Abstract
This study deals with improving the properties of lightweight aggregate by the addition of Ca-bentonite mineral to expandable clays. The addition of bentonite can result in advanced lightweight aggregate materials which meet international standards. Besides bentonite material, twelve different clays (from Egyptian and Hungarian mines) were studied by X-ray fluorescence (XRF), X-ray powder diffraction (XRD, Rietveld method), and a heating microscope (HM). Aggregates were produced and their physical and mechanical properties were measured. The structure and the chemical composition of the clay-Ca bentonite aggregates were studied by scanning electron microscope (SEM) and with energy dispersive spectroscopy (EDAX). The results showed that the addition of 10 wt% bentonite can reduce the bulk density by up to 20%, can increase the compressive strength by up to 180%, and can increase the height of expansion by up to 68% in some samples. It was found that the high kaolinite content of the clay samples was not beneficial for expanding and decreasing the bulk density of aggregates. According to the Rietveld method from XRD and a statistical correlation model shown, the amorphous contents have a reciprocal relationship with the bulk density and a direct relationship with the height expansion. Bentonite, as a natural additive, to some clays, was suitable for producing lightweight aggregates with far better properties, as well as a more economical means of production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to their thermal performance, sound insulation, and good fire resistance, lightweight aggregates (LWA) are becoming the focus of interest all over the world. They are characterized as building materials that have a bulk density of less than 1.2 g/cm3, particle density in the range of 0.8–2 g/cm3, and compressive strength higher than 1 MPa (Choudhry and Hadley 1992; Liao and Huang 2011). Lightweight aggregates are made from materials such as clay, shale, slag, natural pumice, tuff, and perlite. To prepare LWA from expandable clay, the aggregates must be expanded to approximately twice the volume of the original raw material.
During the industrial process, clay is crushed, screened to size, and finally fired in a rotary kiln. Firing temperatures are around 1300 °C (Rashad 2018; Alexander et al. 1999). A clinker cooler is used to decrease the temperature of the aggregates which are finally crushed and screened if necessary (Ozguven and Gunduz 2012; Chandra and Berntsson 2002).
The mechanism of expansion in LWAs, which takes place in several steps, was described by Riley (1951). The first step is the evolution of gasses at a certain temperature where the material is in its visco-plastic state. The sources of gas generation in this process can be the reduction of ferric oxide, the combustion of organic matters or the thermal decomposition of carbonates (Rudnai 1963; Chopra et al. 1964; Lee 2016). In the second step, a partially molten state of the clay prevents the escape of gases. Finally, after cooling, the molten phases remain as glassy phases containing different crystalline compounds depending on the composition of the raw material (Ozguven and Gunduz 2012). When the temperature is too high during sintering, excess gas can evolve through the surface of the aggregates, and this increases the overall porosity and creates continuous pores (Yang et al. 2015). In addition, further increases in temperature beyond the pyro-plastic range can lead to viscous flow, reducing porosity, and pore size (Dondi et al. 2016). Expanded aggregates produced by heat treatment have lower density than natural aggregates and result in lighter concrete products.
For improving the physical, mechanical, or expansion properties of lightweight aggregates, additive materials are required (Kocserha and Gömze 2010; Kocserha et al. 2018). Some of these additives, such as iron, can increase expansion, but bulk density and compressive strength are simultaneously reduced (Bernhardt et al. 2014).
With the addition of CaF2, growth in the glassy phase was achievable, but it decreased the sintering and melting temperature by 125–200 °C lower than the temperature used in the production of LWAs (Lee and Liu 2009; Abdelfattah et al. 2019).
In a previous experiment, carbon fibres were added to the expandable clay. This additive lowered the particle density, and resulted in higher overall porosity and compressive strength (Moreno-Maroto et al. 2017). Additives and their effects mentioned in related studies are summarized in Table 1.
