Berea sandstone rock by using dry CO2 to flush a saturated NaCl core, and discovered a reduction in permeability which was as a result of halite mineral precipitation. On the other hand, a calcite-dolomite cemented sandstone was reported by Ross et al. (1981), it was observed that the increase in permeability of the rock was due to carbonate mineral dissolution. This was credited to an increased pore space alteration due to the carbonate cement bond dissolution. In summary, dissolution and precipitation are the most common mechanism induced by chemical-rock interactions, which remarkably impact of the rock’s petrophysical and geomechanical properties.
Geochemical reactions
Chemical-rock interface induces numerous chemical and physical processes as the oilfield chemicals flow through the porous rock system. The oilfield chemicals may dissolve some solid minerals embedded in the rock (Samuel and Thomas 2018). In addition to ion precipitation in the fluid, downstream transport of precipitates and dissolved minerals can also occur if the concentration exceeds the solubility of the precipitates (Chang and Civan 1991). In most cases, however, only precipitation and dissolution are considered as the geochemical reactions occurring. Investigation on the effects of mineral dissolution on reservoir rock petrophysical properties have been earlier defined by Colón et al. (2004) and Hu et al. (2010). The authors in their conclusion reported that permeability and porosity will increase upon these chemical interactions.
Chemical reaction (dissolution-precipitation)
Mineral precipitation and dissolution involve a concurrent process of solute transport in addition to adsorption and desorption of surface solutes, mineral surfaces and bulk solution, migration of solute on surface and dehydration, and hydration of ions (Appello and Postma 2005). Nonetheless, some of these processes may proceed faster than others. Consequently, clay particles residing in the rock pore spaces are thought to be responsible for the release of fines during fluid-formation interactions. But changes in pH and salinity could result in clay swelling and clay deflocculation. According to hypothesis, fines migration reduces permeability by causing formation damage (Saeedi et al. 2016). As a result of mineral precipitation and fine particles migrating within the host rock pores, formation permeability decreases (Zhao and Yin 2021). There are several types of mineral fines that contribute to permeability reduction, including clay, quartz, feldspar, and carbonate as they can fill or bridge pores. It is well known that rock permeability plays a significant role in fluid flow. On the other hand, the dissolution of rock minerals would result in an increase in formation porosity. The dissolution of formation may lead to a collapse of pore network structures. In addition to the changes in pore space geometry, the fluid-rock interactions also modify rock strength as stated earlier. Nonetheless, the changes in porosity and permeability of the underground formation rock-fluid interaction influences the injection and subsurface movement of the chemicals. Due to fluid-rock interaction, the petrophysical properties of rock may be altered, resulting in lower rates of production than expected. Therefore, the source of the reduction must be identified before any corrective measures can be taken.
The reactive fluid flow momentum through a porous material is accountable for the heterogeneous geochemical reactions like precipitation and dissolution that occur at the rock minerals and the pore-fluids interface (Lamy-Chappuis et al. 2014; Wuyep et al. 2020). In other words, fluid flow can be described as a reactive transport system. Thus, the model development for the chemical-rock interactions comprise precipitation, dissolution, and the transportation of minerals dissolved. As oilfield chemicals flow through a porous medium, it is adsorbed on the rock surface and due to the mineralogical components, it distorts the equilibrium system. As a result, mineral dissolution occurs resulting to a fluid configuration that is saturated with added minerals (Putnis 2015). Although, the rate of rock dissolution is a function of the chemical composition, temperature, ionic strength, chemical concentration, fluid motion, pH, and the hydrodynamic interface between the oilfield chemical and the rock phase that depends on the surface area of the mineral–fluid interface (Lasaga et al. 1994; Lasaga 1998). A rock’s surface roughness is also a significant factor determining its reactivity, with rough surfaces dissolving more rapidly than smooth surfaces (Levenson and Emmanuel 2013).
The dissolution and precipitation reactions that occur as a result of chemical-rock interaction when fluid flows through a porous medium can also be modeled by the discretization of rate laws and chemical law of mass action. Liu et al. (1997) characterized the reactions in two groups: mineral and aqueous reactions. While the aqueous reaction only entails aqueous species such as acid/base dissociation, the mineral counterpart includes both mineral and aqueous species like precipitation and dissolution. The rate of mineral reactions is quick when compared aqueous reactions (Liu et al. 1997). Furthermore, the rate of reactions in reactive chemical-rock surfaces can also be influenced by the concentration of catalysts such as precipitates/dissolves at the surface, or the quantity of microorganisms acting on the rock surface. Saaltink et al. (1998) observed that the concentration of species in the medium contributes to the rate of reaction. Therefore, the rate of reaction is related to change in specie concentration. For instance, considering compound A to be converted to B (Scheme 1), the rate of reaction, r, may be modeled as expressed in Eq. 8. Equation 9 shows the equilibrium constant for the two reacting compounds A and B (scheme 2). The chemical reaction rate (r) describes the quantity of reactants that evolves to products per unit time.
where Keq represents the equilibrium constant, k the rate constant, [A], [B], [C], [D] signifies species concentrations of A, B, C, D, and a, b, c, d are stoichiometric coefficients, n is reaction order. For simple cases, n is equals 0, 1, 2, for zero, first and second order reactions, respectively.
Injecting reactive fluids in a porous medium will lead to the dissolution of several minerals that originally exists and can produce new species, dependent on temperature and chemical composition, the new chemical species may precipitate. The mineral dissolution and precipitation due to geochemical reaction with the reservoir formation is complicated and involves several mechanism rates law. Hence, a rate law would model the combined impact of fluid flow, chemical reaction such as overall rates of precipitation and dissolution of the minerals and factors like ionic strength, temperature, saturation conditions, and pH (Lasaga et al. 1994). Notably, significant changes in permeability and porosity are experienced in the rock due to mineral dissolution and precipitation, thereby modifying fluid flow path features. This establishes a link between the fluid flow and chemistry taking place in the porous medium. To simulate these coupled processes occurring concurrently, different numerical software tools have been developed. TOUGHREACT is a numerical simulation tool developed and deployed to solve the reaction kinetics of precipitation and dissolution resulting from chemical–-rock interaction kinetics in fluid flow through a porous medium, and also to study reactive chemical transport in a porous medium and geochemistry. The overall reaction rate Ri is a combination of heterogeneous mineral reactions which is precipitation–dissolution reaction represented as (Rimin) and homogeneous aqueous reaction (Riaq) as given in Eq. 10. The heterogeneous reaction (Rimin) represents the amount of all the fluid and rock reactions as shown in Eq. 11, whereas the rate of species production j from the aqueous reaction (Riaq) phase as a result of homogeneous reaction is given in Eq. 12.
where rm signifies the rate of dissolution (Rm less than 0) or precipitation (Rm greater than 0) of minerals m per unit volume of formation, vim the mole number of i present in the mineral m, Nm the amount of minerals that exists in the formation.
