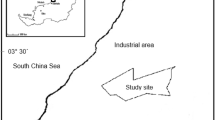
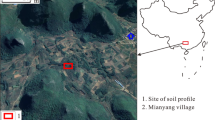
Abstract
Effects of acid rain in soils have received less research attention when compared with other environmental issues. Understanding and simulating the long-term effects of acid rain on the leaching of cations from calcareous soils are essential. Therefore, this study was carried out to determine the effect of simulated acid rain on the cations leaching in a calcareous sandy loam soil at various pH levels of 2.5, 3.0, 3.5, 4.0, 4.5, 5.0, and 7.0. Results showed that the leaching of cations depended on soil pH. After 40 days of leaching, calcium (Ca2+) and magnesium (Mg2+) losses were 361.8 to 116.5, and 156.1 to 64.9 mg kg−1, at pH treatments of 2.5 and 7.0, respectively, while potassium (K+) losses from soil at pH 2.5 and 7.0 were 31.8 and 17.5 mg kg−1, respectively. The amount of sodium (Na+) losses was lower than other cations. A nonlinear correlation was found between the cumulative amounts of cations leached and pH. Additionally, PHREEQC was used to simulate the transport of cations and experimental data sets agree well with the model predictions. Based on this study, the observed concentrations of base cations in the leachates result from the dissolution of soil minerals, such as calcite, dolomite, gypsum, halite, sylvite, and cation exchange in soils. The findings suggested that, in the long-term, acidic inputs would increase cation losses from calcareous soils, potentially leading to plant deficiencies and groundwater quality degradation.







Similar content being viewed by others
Data availability
Not applicable.
References
Allison JD, Brown DS, Novo-Gradac KJ (1991) MINTEQA2/PRODEFA2, A geochemical assessment model for environmental systems: version 3.0 users manual. US Environmental Protection Agency, Athens, GA (EPA/600/3–91/021)
Appelo CAJ, Postma (1996) Geochemistry, groundwater, and pollution. Balkema, Rotterdam, p 536
Colombani N, Pia Gervasio M, Castaldelli G, Mastrocicco M (2020) Soil conditioners effects on hydraulic properties, leaching processes and denitrification on a silty-clay soil. Sci Total Environ 7331:139342
Dan D, Tao Y, Yixiang D, Fuhong S, Jian Z, Chengda H (2019) Acid deposition induced base cation loss and different responses of soils and sediments in Taihu Lake watershed, China. Chemosphere 226:149–158
Ding Z, Wang Q, Hu X (2011) Fractionation of Zn and Pb in bulk soil and size fractions of water-stable micro-aggregates of lead/zinc tailing soil under simulated acid rain. Procedia Environ Sci 10(Part A):325–330
Goulding KWT (2016) Soil acidification and the importance of liming agricultural soils with particular reference to the United Kingdom. Soil Use Manage 32(3):390–399
Guicharnaud R, Paton JL (2006) An evaluation of acid deposition on cation leaching and weathering rates of an Andosol and a Cambisol. J Geochem Explo 88:279–283
Grennfelt P, Engleryd A, Forsius M, Hov Ø, Rodhe H, Cowling E (2020) Acid rain and air pollution: 50 years of progress in environmental science and policy. Ambio 49:49–64
Hesthagen T, Fjellheim A, Schartau AK, Wright RF, Saksgård R, Rosseland BO (2011) Chemical and biological recovery of Lake Saudlandsvatn, a formerly highly acidified lake in southernmost Norway, in response to decreased acid deposition. Sci Total Environ 409:2908–2916
Jalali M, Amirabadi Farhani E, Jalali M (2022) Simulating phosphorus leaching from two agricultural soils as affected by different rates of phosphorus application based on the geochemical model PHREEQC. Environ Monit Assess 194(3):164
Jalali M, Moradi A (2020) Measuring and simulating pH buffer capacity of calcareous soils using empirical and mechanistic models. Arch Agron Soil Sci 66:559–571
Jalali M, Naderi E (2012) The impact of acid rain on phosphorus leaching from a sandy loam calcareous soil of the western Iran. Environ Earth Sci 66:311–317
Jalali M, Ranjbar F (2009) Effects of sodic water on soil sodicity and nutrient leaching in poultry and shep manure amended soils. Geoderma 153:194–204
Jalali M (2007) A study of the quantity/intensity relationships of potassium in some calcareous soils of Iran. Arid Land Res and Manag 21:133–141
Jiang J, Wang Y, Yu M, Cao N, Yan J (2018) Soil organic matter is important for acid buffering and reducing aluminum leaching from acidic forest soils. Chem Geol 501:86–94
Kypritidou K, Doulgeris Ch, Tziritis E, Kinigopoulou V, Jellali S, Jeguirim M (2022) Geochemical modelling of inorganic nutrients leaching from an agricultural soil amended with olive-mill waste biochar. Agronomy 12(2):480
Leys BA, Likens GE, Johnson CE, Craine JM, Lacroix B, McLauchlan KK (2016) Natural and anthropogenic drivers of calcium depletion in a northern forest during the last millennium. Proc Natl Acad Sci USA 113:6934–6938
Li J, Jia Ch, Lu Y, Tang S, Shim H (2015) Multivariate analysis of heavy metal leaching from urban soils following simulated acid rain. Microchem J 122:89–95