In our study, bentonite as a natural additive was used to improve the properties of aggregates. Bentonites are clay soils formed by erosional action on volcanic ash, and consisting of montmorillonite, illite, and a variable composition of other minerals, such as quartz, sodium feldspar, and calcium feldspar according to the Rietveld method (Abu-Jdayil 2011). Structurally, bentonite is classified as a 2:1 layered aluminosilicate and ideally consists of an alumina octahedral sheet between two silica tetrahedral sheets (Ahmad et al. 2011; Paluszkiewicz et al. 2008).
Bentonite is used in several branches of industry. It is used as oil drilling mud, a suspending agent in oil drilling fluids, or as a binder in the production of pellets (Grim 1968). It plays a role in the construction of municipal waste or spent nuclear fuel storage barriers (Pusch 1992; Gens et al. 1998; Yong 1999; Cui et al. 2002; Chegbeleh et al. 2008; Murray 2006; Pusch and Weston 2012). In calcined form, it affects the fresh and strength properties of mortar and concrete (García-García et al. 2006; Ibrahim et al. 2019), or in other studies, it was used as a substitute for cement (Taylor-Lange et al 2015; Rajczyk and Langier 2012). In every case, it produced appropriate results.
In a preliminary study (Abdelfattah et al. 2020), three expandable Hungarian clays were mixed with 5, 10, and 20 wt% Ca-bentonite and the prepared aggregates were heat treated. The results showed that the addition of 10 wt% Ca-bentonite has a beneficial effect on most properties such as bulk density, compressive strength, and the height expansion of the aggregates.
The data in Table 1 show that researchers modified the properties of aggregates with artificial additives. In contrast, a natural additive, bentonite, was used in our experiment. The goals of this study were to produce LWAs from twelve expandable clays originating from different places and to observe and describe the effect of bentonite as a natural material addition on the properties of produced aggregates.
Materials and methods
Clays and bentonite used for our experiments originated from Hungary and Egypt. Three types of clay samples (referred to as yellow (Y), grey (G), and blue (B)) were collected from the Mályi clay mine, in Hungary, and nine were taken from the Giza (marked with 1), Fayum (2), Sinai (5), Helwan (6), and Saf (8,9,10,11,12) deposits, in Egypt (Fig. 1). The bentonite originated from a deposit in Hungary (Istenmezeje). The clays were dried at 105 °C for 24 h in an electric drying chamber. They were ground to fit through a sieve with an aperture of 100 µm for further measurements (Liao and Huang 2011; Bernhardt et al. 2014).
For the chemical analysis of all of the major and minor oxides, and trace elements of the samples, a Rigaku Supermini 200 WDXRF instrument was used. The mineralogical composition of the clays and aggregates was determined by the X-ray diffraction method; measurement was carried out on a Rigaku Miniflex II instrument with Cu-Kα radiation, λ = 1.5418 Å, in a range of 3° ≤ 2Θ ≤ 90. Phase identification and quantitative Rietveld refinements with the calculation of goodness of fit (GOF) were performed using the Profex 4.3 software. Differential thermal analysis (DTA) and thermogravimetric (TGA) measurements were also conducted (MOM Derivatograph C). To observe the melting properties and dimensional changes of the clays, a heating microscope (Camera Electronica) was used up to 1400 °C with 10° C/min heating rate. Image analysis revealed the main characteristic temperatures of the sintering process.
After analysis of the raw materials, clays and 10 wt% Ca-bentonite and water were mixed, and green pellets having a diameter of 6–14 mm were prepared by the hand-rolling method and by wetting the clays (Bernhardt et al. 2014; Fakhfakh et al. 2007). They were dried at 105 °C in a drying chamber. This method was used for clays with and without additives. In order to determine the appropriate sintering temperature, raw aggregates without bentonite were sintered at five different temperatures (1150–1275 °C), in an air atmosphere. The sintering time was 10 min at maximum temperature. After the heating process, the sintered products were cooled naturally in the furnace.
For the Hungarian samples, the best properties were achieved at 1225 °C, and for the Egyptian samples at 1275 °C. These results are presented below.