Mineral precipitation or dissolution from fluid-rock interface occurs simultaneously and through a series of developments like solutes transport between the rock surface and bulk fluid, surface detachment (desorption), and attachment (adsorption) of solutes, surface migration, dehydration, and hydration of ions. Steefel and Lasaga and Lasaga et al. (1994) established a rate law for kinetically controlled mineral precipitation and dissolution reactions which is applied to reactive flow in a porous medium. In this study, the reaction rate is basically a function of all species concentration as expressed in Eq. 13. The overall rate of dissolution or precipitation of rock mineral in aqueous solution is given in Eq. 14.
where f (ai) signifies the function of the activities of different ions in solution, A represents the reactive surface of the mineral (m2/m3 total rock), the rate constant k (mol/m2s) and f (∆G) denotes the free energy of solution.
Based on computational viewpoint, the reaction rate law is governed by fluid flow with surface-controlled reactions like dissolution or precipitation as restrictive steps are much easier to determine. Appello and Postma (2005) also suggested a generic rate law, similar to that shown in equation (12) that takes into consideration changes in solute concentration due to mineral dissolution or precipitation reactions (Eq. 15). Notably, the rate is dependent on the ratio of the surface area A0 to the chemical solution volume V and specific rate.
Given, R represents the general reaction rate (mol/L.s), specific rate k (mol/m2.s), volume of chemical fluid V (m3), initial surface area of the rock A0 (m2), the original moles of solid m0 and the moles of solid at a specific time m. From equation (14), the term (m/m0)n defines change in the surface area, especially the reactive surface sites throughout mineral precipitation/dissolution. Nonetheless, for uniform size of grains precipitating or dissolving cubes and spheres, n = 2/3. For different particles size population, the finer grains will dissolve selectively. Thus, n is a function of the original PSD with a value ≤3.4 (Appello and Postma 2005). The function g(C) considers the effect of the chemical composition on rate, pH, the effects of catalysis and inhibition and the distance from equilibrium (Lasaga 1998). Similarly, Steefel and Lasaga presented another simplified expression that simulates the kinetics of precipitation and dissolution of reservoir rock minerals due to oilfield chemical and reservoir formation interface is shown in Eq. 16. Similarly, Rimstidt and Barnes and Saaltink and co-workers (1998) also reported a similar rate equation. However, since most rate constants are obtained at ambient temperature, the Arrhenius equation as presented in Eq. 17 can be used to study the effect of temperature on reaction rate constant k.
where −1 and + 1 are dissolution and precipitation respectively, k in mol/m2s denotes kinetic rate constant, σ specific reactive surface medium of rock mineral, ψ solution state with regards to the rock mineral (ψ = Q/Keq, Keq, and Q are equilibrium constant and ionic activity product of the formation, and θ and η are empirical parameters determine experimentally, activation energy Ea and the rate of constant k25 given at 25°C, temperature T in degree Kelvin and R1 is gas constant. Note, when dissolution occurs, ψ < 1, > 1 for precipitation, the system will be at equilibrium if equal to 1 and 0 no precipitation/dissolution.
In general, there are many studies on chemical-rock interaction focusing on the influence of chemical interactions on rock geochemicals, and geomechanics (Kan et al. 2005; Tomson et al. 2008; Bybee 2010; Egermann and Vizika 2011; Mohamed and Nasr El-Din 2013; Oluyemi 2014a, 2014b; Wilson 2016; You and Lee 2020; You and Lee 2020). Tables 1 and 2 show a summary of previous empirical and numerical studies on chemical-rock interaction.
Direction for future research
Despite several works on sand production and oilfield interaction, fundamental research is still lacking. The fluid-rock interaction is known to cause several formation damages due to fine migration, clay swelling, geochemical reactions between the invading chemical and the rock, resulting in rock failure (Chang and Civan 1991). Also, in changing the petrophysical properties of the reservoir formation, fluid-rock interaction can significantly alter the rock strength (Corapcioglu et al. 2014; Iriarte and Tutuncu 2018). Although several studies have been conducted regarding oilfield chemical-rock interactions, there is still a serious void that needs to be investigated further. First, further exploration is needed to evaluate the host rock mechanical properties relative to its mineralogy and cementitious content such as clay and the composition of the interacting chemicals. The study must be extended to cover the correlation between rock composition, alterations in the formation properties, formation and fluid interface, and composition of the injected chemical. The reason is that the formation and fluid interaction is subjected to rock mineralogy and the composition of the injected fluid. Moreover, how fluid flow rate, pH, fluid composition, concentration, and temperature affect both petrophysical and geomechanical properties of the formation, and consequently rock damage, failure of the rock, and sand production, needs to be explored. Further investigation with residence time distribution tool to quantify the impact of flow rate on solute and geochemical reaction profiles during the fluid-rock interaction will help provide meaningful insight on pore-scale alterations. As a result, the real-time changes in permeability due to the injection of chemical can be elucidated.
Another area worth investigating is nanofluid formulation and its impact on rock mineral dissolution, resultant properties, and rock formation failure, including experimentation of formulated nanofluids (NFs) which will consist of dispersion of nanoparticles in the base fluid/chemical and their impact on formation upon interaction with the rock relative to standalone counterpart chemical. Without advancing characterization methods for petrophysical and geomechanical properties, it will be challenging to predict fluid-rock interactions and its impact on production and rock formation. Hence, the evolution of the formation microstructure, morphology, and grain-scale changes due to alteration in the formation physical properties as a result of chemical reaction occurring needs to be further examined using the SEM and X-ray microtomography methods. The pore network structure changes, causing changes to the petrophysical properties in the rock needs further investigation with nitrogen adsorption-desorption technique. Furthermore, a combination of modeling and microscopic characterization of the rock is required to predict the transport parameters such as porosity, diffusivity, and permeability. On the other hand, the ability to thoroughly simulate all aspects of fluid-rock interaction is expanding because of sophisticated numerical simulators capable of implementing several methods in modeling multi-scale porous in order to predict changes in the rock properties. The use of numerical simulator provides additional insight to analyze and interpret the fluid-rock interaction. In addition to insufficient database, this study approach faces challenges of limited information on the reaction mechanisms and processes happening at the surface of the mineral. Hence, the experimental measurements should be validated with numerical predictions. Consequently, the kinetic reactions occurring at the interface of the fluid rock need to be studied. Fluid-rock interactions such as wettability, porosity, and permeability changes in sandstone and carbonate reservoir rocks should be addressed experimentally. This should be validated with the combination of core flooding and pore-scale imaging simulation of the fluid-rock interaction.