Liao J, Hu C, Wang M, Li X, Ruan J, Zhu Y, Fairchild IJ, Hartland A (2018) Assessing acid rain and climate effects on the temporal var-iation of dissolved organic matter in the unsaturated zone of a karstic system from southern China. J Hydrol 556:475–487
Liu MX, Song Y, Xu TT et al (2020) Trends of precipitation acidification and determining factors in China during 2006–2015. J Geophys Res: Atmos 125(6):e2019JD031301
Luo WT, Nelson PN, Li M-H, Cai JP, Zhang YY, Zhang YG, Yang S, Wang RZ, Wang ZW, Wu YN, Han XG, Jiang Y (2015) Contrasting pH buffering patterns in neutral-alkaline soils along a 3600 km transect in northern China. Biogeosciences. 12(23):7047–7056
Luo WT, Nelson PN, Li MH, Cai JP, Zhang YY, Zhang YG, Yang S, Wang RZ, Wang Z, Wu YN, Han XG, Jiang Y (2015) Contrasting pH bu ering patterns in neutral-alkaline soils along a 3600 km transect in northern China. Biogeosciences 12:7047–7056
Lu XK, Mo JM, Gundersern P, Zhu WX, Zhou GY, Li DJ, Zhang X (2009) Effect of simulated N deposition on soil exchangeable cations in three forest types of subtropical China. Pedosphere 19:189–198
Lv Y, Wang C, Jia Y, Wang W, Ma X, Du J, Tian X (2014) Effects of sulfuric, nitric, and mixed acid rain on litter decomposition, soil microbial biomass, and enzyme activities in subtropical forests of China. Appl Soil Ecol 79:1–9
Naderi Peikam E, Jalali M (2021) Chemical composition of rainwater at an urban and two rural stations in the west of Iran. Hamedan Environ Earth Sci 80:605
Najafi S, Jalali M (2016) Effect of heavy metals on pH buffering capacity and solubility of Ca, Mg, K, and P in non-spiked and heavy metal-spiked soils. Environ Monit Assess 188:342
Pierret M-C, Viville D, Dambrine E, Cotel S, Probst A (2019) Twenty-five year record of chemicals in open field precipitation and throughfall from a medium-altitude forest catchment (Strengbach-NE France): an obvious response to atmospheric pollution trends. Atmos Environ 202:296–314
Rowell DL (1994) Soil science: methods and applications. Longman Group, Harlow, p 345
Shi Z, Zhang J, Xiao Z, Lu T, Ren X, Wei H (2021) Effects of acid rain on plant growth: a meta-analysis. J Environ Manage 1(297):113213
Starr M, Lindroos A-J, Ukonmaanaho L (2014) Weathering release rates of base cations from soils within a boreal forested catchment: variation and comparison to deposition, litterfall and leaching fluxes. Environ Earth Sci 72:5101–5111
Walna B, Drzymała S, Siepak J (1998) The impact of acid rain on calcium and magnesium status in typical soils of the Wielkopolski National Park. Sci Total Environ 220:15–120
Wang D-Z, Jiang X, Rao W, He J-Z (2009) Kinetics of soil cadmium desorption under simulated acid rain. Ecol Comp 6:432–437
Wayland KG, Long DT, Hyndman DW, Pijanowski BC, Woodhams SM, Haack SK (2003) Identifying relationships between baseflow geochemistry and land use with synoptic sampling and R-mode factor analysis. J Environ Qual 32:180–190
Wei X, Liu Sh, Müller K, Song Z, Guan G, Luo J, Wang H (2019) Urbanization-induced acid rain causes leaching loss of calcium from limestone-derived soil in South China. J Soils Sed 19:3797–3804
Wen F, Hou H, Yao N, Yan Z, Bai L, Li F (2013) Effects of simulated acid rain, EDTA, or their combination, on migration and chemical fraction distribution of extraneous metals in ferrosol. Chemosphere 90:349–357
Xu RK, Yuan JH, Jiang J (2012) pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J Soils Sediments 12:494–502
Yang Y, Wang Y, Peng Y, Cheng P, Li F, Liu T (2020) Acid-base buffering characteristics of non-calcareous soils: correlation with physicochemical properties and surface complexation constants. Geoderma 360:114005
Zha F, Liu C, Kang B, Yang X, Zhou Y, Yang C (2021) Acid rain leaching behavior of Zn-contaminated soils solidified/stabilized using cement-soda residue. Chemosphere 281:130916
Zhao C, Ren S, Zuo Q, Wang S, Zhou Y, Liu W, Liang S (2018) Effect of nanohydroxyapatite on cadmium leaching and environmental risks under simulated acid rain. Sci Total Environ 15(627):553–560
Zhou Y, Li P, Xue L, Dong Z, Li D (2020) Solute geochemistry and groundwater quality for drinking and irrigation purposes: a case study in Xinle City, North China. Geochemistry 80:125609
Zhu H, Wu C, Wang J, Zhang X (2018) The effect of simulated acid rain on the stabilization of cadmium in contaminated agricultural soils treated with stabilizing agents. Environ Sci Pollut Res Int 25(18):17499–17508
Zhang J-E, Ouyang Y, Ling D-J (2007) Impacts of simulated acid rain on cation leaching from the Latosol in south China. Chemosphere 67:2131–2137
Author information
Authors and Affiliations
Contributions
ENP: contributed to the experiments and performed the simulations. MJ: contributed to the design of the study, and supervised the work and writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Amjad Kallel
Rights and permissions
About this article
Cite this article
Jalali, M., Peikam, E.N. Measuring and simulating the effect of acid rain on cations leaching from calcareous soil of Western Iran. Arab J Geosci 15, 1194 (2022). https://doi.org/10.1007/s12517-022-10397-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-10397-8