A scanning electron microscope in high vacuum mode (Carl Zeiss EVO MA10) was used to identify the surface morphology of the different samples of the clay aggregates. Adhesive tape was used to hold the specimens in place on the specimen holder and they were coated with gold according to ion beam sputtering.
The density and water absorption of the aggregates were measured according to the European standard (EN 13055–1 2002 and EN-1097–6:2013). The bulk density was calculated using Eq. (1)
Water absorption of the aggregates was determined according to Eq. (2)
where WD: the dry weight of the LWA, WS: 24-h saturated surface-dry weight, WI: immersed weight in water.
Similar to other studies (Hiramatsu and Oka 1966; Li et al. 2000; Liao and Huang 2011; Yang et al. 2015; Li et al. 2020), the compressive strength of the aggregates was measured on spherical aggregates individually. The aggregates were placed into a cylindrical mold, and an INSTRON universal tester with a cross-head speed of 0.1 mm/s was used to apply and register the fracture force. The results were calculated using the following formula (3)
where F: load belongs to fracture, D: diameter of the aggregates.
Results and discussion
Characterization of raw materials
The chemical compositions of the clay samples are summarized in Table 2. Oxides were classified into three groups such as silica, alumina, and total flux, and they are plotted in the Riley diagram, Fig. 2a, (Riley 1951). The amount of SiO2 in the clays ranged from 55.15 to 64.97%, of Al2O3 17.29–22.53%, the total flux varied between 15.96 and 21.24%. The iron contents of the samples are also indicated (6.3–14.21%). As Fig. 2a shows, each of the 12 clays was in the expanding range (red contour-encased area), which means that clay can be expanded when fired (Kavas et al. 2011; Anan and Abd El-Wahed 2017). Samples can obtain a suitable viscosity to trap a significant amount of the gaseous components, leading to the formation of the expanded structure of LWA (Hung and Hwang 2007).
After a 10% of bentonite addition to the clay sample, the amount of SiO2 in clays ranged from 55.28 to 64.12%, Al2O3 17.53–21.93%, total flux 17.23–21.32%, and the iron content varied from 7.12 to 14.24%.
Some of these values are plotted in the Riley diagram and presented in a magnified format in Fig. 2b. It can be seen that the points representing the different samples shifted toward the direction of fluxing oxides. Also, samples with bentonite contain more iron-oxide than the original raw materials.
A typical curve of DTA/TGA of the blue sample is shown in Fig. 3. Up to 550 °C, the DTA curve has the first wide exothermic peak which represents the burning out of the organic contents, and parallel to this, the decomposition of the structure of the clay minerals begins. At about 800 °C, the decompositions of carbonates ended. This is marked with point 1 on the TG curve. Above 1000 °C, a strong endothermic process begins which indicates that the melting of the sample begins. According to TG test, the total weight loss for the clay samples was between 8 and 19%.
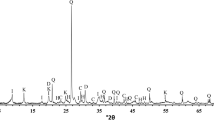
The mineral phases of the collected clay samples were revealed by XRD. Results can be seen in Table 3. Analysis showed that the major mineral phases in all samples are quartz, illite, kaolinite, calcite, albite, and smectite. Other phases like muscovite, halite, orthoclase, goethite, clinochlore, and hematite were also found in the clays.
Characterization of clay samples with bentonite
Expansion extent
The melting behaviour of the clay was studied using a heating microscope. Photos were taken of all samples with and without bentonite additive at different temperatures (T). Image analysis revealed the height expansions (H) and the maximum height of expansion (MHE) of the clay samples (Li et al. 2020; Karaś et al. 2019). The MHE value indicates how much higher the height of the expanded sample is than that of the green sample. Besides the results, Fig. 4 shows the changes that occurred in the shape of sample 1 at different temperatures. For sample 1 from Egypt (Fig. 4a), the value of MHE was 129% at 1270 °C. After the addition of 10% of bentonite (Fig. 4b), the values of MHE increased to 135% at 1241 °C.