Zhong et al. (2019) investigated and reported the impacts of fluid flow and rock interactions on the mechanical and petrophysical properties using core samples rich in clay and another formation sample rich in calcareous material. Findings show that the extent of changes in the formation mechanical properties due to the fracking fluid-rock interaction is dependent on factors such as grain cementation and composition of the rock minerals. Although, there has been many studies on the influence of oilfield chemical and reservoir rock interface on the strength of the formation, permeability, and porosity of a rock, there is limited study on the correlation between rock composition, changes in rock geomechanical properties composition of rock minerals and chemical-rock interactions. Since different rocks will interact differently with injected fluids, it will be a good objective to evaluate the deformation and petrologic features of each type of rock and their likely interactions with various chemicals in the petroleum industry to stimulate the rock. Gomez and He (2012) proposed that the immersion test is the proper approach considered to investigate and observe the evolution of fluid and rock interface and the development of fracture.
The objective of injecting fluids into reservoir is to increase productivity. Therefore, the interface between fluid and reservoir rocks have been modeled to evaluate their contributions to formation damage (Chang and Civan 1991). The authors (Cheng and Civan, Chang and Civan 1991), in their study determined the model parameters through method of optimization by experimental data fitting. Most modeling efforts directed at formation damage addresses aspects such as permeability and porosity alteration and dissolution/precipitation phenomena. In 2019, Gardner and Sitar modeled the interface between rock formations and water by modeling distinct polyhedral blocks with the use of a discrete element method (DEM) and water was modeled with Lattice Boltzmann method (LBM). Though the model captured transient and fluid pressure, it was discovered that additional performance enhancements is required to model an accurate filed scale difficulty (Gardner and Sitar 2019). Hence, the future outlook will be to develop models that couple impacts of the prevailing processes during chemical-rock interface on geomechanical properties of the formation. This approach provides a more realistic and holistic impact of the processes occurring concurrently on the rock properties and the resultant failure rather than modeling single process, whereas the ability to apply single models is restricted in some cases than that of combined effects which cover wider areas. Notwithstanding, combined effects models will demand too much computational effort compared to single models. Consequently, the concentration of ionic species and pH on fine grains release, migration, and deposition also need to be investigated and integrated into the models. Likewise, the impact of precipitates from dissolution/precipitation reactions on rock porosity and network alteration is also another area needing further considerations.
Most instabilities experienced in reservoir performance during production have been ascribed to fluid-rock interactions (Lal 1999; Muniz et al. 2005; Gomez and He 2012). It will be worthwhile therefore to develop criteria for formulating and selecting fluids that will enhance production while minimizing impacts on rock properties leading to formation damage, rock failure, and sand production. Furthermore, studies on mass transport induce changes in pore pressures due to fluid transport and ionic diffusion, and their impacts on rock formation are necessary to obtain a comprehensive stability analysis. Consequently, the microstructural and morphological changes developed within the rock can be evaluated to enhance the interpretation of impact of processes as a result of fluid-rock interaction. However, the intricate relationship between chemical reaction, fluid properties, and rock strength, the evolution of petrophysical properties and mechanical properties leading to rock failure is still poorly understood (Tenthorey et al. 2003). Hence, it will be insightful to further investigate the microstructural changes in response to stress-strain induced by the fluid-rock interaction to understand hydro-mechanical changes during rock failure. On the other hand, it has been found that nanoparticle adsorption on rock surfaces in low salinity water (LSW) and flooding with nanoparticles have proven to minimize cementing material loss, mineral dissolution, ion exchange, and resistance to flow when related to only low salinity water (LSW), thereby minimizing rock formation failure (Abhishek et al. 2018). Hence, instead of using standalone chemical, formulation of nanofluids (NFs) which consist of dispersions of NPs in the base fluid/chemical can be studied as an injection fluid for enhancing production while minimizing the release of fine particles and formation damage. The NPs can alter surface and wettability of the rock (Abhishek et al. 2018); hence, the application of NFs can be considered as an area which further investigation will serve as a guide towards inhibiting the detrimental effects of formation damage associated fluid-rock interactions.
Conclusion
The interaction of oilfield chemicals and rock during enhanced oil recovery (EOR), production, reservoir stimulation, or formation treatment can affect the rock’s petrophysical and geomechanical properties. These chemical injections include chemical flooding (e.g., alkali–surfactant–polymer, surfactant, surfactant-polymer), carbon dioxide flooding and sequestration, reservoir stimulation and formation treatment such as carbonate reservoir acidizing, and hydraulic fracture. It is crucial to know how specific geochemistry reactions and processes determine porosity and permeability evolution as reservoirs are subject to a wide range of chemical-rock interactions. Hence, the review of studies on the impact of oilfield chemicals on rock interface, mechanisms of the interface, and how their interactions affect reservoir rock petrophysical and geomechanical properties was reported in this paper. It was found that the oilfield chemicals exhibited strong interactions on the formation rock resulting in strong cement dissolution, which increases porosity and permeability of the reservoir formation. According to studies, chemically treated formations will release a variety of rock materials when they undergo reactions like adsorption, diffusion, precipitation, and dissolution. The extent of these reactions depends on the particle size distribution within the reservoir rock formation itself. Also, the chemical-rock interactions would vary considerably depending on temperature, the reservoir rock type, pressure, chemical composition and concentration. During these geochemical and petrophysical experiments, the problems of potential chemical-rock interactions were investigated and the consequences of such interactions on rock properties and production were reported. Resultantly, the rock petrophysical and geomechanical properties changes due to the chemical-rock interactions and fluid flow. Fine particles migration along with the fluid flow may occur due to geochemical reactions (dissolution and precipitation). This may either block the rock formation pores or create new pores resulting in changes in the mechanical strength of the reservoir formation. However, it is observed that fluid used in most of the studies are not typical oilfield chemicals and so would present unrelated chemistries since reactions would impact differently on different formation strength after chemical injection. Therefore, oilfield operators who wish to maximize oil production should study the impact of oilfield chemicals on weakening rock UCS in order to assess rock failure. In the sand production process, two stages are involved: firstly, the failure of rock matrix, followed by the detachment of sand grains and their transportation in fluid flow. Furthermore, the prediction of chemical-rock interactions and their impact on production and rock formation will be inconclusive without advances in characterization methods for petrophysical and geomechanical properties. Conclusively, it was discovered that a combination of petrographic observations with aid of advanced characterization techniques, laboratory experiments, and numerical simulations is capable of reconstructing the diagenetic process, as well as evaluating and predicting reservoir petrophysical properties due to chemical-rock interactions.
