Abstract
Bisphenols are a group of chemical compounds widely used in the industry, e.g., in the production of plastics, and as precursor of epoxy resins. The three bisphenols investigated in this study, namely bisphenols A (BPA), F (BPF) and S (BPS), seem to have endocrine activity in the same order of magnitude. Thus, an exposure and risk assessment was performed based on urinary concentrations of these bisphenols in 150 urine samples of 7-year-old children from the Polish Mother and Child Cohort Study (REPRO_PL). Samples were analyzed using high performance liquid chromatography with online sample clean-up coupled to tandem mass spectrometry (online-SPE-LC-MS/MS). BPA, BPF, and BPS were above the LOQ (0.25 µg/L) in 100%, 95%, and 20% of the samples, respectively, with median concentrations of 2.5 µg/L, 1.0 µg/L and < 0.25 µg/L (below the LOQ), respectively. Median Daily Intakes (DIs) were calculated: 45.9 ng/kg bw/day for BPA, at maximum 33.1 ng/kg bw/day for BPF (depending on the urinary excretion fractions used for calculation) and below 4.4 ng/kg bw/day for BPS (calculated at LOQ/2). Hazard quotients (HQ) at the 95th percentile of 0.011 for BPF and 0.63 for BPS did not indicate health risks. However, based on the TDI of 0.2 ng/kg bw/day newly established by EFSA, the HQ for BPA was above 1 in 100% of the samples, thus indicating the exceedance of safe exposure levels. No statistically significant associations were found between bisphenol concentrations and socio-demographic and life-style characteristics, although environmental tobacco smoke at 7 years of age, maternal educational level and socio-economic status showed a positive trend. Further studies are required in order to recognize other BPA replacement exposures among children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bisphenols are a group of chemical compounds mainly used in the manufacture of plastics, resins and thermal paper. The most representative compound of this group is bisphenol A (BPA, 4,4’-(propane-2,2-diyl)diphenol)), which has been used since the decade of the 1960s (Geens et al. 2011; Rochester 2013). BPA has been recently included by the European Chemicals Agency (ECHA) in the Candidate List of substances of very high concern because of its endocrine-disrupting properties for human health, and its use in baby bottles or thermal paper has been banned or restricted (EFSA 2023; Usman and Ahmad 2016).
As a result of BPA restrictions, the industry has been gradually replacing this compound by other bisphenol analogues, such as bisphenol F (BPF, a mixture of the isomeric congeners 2,2’-, 2,4’-, and 4,4’-dihydroxydiphenyl-methane) and S (BPS, 4,4’-sulfonylbisphenol) (Liao et al. 2012a, b; Chen et al. 2016). For instance, BPS is the main replacement in thermal paper manufacture, and its volume doubled between 2016 and 2017 in the EU market (ECHA 2018). BPF is used in a broad range of applications, from lacquers, varnishes and adhesives plastics to water pipes, dental sealants and coatings for food packaging (Chen et al. 2016). Humans are exposed to bisphenols through their daily life. Ingestion is considered the main source of BPA and BPF exposures (Liao et al. 2013). These two compounds were commonly found in foodstuffs. In addition, the 4,4’-isomer of BPF is naturally present in mustard (Zoller et al. 2016). Non-dietary sources such as dermal contact to thermal paper or toys can contribute to the total exposure of these bisphenols (Geens et al. 2012).
As mentioned above, BPA is a recognized endocrine-disrupting chemical (EDC) for human health. As in the case of other EDCs, prenatal and childhood exposures to BPA might pose a higher threat compared to adults (WHO & UNEP 2012). Bisphenols may interact with a variety of hormonal systems that affect growth, metabolism, children’s health and neurodevelopment. Recent reports have explored the human metabolism and distribution of BPS in humans (Oh et al. 2018; Khmiri et al. 2020). Furthermore, the two BPA substitutes BPF and BPS have been found to be as hormonally active as the replaced compound, possessing endocrine-disrupting effects too (Rochester and Bolden 2015; Rocha et al. 2016). The three bisphenols share a close similarity in their molecular structure (Fig. 1) and have been included in the list of prioritised substances to be determined in human biomonitoring studies by the EU (Vaccher et al. 2022). In addition, the European Food Safety Authority (EFSA) has re-evaluated the risks associated with bisphenol A in food and has established a TDI of 0.2 ng/kg bw/day, which is 20,000 times lower than the previous temporary TDI (t-TDI) of 4,000 ng/kg bw/day (EFSA 2015; 2023).
The aim of this study is to determine the presence and distribution of BPA and the two main substitutes BPF and BPS in 150 urine samples of school-age children from Poland. In addition, our purpose is to assess the main socio-demographic and life-style determinants of bisphenol exposures and calculate the overall risk assessment.
Methodology
Study Population and Sampling
The study is based on the Polish Mother and Child Cohort (REPRO_PL), a prospective birth cohort established in 2007 in Poland. The details regarding the cohort methodology have been published previously (Polanska et al. 2009; 2011; 2016). Out of 407 children who were followed-up until the age of 7 years (2014–19), urine samples from 150 children (37%) were randomly selected for the analysis of bisphenols (Polanska et al. 2016). Written informed consent was obtained from the parents of each child before the study, which was approved by the Ethical Committee of the Nofer Institute of Occupational Medicine, Lodz, Poland (Decision No. 22/2014).