In the Riley diagram, a shift toward the direction of fluxing oxides due to the addition of bentonite was clearly observable, according to the results of heating microscope measurements. Based on the comparison of Fig. 4a and b, the temperature which belongs to MHE decreased by about 30 °C and the temperature at which a 10% increase in the height of the sample occurred was 75 °C lower with the addition of bentonite. Numerical values for all samples are summarized in the first columns in Table 4, while the results can be compared with the help of Fig. 5. The MHE value of the Hungarian samples without bentonite was between 22 and 38% while for the Egyptian samples, it ranged from 12 up to 44%. After the addition of 10% of bentonite to the clays, the value of MHE was higher in most cases except for samples 8 and 10. For the Hungarian clays, MHE increased from 33 to 64% while the increase was between 20 and 35% for the Egyptian samples.
Bulk density and compressive strength
The bulk density for pelletized aggregates without any additive in this study was found to be less than 1.2 g/cm3. For the Hungarian samples, the range of density was between 0.57 and 1.2 g/cm3 at 1225 °C, while for the Egyptian samples at 1275 °C, the lowest and the highest values were 0.66 and 1.11 g/cm3, respectively. Among samples without any additive, the blue clay sample had the lowest density. This was probably related to the greater amount of its low viscosity glassy phase during firing. The numerical values can be found in Table 4. The results of the compressive strength measurement were in the interval of 1.54–4.82 MPa for the Hungarian aggregates and 1.18–10.4 MPa for the Egyptian clay. All strength values are higher than 1 MPa, so these are strong enough to be used as LWAs (Liao and Huang (2011)).
Figure 6a, b show the change in bulk density and compressive strength before and after the addition of 10 wt% Ca-bentonite. The addition of bentonite had a great effect on the density and compressive strength of the aggregates. The bulk density of the Hungarian samples changed to 0.55–0.95 g/cm3, which is a 4–26% decrease. With decreasing density, there was a significant improvement in compressive strength. The strength of the aggregates was 48–112% higher. This is a beneficial change for LWAs, as it can result in lighter building blocks and concrete elements with elevated strength.
The bulk density of the Egyptian clays with 10% bentonite changed in a different way, ranging from 0.65 to 1.02 g/cm3. The change was both decrease and increase depending on the sample. Compressive strength increased in five of the nine Egyptian clays tested, while it decreased by 18–70% in the four other cases. The change in density and compressive strength of the aggregates is related partly to the amount of kaolinite phase in the samples. Kaolinite was present in high percentages in samples 1, 8, 9, 10, and 11 (> 25 wt%). The compressive strength of sample 5 decreased due to its 10.92% calcite content. With increasing temperature, the decomposition of calcite increases CO2 emission, causing more porosity and a decrease in density and compressive strength.
For the samples (8, 9, and 11) where the quartz phase was low, adding Ca-bentonite resulted in an increase in the bulk density, and for samples 9 and 11, compressive strength decreased remarkably.
The range of water absorption for these aggregates is 0–30% (Table 4). Some of these samples, such as the blue and the grey, had very low water absorption because the aggregates were covered with a glazed surface which formed from the amorphous contents, and such a surface affects the measured water absorption.
Plotting the MHE as a function of density (Fig. 7a and b), the results of the samples without bentonite do not show a statistical correlation (R2 = 0.21), but a correlation (R2 = 0.77) can be assumed when bentonite is added.
Comparing our results to studies (Table 1) where artificial additives were applied, it can be said that bentonite as natural additive is also suitable for enhancing the properties of LWAs.
Mineral phases and microstructure of lightweight aggregates
To define the mineral phases in the LWAs, 12 samples were selected. Heat treatment (Hungarian clays at 1225 °C and Egyptian clays at 1275 °C) of the clay aggregates resulted in the amorphous phase being the main phase in the samples. The mineral phases for the additive free aggregates were the same as the crystalline phases of the aggregates with Ca bentonite.
Besides the 66–78% glassy phase, some crystalline phases were also found. In the Hungarian samples, quartz, hematite, and mullite were the main phases (Table 5). In most of the Egyptian samples, mullite was the main crystalline phase, thanks to the significant kaolinite content. Besides quartz and hematite and feldspars, other minerals like cordierite, cristobalite, and tridymite were also found.