References
Abass H, Nasr-El-Din H, BaTaweel M (2002) Sand control: sand characterization, failure mechanisms, and completion methods. Society of Petroleum Engineers, San Antonio
Abhishek R, Hamouda AA, Murzin I (2018) Adsorption of silica nanoparticles and its synergistic effect on fluid/rock interactions during low salinity flooding in sandstones. Coll Surf A 555:397–406
Ahmad KM, Kristály F, Turzo Z (2018) Effects of clay mineral and physico-chemical variables on sandstone rock permeability. J Oil Gas Petrochem Sci 1(1):18–26. https://doi.org/10.30881/jogps.00006
Aladejare AE (2020) Evaluation of empirical estimation of uniaxial compressive strength of rock using measurements from index and physical tests. J Rock Mech Geotech Eng 12(2):256–268
Aladejare AE, Wang Y (2019) Probabilistic characterization of Hoek–Brown constant mi of rock using Hoek’s Guideline Chart, Regression Model, and Uniaxial Compression Test. Geotech Geol Eng 37:5045–5060
Alonso EECR (2010) Behavior of materials for earth and rockfill dams: perspective from unsaturated soil mechanics. Front Archit Civ Eng China 4:1–39
Alyafei N (2019) Fundamentals of reservoir rock properties . In: Fundamentals of reservoir rock properties. Doha, Qatar: Hamad Bin Khalifa University Press , pp. 11-19
Amiri M, Moomivand H (2019) Development of a new physical modeling method to investigate the effect of porosity on the parameters of intact rock failure criteria. Arab J Geosci 12:(317. https://doi.org/10.1007/s12517-019-4494-x)
André P, Audigane P, Azaroual M, Menjoz A (2007) Numerical modeling of fluid–rock chemical interactions at the supercritical CO2–liquid interface during CO2 injection into a carbonate reservoir, the Dogger aquifer (Paris Basin, France). Energy Conversion and Management, vol 48 no. 6, https://doi.org/10.1016/j.enconman.2007.01.006), pp. 1782-1797
Appello C, Postma D (2005) Geochemistry, groundwater and pollution, 2nd edition, Amsterdam, the Netherlands: A.A. Balkema Publishers
Atapour H, Mortazavi A (2018) The influence of mean grain size on unconfined compressive strength of weakly consolidated reservoir sandstones. J Petrol Sci Eng 171. https://doi.org/10.1016/j.petrol.2018.07.029
Bahrani N, Kaiser P (2016) Strength degradation approach (SDA) for estimation of confined strength of micro-defected rocks. Houston, American Rock Mechanics Association
Barclay S, Worden R (2000) Geochemical modelling of diagenetic reactions in a sub-arkosic sandstone. Clay Miner 35(1):57–67
Barsotti E, Qin Z, Piri M (2021) In situ investigation of fluid-rock interactions at ångstrom resolution. Journal of Geophysical Research: Solid Earth 126(2)
Bertaux J, Lemanczyk Z (1987) Importance of dissolution/precipitation mechanisms in sandstone-alkali interactions. Society of Petroleum Engineers, San Antonio
Burclaff N (2020) Oil and gas industry: a research guide. [Online] Available at: https://guides.loc.gov/oil-and-gas-industry. Accessed 20 July 2021
Bybee K (2010) Two-phase cement/CO2/brine interaction in wellbore environments. J Pet Technol 62(5):78–80
Chang C, Z MD, Khaksar A (2006) Empirical relations between rock strength and physical properties in sedimentary rocks. J Pet Sci Eng 51:223–237
Chang FF, Civan F (1991) Modeling of formation damage due to physical and chemical interactions between fluids and reservoir rocks. Dallas, Texas, This paper was prepared for presentation at the 66th Annual Technical Conlerence and Exhibition of the Society of Petroleum Engineers
Chen YL (2018) Permeability evolution and particle size distribution of saturated crushed sandstone under compression. Geofluids, pp. 1–2
Chun’an T, Hudson J (2012) Rock failure mechanisms. New York: Taylor & Francis Group, LLC
Colón CFJ, Oelkers EH, Schott J (2004) Experimental investigation of the effect of dissolution on sandstone permeability, porosity, and reactive surface area. Geochim Cosmochim Acta 68(4):805–817
Coneybeare C (1967) Influence of compaction on stratigraphic analysis:. Canadian Petroleum Geology Bulletin, vol 15, pp. 331–345
Corapcioglu H, Miskimins J, Prasad M (2014) Fracturing fluid effects on Young’s modulus and embedment in the niobrara formation. In: SPE annual technical conference and exhibition. Amsterdam, Society of Petroleum Engineers.https://doi.org/10.2118/170835
Crawford B (2011) Mechanical rock properties prediction: deriving rock strength and compressibility from petrophysical properties. Beijing, ISRM Congress
Davidova I, Hicks M, Fedorak P, Suflita J (2001) The influence of nitrate on microbial processes in oil industry production waters. J Ind Microbiol Biotechnol 27:80–86
Deglint HJ (2018) Use of imaging techniques to quantify fluid-rock interaction and petrophysical properties in low permeability hydrocarbon reservoirs (Unpublished doctoral thesis). University of Calgary, Calgary, AB. https://doi.org/10.11575/PRISM/33240
Denney D (2013) Simulating the chemical interaction of injected CO2 and carbonic acid. J Pet Technol 65:125–127. https://doi-org.ezproxy.rgu.ac.uk/10.2118/0713-0125-JPT)
Dennis D, Hitzman D (2007) Advanced nitrate-based technology for sulfide control and improved oil recovery. Houston: Society of Petroleum Engineers
Dernaika M (2015) Petrophysical and fluid flow properties of a tight carbonate source rock using digital rock physics.. San Antonio, SPE/AAPG/SEG Unconventional Resources Technology Conference
Dunn D, LaFountain L, Jackson R (1973) Porosity dependence and mechanisms of brittle fracture in sandstones. J Geophys Res 78:2403–2417
Dvorkin J, Gutierrez M (2002) Grain sorting, porosity and elasticity. Petrophysics 43:185–196
Dyke C, Dobereiner L (1991) Evaluating the strength and deformability of sandstones. Q J Eng Geol Hydrogeol 24(1):123–134
Egermann P, Bekri S, Vizika O (2010) An integrated approach to assess the petrophysical properties of rocks altered by rock-fluid interactions (CO2 injection). Society of Petrophysicists and Well-Log Analysts, vol 51 no. 1
Egermann PBS, Vizika O (2011) An integrated approach to assess the petrophysical properties of rocks altered by rock-fluid interactions (CO2 injection). Intl J Greenhouse Gas Control 5(3):579–588
El-Hajj H, Odi U, Gupta A (2013) Carbonate reservoir interaction with supercritical carbon dioxide. Beijing, International Petroleum Technology Conference
Eshiet K (2012) Modelling of hydraulic fracturing and its engineering application. Leeds, UK: PhD thesis University of Leeds
Faegestad I (2016) The defining series formation damage Irene Faergestad Editor. Oilfield Review. Https://Www.Slb.Com/-/Media/Files/Oilfield-Review/Defining-Heavy-Oil.Ashx (Accessed 10/10/2021)., 1–46. www.slb.com/defining
Fjær E et al (1992) Petroleum related rock mechanics. Elsevier, Amsterdam
Frank F, Faruk C (1991) Modeling of formation damage due to physical and chemical interactions between fluids and reservoir rocks. Dallas, Annual Technical Conference and Exhibition
Friedman M (1976) Porosity, permeability, and rock mechanics - a review. American Rock Mechanics Association
Fritz B, Jacquot E, Jacquemont B, Baldeyrou-Bailly A, Rosener M, Vidal O (2010) Geochemical modelling of fluid-rock interactions in the context of the Soultz-sous-Forêts geothermal system. Compt Rendus Geosci 342(7–8):653–667. https://doi.org/10.1016/j.crte.2010.02.005
Gharagheizi F, Mohammadi AH, Arabloo M, Shokrollahi A (2017) Prediction of sand production onset in petroleum reservoirs using a reliable classification approach. Petroleum 3(2):280–285. https://doi.org/10.1016/j.petlm.2016.02.001
Gamier A, Van Lingen N (1959) Phenomena affecting drilling rates at depth. Presented at AIME, 216, In: Paper SPE-1094-g
Gardner M, Sitar N (2019) Modeling of dynamic rock–fluid interaction using coupled 3-D discrete element and lattice boltzmann methods. Rock Mech Rock Eng 52:5161–5180
Gholami R, Fakhari N (2017) Support vector machine: principles, parameters and applications. In: S. S. V. E. B. Pijush Smui, ed. Handbook of Neural Computation. s.l.:s.n., pp. 515-535
Giangiacomo L, Dennis D (1997) Field testing of the biocompetitive exclusion process for control of iron and hydrogen sulfides. Casper, Wyoming, Society of Petroleum Engineers
Gieg L, Jack T, Foght J (2011) Biological souring and mitigation in oil reservoirs. Appl Microbiol Biotechnol 92:263–282
Gomez S, He W (2012) Fighting wellbore instability: customizing drilling fluids based on laboratory studies of shale-fluid interactions. Tianjin, China, SPE-155536-MS presented at the IADC/SPE Asia Pacific Drilling Technology Conference and Exhibition. 10.2118/155536-MS.
