Abstract
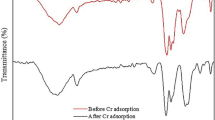
In the present study, iron-pillared clay was prepared using ferrous nitrate Fe(NO3)3. The removal of Reactive Blue (RB4) dye by ultrasound-assisted sorption onto the synthesized pillared Tunisian clay in aqueous solutions was studied. The natural and modified clay samples were characterized using XRD, FTIR TEM, and nitrogen adsorption measurement methods. Mineralogical data show that smectites are the main minerals of clay samples. FTIR spectra indicate that the stretching vibration bands of the structural hydroxyl groups are broad after treatments. A new band appears at 1570 cm−1, after the sorption of RB4 dye, attributed to aromatic C = C stretching vibration, which suggests the adsorption of RB4. A remarkable decrease was detected in the BET surface after sono-adsorption indicating that the sites of modified clay are almost occupied by the dye. The maximum Reactive Blue 4 quantity was removed at pH 8. Meanwhile, more active Fe–C surface sites were available for both adsorption and sono-adsorption with increasing RB4 concentration, thereby increasing the removal efficiency. Monolayer adsorption capacity via the sono-assisted method also showed a high removal amount of 46.51 mg/g. Langmuir’s model describes the experimental data better than Freundlich’s. A comparative study confirmed that RB4 sono-adsorption by a Tunisian clay sample was higher than others used in other relevant studies, highlighting that these clay deposits may be applied to the dyes’ elimination from wastewaters.










Similar content being viewed by others
References
Adeogun A, Osideko OA, Idowu M, Shappur V, Akinloye O, Babu BR (2020) Chitosan supported CoFe2O4 for the removal of anthraquinone dyes: kinetics, equilibrium and thermodynamics studies. SN Appl Sci 2. https://doi.org/10.1007/s42452-020-2552-3
Ali F, Reinert L, Levêque J-M, Levêque JM, Duclaux L, Muller F, Saeed S, Shah SS (2014) Effect of sonication conditions: solvent, time, temperature and reactor type on the preparation of micron sized vermiculite particles. Ultrason Sonochem 21:1002–1009
Almazán-Sánchez PT, Linares-Hernández I, Marcos J, Solache-Ríos M-M (2016) Textile wastewater treatment using iron-modified clay and copper-modified carbon in batch and column systems. Water Air Soil Pollut 227:100. https://doi.org/10.1007/s11270-016-2801-7
Amami-Hamdi A, Dhahri F, Jomaa-Salmouna D, Ben Ismail-Lattrache K, Ben Chaabane N (2016) Quantative analysis and paleoecology of Middle to Upper Eocene Ostracods from Jebel Jebil, central Tunisia. Rev Micropaléontologie 59:409–424
Asfaram A, Ghaedi M, Hajati S, Goudarzi A, Bazrafshan AA (2015) Simultaneous ultrasound-assisted ternary adsorption of dyes onto copper-doped zinc sulfide nanoparticles loaded on activated carbon: Optimization by response surface methodology. Spectrochim Acta Part A Mol Biomol Spectrosc 145:203–212
Becelic-Tomin M, Dalmacija B, Rajic L, Tomasevic D, Kerkez D, Watson M, Prica M (2014) Degradation of anthraquinone Dye Reactive Blue 4 in pyrite ash catalyzed Fenton reaction. Sci World J. https://doi.org/10.1155/2014/234654
Bel Hadjltaief H, Ben Zina M, Galvez ME, Da Costa P (2016) Photocatalytic degradation of methyl green dye in aqueous solution over natural clay-supported ZnO–TiO2 catalysts. J Photochem Photobiol A Chem 315:25–33
Belachew N, Bekele G (2019) Synergy of magnetite intercalated bentonite for enhanced adsorption of congo red dye. SILICON 12:603–612. https://doi.org/10.1007/s12633-019-00152-2
Ben Ameur S, Barhoumi A, Bel hadjltaief H, Mimouni R, Duponchel B, Leroy G, Amlouk M, Guermazi H (2017) Physical investigations on undoped and Fluorine doped SnO2 nanofilms on flexible substrate along with wettability and photocatalytic activity tests. Mater Sci Semicond Process 61:17–26