Iron-containing phases or pure iron powder play an important role in the expansion mechanism of the clays and affect the properties of aggregates (Bernhardt et al. 2014). As the iron oxide content of Ca-bentonite was relatively high (14.5%), this favoured the formation of hematite. Figure 8 shows the change in the amount of hematite for samples with and without additives. With the exception of samples 2 and 9, it was higher in all cases where bentonite was used.
Another factor that may affect the properties of the aggregates is the amorphous phase. The amount of amorphous content was much higher compared to the crystalline phase. In the case of clay ceramics, the increasing amorphous content with increasing temperature generally increases the density, as it is accompanied by shrinkage. This usually means a more compact structure. However, in the case of LWAs, the pressure of the gas phase at the firing temperature and the amorphous phase in the plastic state together result in an increase in the volume of the samples. Using correlation analysis, we can search for correlations between the properties of LWAs and amorphous content. Table 6 shows a correlation model (MS Excel) for the clays studied. From the correlation for regression values, the amorphous glassy contents have a good reciprocal relationship with the bulk density, with regression values of − 0.8, and there is a direct relationship with the height of expansion. Regarding the latter, the regression coefficient is only 0.64, and this means it does not have a strong effect.
In order to observe the pore distribution and the microstructure of the LWAs, we prepared and analysed aggregates with SEM. The structure of LWA is consistent with the phase composition in all of the samples. The structure of Sample 1 can be seen in Figs. 9 and 10 before and after the addition of bentonite. It can be seen in Fig. 9a that the cross-section of the aggregates consisted of two layers. Inside the outer layer, there are small pores with an irregular distribution. In contrast to this, the inner side is one large pore. This type of structure develops if the outer layer of the aggregates is closed by a silicate melt, and the evolved gas trapped inside and the amount of it that reaches the outer layer is very little. The process produces higher pressure inside the aggregate, so MHE increases and the density decreases.
After the addition of 10 wt% of bentonite, the structure of the sample was different. It can be observed in Fig. 9b that the pore distribution became homogeneous; the pore size distribution seems to be identical throughout the cross-section and the surface of aggregates is also smoother. The new structure with bentonite is better, because regular pore size can increase the compressive strength of LWA, as it causes a more homogeneous stress distribution throughout the microstructure (Bernhardt et al. 2014; Cheeseman and Virdi 2005).
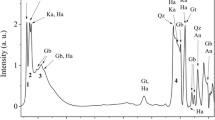
SEM images showed large amounts of white spots on the fracture surfaces of the samples, and these multiplied around the pores and on the surface of the pores. Figures 10a and b provide an example. EDAX studies have revealed that these are high iron phases. In the pictures, numbers indicate the studied areas; the chemical compositions of which are given in Table 7. Point 1, measured by EDAX, can be found in Fig. 9a.
Conclusion
In contrast to previous research (Table 1) which used artificial additives to modify the properties of the expandable clays, in this study, a natural additive, bentonite, was used. The effect of Ca-bentonite as a natural additive on the properties of light expanded clay aggregate was studied. The addition of bentonite is intended to improve the properties of LWA and reduce the energy required for production. The behaviour of twelve clay-bentonite mixtures (three Hungarian and nine Egyptian clay samples) was examined.
Bentonite as an additive with a high iron oxide and clay mineral content clearly influenced and changed the properties of each clay. With the addition of bentonite, not only was more iron added to the mixture, but the decomposition of the introduced clay minerals resulted in additional alkali and alkaline earth metal oxides, which lowered the expansion temperature of the clay mixtures.
In the case of the Hungarian clays, the densities decreased, and the strength values doubled in some samples. Bentonite allowed for the production of much better quality LWA at lower temperatures for these clays. In addition to the economic benefits of lower combustion temperatures, CO2 emissions can also be reduced.