Greene E, Brunelle V, Jenneman G, Voordouw G (2006) Synergistic inhibition of microbial sulfide production by combinations of the metabolic inhibitor nitrite and biocide. Appl Environ Microbiol 72:7897–7901
Grigoryan A et al (2008) Competitive oxidation of volatile fatty acids by sulfate- and nitrate-reducing bacteria from an oil field in Argentina. Appl Environ Microbiol 74:4324–4335
Guodong Y et al (2017) Numerical investigation into the impact of CO2-water-rock interactions on CO2 injectivity at the Shenhua CCS demonstration project, China. Geofluids.
Hamada GM, Al-Blehed MS, Al-Awad MNJ (1999) determining petrophysical properties of low resistivity reservoirs using nuclear magnetic resonance logs. Houston, Texas, SPE Annual Technical Conference and Exhibition, https://doi-org.ezproxy.rgu.ac.uk/10.2118/56789-MS
Herz-Thyhsen RJ, Kaszuba JP, Dewey JC (2020) Mineral dissolution and precipitation induced by hydraulic fracturing of a mudstone and a tight sandstone in the Powder River Basin, Wyoming, USA. Appl Geochem 119:1–13
He W, Stephens M (2011) Bridging particle size distribution in drilling fluid and formation damage. Society of Petroleum Engineers
Hill S, Villeneuve M, McNamara D (2018) The impact of physical and geomechanical rock properties on fluid flow in Sandstone Reservoirs: Taranaki Basin, NZ. s.l., EGU General Assembly Conference Abstracts
Hitzman D, Sperl G (1994) A new microbial technology for enhanced oil recovery and sulfide prevention and reduction. Oklahoma, Society of Petroleum Engineers, Tulsa
Horsrud P, Bostrom B, Sonstebo E, Holt R (1998) Interaction between shale and water-based drilling fluids: laboratory exposure tests give new insight into mechanisms and field consequences of KCL contents. Society of Petroleum Engineers
Hu DW, Zhou H, Zhang F, Shao JF (2010) Evolution of poroelastic properties and permeability in damaged sandstone. Int J Rock Mech Min Sci 47:962–973
Hubert C (2010) Microbial ecology of oil reservoir souring and its control by nitrate injection. In: Handbook of hydrocarbon and lipid microbiology. s.l.:Springer, Berlin, Heidelberg
Hubert C (2017) Sulphate-reducing bacteria: terrors of the deep. s.l., Offshore Technology
Hussain M, El H, Wm AA (2006) Controls of grain-size distribution on geomechanical properties of reservoir rock: a case study: Cretaceous Khafji Member, Zuluf Field offshore Arabian Gulf. Mar Pet Geol 23:703–713
Ilgen A, Cygan R (2016) Mineral dissolution and precipitation during CO2 injection at the Frio-I Brine Pilot: geochemical modeling and uncertainty. Intl J Greenhouse Gas Control 44:166–174
Iriarte J, Tutuncu AN (2018) Geochemical and geomechanical alterations related to rock–fluid–proppant interactions in the Niobrara Formation. Lafayette, In: Paper presented at the SPE international conference and exhibition on formation damage control, Society of Petroleum Engineers. 10.2118/18953 6-MS
Israeli Y, Emmanuel S (2018) Impact of grain size and rock composition on simulated rock weathering. Earth Surface Dynamics 6:319–327
Jegarluei S, Moazzeni A (2010) Effect of cementation on mechanical properties of reservoir rocks and hydrocarbon production. Society of Petroleum Engineers.
Jessica I, Azra NT (2018) Geochemical and geomechanical alterations related to rock-fluid-proppant interactions in niobara formations. Society of Petroleum Engineers, Lafayette, pp 1–16
Jiahui Y, Kyung JL (2021) Analyzing the dynamics of mineral dissolution during acid fracturing by pore-scale modeling of acid-rock interaction. SPE J Issue 1–14. https://doi-org.ezproxy.rgu.ac.uk/10.2118/200406-PA
Jin L, Zeng Y, Zhang S (2017) Large scale triaxial tests on effects of rock block proportion and shape on mechanical properties of cemented soil–rock mixture. Rock Soil Mech 38:141–149
Jongpradist P, Youwai S, Jaturapitakkul C (2011) Effective void ratio for assessing the mechanical properties of cement–clay admixtures at high water content. J Geotech Geoenviron 137:621–627
Kan A, Fu G, Tomson M (2005) Adsorption and precipitation of an aminoalkylphosphonate onto calcite. J Colloid Interface Sci 281(2):275–284
Keelan D (1982) Core analysis for aid in reservoir description. J Pet Technol 34:2483–2491
Kitamura M, Hirose T (2017) Strength determination of rocks by using indentation tests with a spherical indenter. J Struct Geol 98:1–11
Kwan MF, Surip N (2020) Analysis of mechanical behaviour of reservoir rock properties. In: Lin J (eds) Proceedings of the International Field Exploration and Development Conference 2019. IFEDC 2019, Singapore. Springer Series in Geomechanics and Geoengineering. Springer. https://doi.org/10.1007/978-981-15-2485-1_334.