Bethi B, Manasa V, Srinija K, Sonawane SH (2018) Intensification of Rhodamine-B dye removal using hydrodynamic cavitation coupled with hydrogel adsorption. Chem Eng Process - Process Intensif 134:51–57
Chatel G, Novikova L, Petit S (2016) How efficiently combine sonochemistry and clay science. Appl Clay Sci 119:193–201
Chu KH, Al-Hamadani YAJ, Park CM, Lee G, Jang M, Am J, Her NG, Son A, Yoon Y (2017) Ultrasonic treatment of endocrine disrupting compounds, pharmaceuticals, and personal care products in water: a review. Chem Eng J 327:629–647
Dietel J, Warr LN, Bertmer M, Steudel A, Grathoff G, Emmerich K (2017) The importance of specific surface area in the geopolymerization of heated illitic clay. Appl Clay Sci 139:99–107. https://doi.org/10.1016/J.CLAY.2017.01.001
Dil AA, Vafaei A, Ghaedi AM, Ghaedi M, Dil EA (2018) Multi-responses optimization of simultaneous adsorption of methylene blue and malachite green dyes in binary aqueous system onto Ni:FeO(OH)-NWs-AC using experimental design: derivative spectrophotometry method. Appl Organomet Chem 32:4149
Eloussaief M, Bel Hadjltaief H, Dammak N, Benzina M (2018) Valorisation of green algae from Tunisian littoral on dye elimination. Arab J Geosci 11:730
Eloussaief M, Chakroun S, Kallel N, Benzina M (2020a) Efficiency of clay materials collected from Ain Jaloula (Central Tunisia) in sunflower oil decolorization. EuroMediterr J Environ Integr 5:33
Eloussaief M, Hamza W, Ghorbali G, Kallel N, Benzina M (2020b) Fe-rich aragonite concretion applied to industrial dye purification using Fenton and photo-Fenton technologies. Waste Biomass Valorization 12:3303–3313
Eren Z (2012) Ultrasound as a basic and auxiliary process for dye remediation: a review. J Environ Manage 104:127–141
Fabryanty R, Valencia C, Soetaredjo FE, Putro JN, Santoso SP, Kurniawan A, Ju Y-H, Ismadji S (2017) Removal of crystal violet dye by adsorption using bentonite-alginate composite. J Environ Chem Eng 5:5677–5687
Hamza W, Chtara C, Benzina M (2016) Purification of industrial phosphoric acid (54%) using Fe-pillared bentonite. Environ Sci Pollut Res 23:15820–15831
Hamza W, Fakhfakh N, Dammak N, Bel Hadjltaeif H, Benzina M (2020) Sono-assisted adsorption of organic compounds contained in industrial solution on iron nanoparticles supported on clay: optimization using central composite design. Ultrason Sonochemistr 67:105–134. https://doi.org/10.1016/j.ultsonch.2020.105134
Jarraya I, Fourmentin S, Benzina M, Bouaziz S (2016) VOC adsorption on raw and modified clay materials. Chem Geol 275:1–8
Jorfi S, Darvishi Cheshmeh Soltani R, Ahmadi M, Khataee A, Safari M (2017) Sono-assisted adsorption of a textile dye on milk vetch-derived charcoal supported by silica nanopowder. J Environ Manage 187:111–121
Karmakera S, Naga AJ, Sahaa TK (2020) Adsorption of Reactive Blue 4 Dye onto Chitosan 10B in aqueous solution: kinetic modeling and isotherm analysis. Russ J Phys Chem A 94:2349–2359. https://doi.org/10.1134/S0036024420110126
Kashif Uddin M (2016) A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem Eng J 308:438–462. https://doi.org/10.1016/j.cej.2016.09.029
Khoualdia B, Loungou M, Elaloui E (2017) Adsorption of organic matter from industrial phosphoric acid (H3PO4) onto activated bentonite. Arab J Chem 10:1073-S1080
Machado AT, Valenzuela-Diaz FR, de Souza CAC, de Andrade Lima LRP (2011) Structural ceramics made with clay and steel dust pollutants. Appl Clay Sci 51:503–506
Milenković DD, Bojić AL, Veljković VB (2013) Ultrasound-assisted adsorption of 4-dodecylbenzene sulfonate from aqueous solutions by corn cob activated carbon. Ultrason Sonochem 20:955–962