In the case of the Egyptian clays, bentonite did not improve the properties in all cases. Of the nine Egyptian clays tested, five showed better sample strength (2–180% improvement), while four had a significant decrease (23–70% reduction). From the measured data, it can be seen that above 25% kaolinite content, in the case of Egyptian clays, bentonite addition increased the density of the samples, while at 20% lower kaolinite content, the density clearly decreased and the strength increased.
Not all clay is affected in the same way by the use of bentonite, as of course the constituent minerals of the clay also affect the results. It can be said that it is possible to improve the properties of LWAs with a natural additive like bentonite, but the appropriate amount must be selected for each clay and the sintering temperature of the mixture must be determined.
Change history
29 July 2022
Missing Open Access funding information has been added in the Funding Note.
References
Abdelfattah M, Kocserha I, Géber R, Móricz F (2020) Evaluating the properties and mineral phases of the expanded clay aggregates with the bentonite additive material. J Phys: Conf Ser 1527:012030. https://doi.org/10.1088/1742-6596/1527/1/012030
Abdelfattah M, Kocserha I, Géber R (2019) The effect of calcium fluoride on mineral phases and properties of lightweight expanded clay aggregates. XIIIth International Conference preparation of ceramic materials. P 141–146. ISBN 978–80–553–3314–4
Abu-Jdayil B (2011) Rheology of sodium and calcium bentonite–water dispersions: effect of electrolytes and aging time. Int J Miner Process 98(3–4):208–213. https://doi.org/10.1016/j.minpro.2011.01.001
Ahmad S, Barbhuiya SA, Elahi A, Iqbal J (2011) Effect of Pakistani bentonite on properties of mortar and concrete. Clay Miner 46(01):85–92. https://doi.org/10.1180/claymin.2011.046.1.85
Alexander MG, Arliguie G, Ballivy G, Bentur A, Marchand J, (1999) Engineering and transport properties of the interfacial transition zone in cementitious composites. RILEM Publications. s S.A.R.L.
Anan TI, Abd El-Wahed AG (2017) The Maastrichtian-Danian Dakhla Formation, Eastern Desert, Egypt: utilization in manufacturing lightweight aggregates. Appl Clay Sci 150:10–15. https://doi.org/10.1016/j.clay.2017.08.027
Bernhardt M, Justnes H, Tellesbø H, Wiik K (2014) The effect of additives on the properties of lightweight aggregates produced from clay. Cement Concr Compos 53:233–238. https://doi.org/10.1016/j.cemconcomp.2014.07.005
Chandra S, Berntsson L (2002) Production of lightweight aggregates and its properties. Lightweight Aggregate Concrete 21–65.https://doi.org/10.1016/b978-081551486-2.50005-5
Cheeseman CR, Virdi GS (2005) Properties and microstructure of lightweight aggregate produced from sintered sewage sludge ash. Resour Conserv Recycl 45(1):18–30. https://doi.org/10.1016/j.resconrec.2004.12.006
Chegbeleh LP, Nishigaki M, Akudago JA, M. Alim A, Komatsu M,(2008) Concepts of repository and the functions of bentonite in repository environments: a state of the art review. J Fac Environ Sci Technol. Okayama Univ. 13(1):1–5. doi=10.1.1.1045.823&rep=rep1&type=pdf
Chopra S, Lal K, Ramachandran V (1964) Gas-producing agents in the production of lightweight aggregates. J Chem Technol Biotechnol 14(5):181–185
Choudhry V, Hadley SR (1992) Utilization of coal gasification slag: overview. Praxis Engineers Inc. Milpitas Ch 20:253–263. https://doi.org/10.1021/bk-1992-0515.ch020
Cui Y, Yahia-Aissa M, Delage P (2002) A model for the volume change behaviour of heavily compacted swelling clays. Eng Geol 64(2–3):233–250. https://doi.org/10.1016/s0013-7952(01)00113-2