Lade PV, Yamamuro JA, Bopp PA (1996) Significance of particle crushing in granular materials. J Geotech Eng 122:309–316
Lal M (1999) Shale stability: drilling fluid interaction and shale strength. Jakarta, Indonesia, SPE-54356-MS presented at the SPE Asia Pacific Oil and Gas Conference and Exhibition, https://doi.org/10.2118/54356-MS
Lamy-Chappuis B et al (2014) Rapid porosity and permeability changes of calcareous sandstone due to CO2 enriched brine injection. Geophys Res Lett 41(2):399–406
Larsen J (2002) Downhole nitrate applications to control sulfate reducing bacteria. Houston Texas, NACE International
Lasaga AC (1998) Kinetic theory in the earth sciences. Princeton University Press, JSTOR. http://www.jstor.org/stable/j.ctt7zvgxm. Accessed 25 June 2022
Lasaga AC et al. (1994) Chemical weathering rate laws and global geochemical cycles. Geochim Cosmochim Acta 58(10):2361–2386
Levenson Y, Emmanuel S (2013) Pore-scale heterogeneous reaction rates on a dissolving limestone surface. Geochim Cosmochim Acta 119:188–197
Li L, Aubertin M (2003) A general relationship between porosity and uniaxial strength of engineering materials. Can J Civ Eng 30(4):644–658
Liou MF (2005) A numerical study of transport phenomena in porous media. Department of Mechanical and Aerospace Engineering, Case Western Reserve University
Li SH et al (2019) Experimental study on the impact of CO2–brine–rock interaction on rock properties and fracture propagation during supercritical CO2 fracturing in Chang-7 tight sandstone formation. New York City, New York, Paper presented at the 53rd U.S. Rock Mechanics/Geomechanics Symposium, pp. 1-8
Liu X et al (1997) A geochemical reaction-transport simulator for matrix acidizing analysis and design. J Pet Sci Eng 17:181–196
Li X, Wang G, Cao L (2014) Test research on influence of water and mineral composition on physical and mechanical properties of phyllite. In: 4th international conference on frontiers of manufacturing and design science, ICFMD. Hong Kong, China, Trans Tech Publications Ltd.
Liu X, Zhuang Y, Liang L Xiong J (2019) Investigation on the Influence of water-shale interaction on stress sensitivity of organic-rich shale. Geofluids
Maurer J (1965) Bit-tooth penetration under simulated borehole conditions. J Pet Technol 17(12):1433–1442
Mohamed I, Nasr-El-Din H (2013) Fluid/rock interactions during CO2 sequestration in deep saline carbonate aquifers: laboratory and modeling studies. Soc Petrol Eng 18(3):468–485
Mohammed M (2020) Estimating petrophysical properties of shale rock using conventional neural networks CNN. virtual, SPE annual technical conference and exhibition
Moos D, Zoback M, Bailey L (1999) Feasibility study of the stability of open hole multilaterals. Oklahoma City, OK, SPE Mid-Continent Operations Symp
Muller N et al (2009) CO2 injection impairment due to halite precipitation. Energy 112:3507–3514
Muniz SE, Fontoura ABS, Lomba TFR (2005) Rock-drilling fluid interaction studies on the diffusion cell. SPE-94768-MS presented at the SPE Latin American and Caribbean Petroleum Engineering Conference, Rio de Janeiro, Brazil. 10.2118/54356-MS
Muqtadir A et al (2018) Effect of saturating fluid on the geomechanical properties of low permeability scioto sandstone rocks. In Proceedings of the 52nd US Rock Mechanics/Geomechanics Symposium, Seattle, WA, USA
Negara A et al (2017) Unconfined compressive strength prediction from petrophysical properties and elemental spectroscopy using support-vector regression. Society of Petroleum Engineers
Nunez R et al (2017) Investigation of dissolution effects on dolomite porous media under carbonated water injection CWI. International Petroleum Exhibition & Conference, Abu Dhabi
Oelkers E, Bénézeth P, Pokrovski G (2009) Thermodynamic, databases for water-rock interaction. Rev Mineral Geochem 70(1):1–46
Oluyemi G (2014a) Conceptual physicochemical models for scale inhibitorformation rock interaction. Pet Sci Technol 32(3):253–260
Oluyemi G (2014b) Chemical injection related sand failure: an experimental investigation. 6th European Sand Management Forum, Aberdeen
Oluyemi G, Oyeneyin B, MacLeod C (2006) Prediction of directional grain size distribution: an integrated approach. Society of Petroleum Engineers, Nigeria
Oyeneyin M, Macleod C, Oluyemi G, Onukwu A (2005) Intelligent sand management. Proceedings of Nigeria annual international conference and exhibition, Abuja, Nigeria
Pal S, Mushtaq M, Banat F, Al Sumaiti AM (2018) Review of surfactant-assisted chemical enhanced oil recovery for carbonate reservoirs: challenges and future perspectives. Pet Sci 15(1):77–102. https://doi.org/10.1007/s12182-017-0198-6
Palmstrom A (1996) RMi - A system for characterizing rock mass strength for use in rock engineering. Journal of Rock Mechanics and Tunneling Technology 1:69–108
Pan H et al (2019) Joint Interpretation of elastic and electrical data for petrophysical properties of gas-hydrate-bearing sediments using inverse rock physics modeling method. Petrophysics 60(6):854–871. https://doi-org.ezproxy.rgu.ac.uk/10.30632/PJV60N6-2019a9
Pan R et al (2016) Influence of mineral compositions of rocks on mechanical properties. In 50th US Rock Mechanics/Geomechanics Symposium 2016,. Houston, TX: , American Rock Mechanics Association (ARMA)
Patrick J, Yang C, Lu J, Kimberly D (2014) Laboratory batch experiments and geochemical modelling of water rock super critical CO2 reactions in Gulf of Mexico Miocene rocks: implications for future CCS projects. Energy Procedia 63:5512–5521
Penberthy W, Jr Shaughnessy C (1992) Sand control. Society of Petroleum Engineers, TX, USA
Petrozziello L et al (2020) Iron sulfide scale inhibition: squeeze life extension through improved interaction between scale inhibitor and rock. Paper presented at the SPE International Oilfield Scale Conference and Exhibition, Society of Petroleum Engineers (SPE)
Priscilla, G (2021) Petroleum production. https://www.britannica.com/technology/petroleum-production. Accessed 22 Feb 2022
Putnis A (2015) Transient porosity resulting from fluid–mineral interaction and its consequences. Rev Mineral Geochem 80(1):1–23
Rahim M et al (2013) Simulation of chemical interaction of injected CO2 and carbonic acid based on laboratory tests in 3D coupled geomechanical modeling. Beijing, International Petroleum Technology Conference
Rahmati H, Nouri A, Vaziri H, Chan D (2012) Validation of predicted cumulative sand and sand rate against physical-model test. J Can Pet Technol 51(5):403–410