Mittal A, Mittal J, Malviya A (2010) Adsorption of hazardous dye crystal violet from wastewater by waste materials. J Colloid Interface Sci 343:463–473
Miyah Y, Lahrichi A, Idrissi M, Boujraf S, Taoudad H, Zerrouq F (2017) Assessment of adsorption kinetics for removal potential of Crystal Violet dye from aqueous solutions using Moroccan pyrophyllite. J Assoc Arab Univ Basic Appl Sci 23:20–28
Oukebdane K, Lacene Necer I, Didi MA (2022) Binary comparative study adsorption of anionic and cationic Azo-dyes on Fe3O4-bentonite magnetic nanocomposite: kinetics, equilibrium, mechanism and thermodynamic study. SILICON. https://doi.org/10.1007/s12633-022-01710-x
Roosta M, Ghaedi M, Shokri N, Daneshfar A, Sahraei R, Asghari A (2014) Optimization of the combined ultrasonic assisted/adsorption method for the removal of malachite green by gold nanoparticles loaded on activated carbon: experimental design. Spectrochim Acta Part A Mol Biomol Spectrosc 118:55–65
Sabna V, Thampi SG, Chandrakaran S (2016) Adsorption of crystal violet onto functionalised multi-walled carbon nanotubes: equilibrium and kinetic studies. Ecotoxicol Environ Saf 134:390–397
Sajjadi S, Khataee A, Kamali M (2017) Sonocatalytic degradation of methylene blue by a novel graphene quantum dots anchored CdSe nanocatalyst. Ultrason Sonochem 39:676–685
Sarma GK, Sen Gupta S, Bhattacharyya KG (2016) Adsorption of Crystal violet on raw and acid-treated montmorillonite, K10, in aqueous suspension. J Environ Manage 171:1–10
Sellaoui L, Dhaouadi F, Reynel-Avila HE, Mendoza-Castillo DI, Bonilla-Petriciolet A, Trejo-Valencia R, Taamalli S, Louis F, El Bakali A, Chen Z (2021) Physicochemical assessment of anionic dye adsorption on bone char using a multilayer statistical physics model. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-021-15264-9
Soltani RDC, Jorfi S, Safari M, Rajaei M-S (2016) Enhanced sonocatalysis of textile wastewater using bentonite-supported ZnO nanoparticles: response surface methodological approach. J Environ Manage 179:47–57
Ullhyan A (2014) Adsorption of reactive blue-4 dye from aqueous solution onto acid activated mustard stalk: equilibrium and kinetic studies. Glob J Biol Agric Health Sci 3:98–105
Wu TY, Guo N, Teh CY, Hay JXW (2013) Theory and fundamentals of ultrasound. In: Advances in utrasound technology for environmental remediation. Springer Briefs in Molecular Science, Green Chemistry for Sustainability. https://doi.org/10.1007/978-94-007-5533-8
Younes H, El Etriby H, Kh MH (2021) High removal efficiency of reactive yellow 160 dye from textile wastewater using natural and modified glauconite. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-021-03528-3
Zhai Y, Qu H, Li Z, Zhang B, Cheng J, Zhang J (2020) Rapid and efficient adsorption removal of Reactive Blue 4 from aqueous solution by cross-linked microcrystalline cellulose-epichlorohydrin polymers: isothermal, kinetic, and thermodynamic study. Transactions of Tianjin University 27:77–86. https://doi.org/10.1007/s12209-020-00245-9
Acknowledgements
The authors thank Mr Nidhal Baccar, Laboratory technician (Center of Biotechnology of Sfax) for analyzing FTIR samples. They would also like to express their thanks to Dr. Abdelmajid Dammak (Professor at the University of Sfax) for the English revision of this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Responsible Editor: Amjad Kallel
Rights and permissions
About this article
Cite this article
Eloussaief, M., Hamza, W., Fakhfakh, N. et al. Characterization of iron-modified natural clay for textile dye retention by sono-adsorption technology. Arab J Geosci 15, 1109 (2022). https://doi.org/10.1007/s12517-022-10400-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-10400-2