Dondi M, Cappelletti P, D’Amore M, de Gennaro R, Graziano SF, Langella A, Raimondo M, Zanelli C (2016) Lightweight aggregates from waste materials: reappraisal of expansion behavior and prediction schemes for bloating. Constr Build Mater 127:394–409
EN 13055–1 (2002) Lightweight aggregates - Part 1: Lightweight aggregates for concrete, mortar and grout
Fakhfakh E, Hajjaji W, Medhioub M, Rocha F, López-Galindo A, Setti M, Kooli F, Zargouni F, Jamoussi F (2007) Effects of sand addition on production of lightweight aggregates from Tunisian smectite-rich clayey rocks. Appl Clay Sci 35:228–237. https://doi.org/10.1016/j.clay.2006.09.006
García-García S, Jonsson M, Wold S (2006) Temperature effect on the stability of bentonite colloids in water. J Colloid Interface Sci 298(2):694–705. https://doi.org/10.1016/j.jcis.2006.01.018
Gens A, Garcia-Molina AJ, Olivella S, Alonso EE, Huertas F (1998) Analysis of a full scale in situ test simulating repository conditions. Int J Numer Anal Meth Geomech 22(7):515–548
Grim RE (1968) Clay Mineralogy International Series in the Earth and Planetary Sciences, 2nd edn. McGraw-Hill, New York
Hiramatsu Y, Oka Y (1966) Determination of the tensile strength of rock by a compression test of an irregular test piece Int. J Rock Mech Min Sci 3:89
Hung MF, Hwang CL (2007) Study of fine sediments for making lightweight aggregate. Waste Manage Res 25(5):449–456. https://doi.org/10.1177/0734242x07077615
Ibrahim JFM, Kurovics E, Gömze LA (2019) New zeolite-alumina composite materials -development and investigation. Preparation of Ceramic Materials Proceedings of the XIIIth International Conference. Jahodná, ISBN: 978–80–553–3314–4
Islam M, Sagirul SN, Moniruzzaman M, Umme AUS (2016) Effect of soda lime silica glass waste on the basic properties of clay aggregate. Int J Sci Eng Res 7(4):149–153 (ISSN 2229-5518)
Karaś KP, Klein M, Hupka J, Łuczak J (2019) Utilization of shale cuttings in production of lightweight aggregates. J Environ Manage 231:232–240. https://doi.org/10.1016/j.jenvman.2018.09.101
Kavas T, Christogerou A, Pontikes Y, Angelopoulos GN (2011) Valorisation of different types of boron-containing wastes for the production of lightweight aggregates. J Hazard Mater 185(2–3):1381–1389. https://doi.org/10.1016/j.jhazmat.2010.10.059
Kocserha I, Gömze LA (2010) Friction properties of clay compounds. Appl Clay Sci 48:425–430. https://doi.org/10.1016/j.clay.2010.01.017
Kocserha I, Hamza A, Géber R (2018) The effects of red mud on clay compounds. Material Science and Engineering 426:2018. https://doi.org/10.1088/1757-899X/426/1/012026
Lee TC, Liu FJ (2009) Recovery of hazardous semiconductor-industry sludge as a useful resource. J Hazard Mater 165(1–3):359–365. https://doi.org/10.1016/j.jhazmat.2008.10.105
Lee KG (2016) Bloating mechanism of lightweight aggregate with the size. J Korean Ceram Soc 53(2). https://doi.org/10.4191/kcers.2016.53.2.241
Li Y, Wu D, Zhang J, Chang L, Wu D, Fang Z, Shi Y (2000) Measurement and statistics of single pellet mechanical strength of differently shaped catalysts. Powder Technol 113(1–2):176–184. https://doi.org/10.1016/s0032-5910(00)00231-x
Li X, He C, Lv Y, Jian S, Liu G, Jiang W, Jiang D (2020) Utilization of municipal sewage sludge and waste glass powder in production of lightweight aggregates. Constr Build Mater 256:119413. https://doi.org/10.1016/j.conbuildmat.2020.119413
Liao Y, Huang C (2011) Effects of heat treatment on the physical properties of lightweight aggregate from water reservoir sediment. Ceram Int 37:3723–3730. https://doi.org/10.1016/j.ceramint.2011.04.122