Rahmati H, Jafarpour M, Azadbakht S, Nouri A, Vaziri H, Chan D, Xiao Y (2013) Review of sand production prediction models. J Petrol Eng 2013:1–16. https://doi.org/10.1155/2013/864981
Rampersad P, Hareland G, Boonyapaluk P (1994) Drilling optimization using drilling data and available technology. Latin America/Caribbean, In: Paper SPE-27034 Presented at the SPE Latin America/Caribbean Petroleum Engineering Conference
Rashidi B, Hareland G, Nygaard R (2008) Real-time drill bit wear prediction by combining rock energy and drilling strength concepts. International Petroleum Exhibition and Conference, Abu Dhabi
Reinsel M, Sears J, Stewart P, McInerney M (1996) Control of microbial souring by nitrate, nitrite or glutaraldehyde injection in a sandstone column. J Ind Microbiol 17:128–136
Romana M, Vásárhelyi B (2007) A discussion on the decrease of unconfined compressive strength between saturated and dry rock samples. In: Proceedings of the international society for rock mechanics and rock. 11th ISRM Congress, Lisbon, Portugal
Ross G, Todd A, Tweedie J (1981) The effect of CO2, flooding on the permeability of reservoir rocks. Dev Pet Sci 13:351–366
Saaltink MW, Ayora C, Carrera J (1998) A mathematical formulation for reactive transport that eliminates mineral concentrations. Water Resour Res 34(7):1649–1656
Saeedi A, Delle Piane C, Esteban L, Xie Q (2016) Flood characteristic and fluid rock interactions of a supercritical CO2, brine, rock system: South West Hub, Western Australia. Intl J Greenhouse Gas Control 54:309–321. https://doi.org/10.1016/j.ijggc.2016.09.017
Samuel WS, Thomas D (2018) Permeability changes resulting from quartz precipitation and dissolution around upper crustal intrusions. Geofluids, Issue https://doi.org/10.1155/2018/6957306
Sandbeck K Hitzman D (1995) Biocompetitive exclusion technology: a field system to control reservoir souring and increasing production. Oklahoma, United States, U.S. Department of Energy. Office of Scientific and Technical Information
Sarda JP et al (1993) Use of porosity as an indicator for sand production evaluation. Paper No. SPE 26454, SPE Annual Technical conference and exhibition. Houston, TX, Paper No. SPE 26454, SPE
Seely D, Johns K (2018) Full-scale permeability, porosity, particle size distribution of an oil shale rockfill. In 52nd US Rock Mechanics/Geomechanics Symposium. Seattle, WA, American Rock Mechanics Association (ARMA)
Sergi M, David T, Carl IS, Chaopeng S (2012) An investigation of the effect of pore scale flow on average geochemical reaction rates using direct numerical simulation. Water Resour Res 48:1–11. https://doi.org/10.1029/2011WR011404
Shebl M, Surdam R (1995) Hydrocarbon-water-rock interaction: redox reaction as a mechanism for sandstone reservoir porosity enhancement. Am Assoc Petrol Geol (AAPG) 79:no. 6
Shimizu H, Murata S, Ishida T (2010) Effects of particle number and size distribution on macroscopic mechanical properties of rock models in DEM. J Soc Mater Sci Jpn 59:219–226
Shiraki R, Dunn T (2000) Experimental study on water-rock interactions during CO2 flooding in the Tensleep Formation, Wyoming, USA. Appl Geochem 15:265–279
Shi X et al (2015) Confined compressive strength model of rock for drilling optimization. Petroleum 1:40–45
Smorodinov M, Motovilov E, Volkov V (1970) Determinations of correlation relationships between strength and some physical characteristics of rocks. Belgrade, Yugoslavia, Proc. 2nd ISRM Cong
Sonmez H, Gokceoglu C, Nefeslioglu H, Kayabasi A (2006) Estimation of rock modulus: for intact rocks with an artificial neural network and for rock masses with a new empirical equation. Int J Rock Mech Min Sci 43(2):224–235
Steefel CI, Mäher K (2009) Fluid-rock interaction: A reactive transport approach. Rev Mineral Geochem 70:485–532. https://doi.org/10.2138/rmg.2009.70.11
Sumit M, Liu HH, Spycher N, Kennedy BM (2013) Impact of fluid–rock chemical interactions on tracer transport in fractured rocks. J Contam Hydrol 154:42–52. https://doi.org/10.1016/j.jconhyd.2013.08.008
Sunde E, Lillebo B, Thorstenson T, Bødtker G (2004) H2S inhibition by nitrate injection on the gullfaks field, corrosion., s.l.: NACE International
Sun W, Wang L, Wang Y (2017) Mechanical properties of rock materials with related to mineralogical characteristics and grain size through experimental investigation: a comprehensive review. Front Struct Civ Eng 11:322–328
Tabari K, Tabari M, Orod T (2011) Application of biocompetitive exclusion in prevention and controlling biogenetic H 2S of petroleum reservoirs. Aust J Basic Appl Sci 5(12):715–718
Telang A et al (1998) Effects of two diamine biocides on the microbial community from an oil field. Can J Microbiol 44:1060–1065
Tenthorey E, Cox FS, Todd FH (2003) Evolution of strength recovery and permeability during fluid-rock reaction in experimental fault zones. Earth Planet Sci Lett 206:161–172
Tianfu X, Guanhong F, Yan S (2014) On fluid–rock chemical interaction in CO2-based geothermal systems. J Geochem Explor 144:179–193. https://doi.org/10.1016/j.gexplo.2014.02.002
Tixier M, Loveless G, Anderson R (1973) Estimation of formation strength from the mechanical properties log. J Pet Technol 27:283–293
Tomson M et al (2008) Mechanistic understanding of rock/phosphonate interactions and effect of metal ions on inhibitor retention. SPE J 13(3):325–336
Tronvoll J, Fjaer E (1994) Experimental study of sand production from perforation cavities. Int J Rock Mech Min Sci Geomech Abstr 31(5):393–410
UKOG (2015) Oil and gas in UK: Economic report. UK Oil & Gas PLC, London
Vasarhelyi B, Van P (2006) Influence of water content on thestrength of rock. Eng Geol 84(1–2):70–74
Vidrine D, Benit E (1968) Field verification of the effect of differential pressure on drilling rate. J Pet Technol 20(7):676–682
Voordou WG et al (2011) Souring treatment with nitrate in fields from which oil is produced by produced water reinjection. Society of Petroleum Engineers, Woodland
Wang H, Cardiff P, Sharma M (2016) A 3-D poro-elasto-plastic model for sand production around open-hole and cased and perforated wellbores. Houston, TX, USA , Proceedings of the 50th US Rock Mechanics/Geomechanics Symposium
Wang J (1994) Effect of grain size distribution on the rheological behavior of polycrystalline materials. J Struct Geol 16:961–970
Wang Q, Zhou W, Hu Q, Xu H, Meendsen F, Shu Y, Qiao H (2021) Pore geometry characteristics and fluid-rock interaction in the Haynesville Shale, East Texas, United States. Energy and Fuels 35(1):237–250. https://doi.org/10.1021/acs.energyfuels.0c02423
Wei W et al (2020) Rock-fluid interaction and its application in unconventional production. Paper presented at the SPE/AAPG/SEG Unconventional Resources Technology Conference, Unconventional Resources Technology Conference. https://doi-org.ezproxy.rgu.ac.uk/10.15530/urtec-2020-1050
William E, Glassley LJ, Crossey I, Montanez P (2016) Fluid–rock interaction. In: White W (ed) Fluid–rock interaction. Springer International Publishing, Switzerland
Willson S, Moschovidis Z, Cameron J, Palmer I (2002) New model for predicting the rate of sand production. In: SPE/ISRM Rock Mechanics Conference, Irving, pp 152–160
Wilson A (2016) Chemical analysis of flowback water and downhole gas-shale samples. J Pet Technol 68(9):114–115
Wong LNY, Maruvanchery V, Liu G (2015) Water effects on rock strength and stiffness degradation. Acta Geotech 11(4). https://doi.org/10.1007/s11440-015-0407-7
WPC (2021) World petroleum council. https://www.world-petroleum.org/edu/221-why-are-oil-and-gas-important. Accessed Mar 14 2022
Wu G, Sharma M (1989) Model for precipitation and dissolution processes with precipitate migration. Am Inst Chem Eng 35(8)
Wu J et al (2018) Particle size distribution of aggregate effects on mechanical and structural properties of cemented rockfill: experiments and modeling. Constr Build Mater 193:295–311
Wuyep E, Oluyemi G, Yates K, Akisanya A (2018) Geomechanical effects of oilfield chemicals on sand failure in reservoir rocks. J Petrol Sci Eng
Wuyep E, Oluyemi G, Yates K, Akisanya A (2019) Evaluation of interactions between oilfield chemicals and reservoir rocks. Nat Resour Res 29(2):1239–1258
Wuyep E, Oluyemi G, Yates K, Akisanya AR (2020) Sand failure: effect of biocide on the geomechanical properties of outcrop carbonate rock under static condition. Arab J Geosci 13:1239. https://doi.org/10.1007/s12517-020-06176-y
Xu F et al (2018) Effect of the drilling fluid on hardness characteristics of tight sandstone. Curr Sci 115:2015–2018
Yadav PK et al (2016) Effect of drilling fluid on rock mechanical properties at near-drilling conditions: an implication of fluid design on wellbore stability. Kuala Lumpur, Malaysia, In proceedings of the technology conference, Asia
Yang G et al (2017a) Numerical investigation into the impact of CO2-water-rock interactions on CO2 injectivity at the Shenhua CCS demonstration project, China. Geofluids :1468-8115
Yang W, Brownlow JW, Walker DL, Lu J (2021) Effect of surfactant-assisted wettability alteration on immiscible displacement: a microfluidic study. Water Resour Res 57(8):1–18. https://doi.org/10.1029/2020WR029522
Yang L et al (2017b) Fluid–rock interactions during continuous diagenesis of sandstone reservoirs and their effects on reservoir porosity. J Intl Assoc Sedimentol 64(5):1303–1321
Yang L, Xu T, Liu K, Peng B, Yu Z, Xu X (2017c) Fluid–rock interactions during continuous diagenesis of sandstone reservoirs and their effects on reservoir porosity. Sedimentology 64(5):1303–1321. https://doi.org/10.1111/sed.12354
You J, Lee KJ (2020) Analyzing the dynamics of mineral dissolution during acid fracturing by pore–scale modeling of acid–rock interaction. Paper presented at the SPE improved oil recovery conference, society of petroleum engineers (SPE)
Yu B et al (2016) Experimental study of compaction and fractal properties of grain size distribution of saturated crushed mudstone with different gradations. Rock Soil Mech 37:1887–1894
Zhang HZLJ (2018) Microstructures, mineral compositions, and mechanical properties of red layers in Southern China. Adv Mater Sci Eng :1–9
Zhang J, Wang H, Chen S, Li Y (2018) Bearing deformation characteristics of large-size broken rock. J China Coal Soc 43:1000–1007
Zhang M, Huang S, Wang Q (2006) Thermodynamics model for the characteristic of the dissolution of primary minerals related to clastic diagenesis. Xinjiang Geol 24(2):187–191
Zhang S, Yang L, DePaolo D, Steefel C (2015) Chemical affinity and pH effects on chlorite dissolution kinetics under geological CO2 sequestration related conditions. Chem Geol 396:208–217
Zhao D, Yin D (2021) Effect of fluid/rock interactions on physical character of tight sandstone reservoirs during CO2 flooding. Geofluids :5552169. https://doi.org/10.1155/2021/5552169
Zhao F et al (2016) Simultaneous inhibition of sulfate-reducing bacteria, removal of H2S and production of rhamnolipid by recombinant Pseudomonas stutzeri Rhl: applications for microbial enhanced oil recovery. Bioresour Technol 207:24–30
Zhao W, Huang J, Su Q, Liu T (2018) Models for strength prediction of high-porosity cast-in-situ foamed concrete. Adv Build Technol Constr Mater. https://doi.org/10.1155/2018/3897348
Zhong Y et al (2019) Effect of Fracturing fluid/shale rock interaction on the rock physical and mechanical properties, the proppant embedment depth and the fracture conductivity. Rock Mech Rock Eng 52:1011–1022
Zhong H, Yang T, Yin H, Lu J, Zhang K, Fu C (2020) Role of alkali type in chemical loss and ASP-flooding enhanced oil recovery in sandstone formations. SPE Reserv Eval Eng 23(02):431–445
Zhou S, Sun F (2016) Sand production mechanism and changes of rock properties affected by sand production. In: Sand production management for unconsolidated sandstone reservoirs. s.l, pp. 31-55. https://doi.org/10.1002/9781118961865.ch2
Zhu G et al (2016) New experiment system for the interaction between soft rock and water: a case study on the Mogao Grottoes support rock. Colombia, XV Colombian Geotechnical Congress & II International Specialized Conference of Soft Rocks
Zoback M (2010) Reservoir geomechanics. In: Reservoir geomechanics. New York, NY, USA: 1st ed.; Cambridge University Press
Funding
The authors recognize the Tertiary Education Trust Fund (TETFund), Nigeria, for their sponsorship of the Research study. The Engineering school, Robert Gordon University, Aberdeen is also acknowledged for resources made available towards this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Zeynal Abiddin Erguler
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Peretomode, E., Oluyemi, G. & Faisal, N.H. Oilfield chemical-formation interaction and the effects on petrophysical properties: a review. Arab J Geosci 15, 1223 (2022). https://doi.org/10.1007/s12517-022-10469-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-10469-9