Liu P, Farzana R, Rajarao R, Sahajwalla V (2017) Lightweight expanded aggregates from the mixture of waste automotive plastics and clay. Constr Build Mater 145:283–291. https://doi.org/10.1016/j.conbuildmat.2017.04.009
Moreno-Maroto JM, González-Corrochano B, Alonso-Azcárate J, Rodríguez L, Acosta A (2017) Manufacturing of lightweight aggregates with carbon fiber and mineral wastes. Cement Concr Compos 83:335–348. https://doi.org/10.1016/j.cemconcomp.2017.08.001
Murray HH (2006) Bentonite applications, Developments in Clay Science. 2:111–130.https://doi.org/10.1016/S1572-4352(06)02006-X
Ozguven A, Gunduz L (2012) Examination of effective parameters for the production of expanded clay aggregate. Cement and Concrete Composite 34:781–787. https://doi.org/10.1016/j.cemconcomp.2012.02.007
Paluszkiewicz C, Holtzer M, Bobrowski A (2008) FTIR analysis of bentonite in moulding sands. J Mol Struct 880(1–3):109–114. https://doi.org/10.1016/j.molstruc.2008.01.028
Pusch R (1992) Use of bentonite for isolation of radioactive waste products. Clay Miner 27(03):353–361. https://doi.org/10.1180/claymin.1992.027.3.08
Pusch R, Weston R (2012) RETRACTED: Superior techniques for disposal of highly radioactive waste (HLW). Prog Nucl Energy 59:75–85. https://doi.org/10.1016/j.pnucene.2012.01.005
Rajczyk J, Langier B (2012) Concrete composite properties with modified sodium bentonite in material application engineering. Adv Mater Res 583:154–157. https://doi.org/10.4028/www.scientific.net/amr.583.154
Rashad AM (2018) Lightweight expanded clay aggregate as a building material – an Overview. Constr Build Mater 170:757–775. https://doi.org/10.1016/j.conbuildmat.2018.03.009
Riley CM (1951) Relationship of chemical properties to bloating of clays. J Am Ceram Soc 34:121–128
Rudnai G (1963) Lightweight concretes, Akadémiai Kiadó, Budapest
Taylor-Lange SC, Lamon EL, Riding KA, Juenger MCG (2015) Calcined kaolinite–bentonite clay blends as supplementary cementitious materials. Appl Clay Sci 108:84–93. https://doi.org/10.1016/j.clay.2015.01.025
Wei Y, Weng S, Xie X (2018) Reduction of sintering energy by application of calcium fluoride as flux in lightweight aggregate sintering. Constr Build Mater 190:765–772. https://doi.org/10.1016/j.conbuildmat.2018.09.134
Yang C, Cuin C, Qin J (2015) Recycling of low-silicon iron tailings in the production of lightweight aggregates. Ceram Int 41:1213–1221. https://doi.org/10.1016/j.ceramint.2014.09.050
Yong RN (1999) Overview of modeling of clay microstructure and interactions for prediction of waste isolation barrier performance. Eng Geol 54(1–2):83–91. https://doi.org/10.1016/s0013-7952(99)00064-2
Funding
Open access funding provided by University of Miskolc. This research was supported by the European Union and the Hungarian State, co-financed by the European Regional Development Fund in the framework of the GINOP-2.3.4–15-2016–00004 project, aimed to promote the cooperation between higher education and the industry. The researcher [Mohamed Abdelfattah] is funded by Stipendium Hungaricum Scholarship [11729] under the joint exchange program between the Arab Republic of Egypt and Hungary.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Zeynal Abiddin Erguler
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdelfattah, M.M., Géber, R., Abdel-Kader, N.A. et al. Assessment of the mineral phase and properties of clay-Ca bentonite lightweight aggregates. Arab J Geosci 15, 205 (2022). https://doi.org/10.1007/s12517-022-09538-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12517-022-09538-w