Analysis of Bisphenols in Urine
The bisphenols A, F, and S were analyzed in urine using a so far unpublished method, validated according to the guidelines of the working group “Analyses in Biological Materials” of the Permanent Senate Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area of the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG; Bader et al. 2010). For sample preparation, 150 µL 1 M ammonium acetate buffer (pH 5.0), 6 µL β-glucuronidase (aryl sulfatase-free), and 30 µL internal standard solution (containing 100 µg/L of each labelled standard in acetonitrile) were added to 300 µL of urine. The mixture was incubated at 37 °C for 4 h and then stored at -20 °C overnight. After thawing and equilibration to room temperature, the mixture was centrifuged (2600 g, 10 min) and the supernatant transferred into a new HPLC vial for instrumental analysis. Calibration solutions and quality control materials were treated in the same manner.
Instrumental analysis was performed by online-SPE-LC-MS/MS with peak focusing during analyte transfer from the enrichment column onto the analytical column. A molecular imprinted polymer phase for bisphenols was used in the enrichment column and a mixed-mode phase (reversed-phase and anion exchange) was used for the separation of the bisphenols. Ammonium fluoride was used for signal enhancement, as already described by van der Meer et al. (2019). To reduce contamination of the mass spectrometer, flow from the LC column was diverted into waste, except within the retention time windows of the bisphenols. For a detailed description of the analytical method, as well as validation data, see the supplementary material. The limit of quantification (LOQ, based on a signal-to-noise ratio of 10 in matrix—see Bury et al. 2018 for details) for all three bisphenols was 0.25 µg/L. For external quality control, using this method, IPA successfully participated in the 64th round (in Q4 of 2019) of the biannual ring trials of the German External Quality Assessment Scheme for analyses in biological materials (G-EQUAS) for BPA (BPF and BPS were not offered) in the environmental medical field. For each of the bisphenols, the five urine samples with the highest measured concentrations (except for BPA—see below) were analyzed again without the addition of β-glucuronidase to exclude artifacts from external (pre-analytical) contamination (as bisphenols are expected to be primarily excreted in urine as glucuronides; Völkel et al. 2002; Khmiri et al. 2020). In case of BPA, the volume of the urine sample with the third-highest concentration was insufficient for an additional analysis. Thus, the urine sample with the sixth-highest concentration was analyzed instead.
Covariates
The following socio-demographic information was obtained by questionnaires filled out by mothers at child examination: place of residence (rural, urban); socio-economic status (SES) of the family (least affluent, affluent, most affluent); parental educational level (years of completed education: ≤ 9, 10–12, > 12); parental occupational activity (yes, no); child sex and parental age (calculated for date at child birth). Information on children’s passive smoking at age of 7 years was extracted from cotinine levels in urine as described by Stragierowicz et al. (2013). A cut off value at 2.1 ng/ml for child environmental tobacco smoke (ETS) exposure was selected (Lupsa et al. 2015). Child height and weight was measured at age of 7 years by trained staff based on standard protocol (Polanska et al. 2016). Body mass index (BMI) categories (underweight boys < 13.95 kg/m2, girls < 13.80 kg/m2; recommended weight boys 13.96–18.64 kg/m2, girls 13.81–18.19 kg/m2; overweight/obese boys > 18.65 kg/m2, girls > 18.20 kg/m2) were based on Polish reference data BMI z-scores at age 7 (Kulaga et al. 2015).
Daily Intakes and Overall Risk Assessment
The daily intakes of bisphenols were estimated from spot urine samples using the following equation, taken from Koch et al. (2011) and Covaci et al. (2015):
where BPscreatinine is the creatinine-adjusted bisphenols concentrations (in μg bisphenol/g creatinine); CErate is the child individual body height and gender-based reference values for urinary creatinine excretion rate (in g/day), taken from Remer et al. (2002); Fue is the urinary excretion fraction for bisphenols, and bw is the body weight for each child (in kg). Fue is assumed to be 1 for BPA (Koch et al. 2012). For BPS, two Fue values have been used: 0.82 as for a best-case assumption (Oh et al. 2018) and 0.56 as a worst-case assumption (Khmiri et al. 2020). Fue values for BPF have not been estimated yet, and three values have been used: 1, as for BPA in the best-case scenario, and 0.82 and 0.56, as for BPS, representing intermediate and worst-case scenarios, respectively. Afterwards, hazard quotients (HQ) for exposure levels of BPA and BPF in the population were calculated as the quotients between the daily intake rates and available estimated Tolerable Daily Intakes (TDI). In the case of BPA, a TDI of 0.2 ng/kg bw/day has been recently established by EFSA (EFSA 2023), a value well below the previous temporary TDI of 4000 ng/kg bw/day (EFSA 2015). For BPF, a TDI of 11,000 ng/kg bw/day was suggested by Dietrich and Hengstler (2016), based on the risk assessment performed by Zoller et al. (2016). For BPS, no TDI is established yet. Accordingly, for risk assessment the HQ was calculated based on urinary BPS concentrations in conjunction with the HBM-GV of 1 µg/L. For a cumulative risk assessment of the three bisphenols, the Hazard Index (HI) was estimated using the sum of the HQs. For the above calculations, urinary concentrations below the LOQ were set to 0.125 µg/L (LOQ/2).
Data analysis
Data analysis and graphics were performed using the statistical software R (R Development Core Team 2021) and ggplot2 package (Wickham 2016). For descriptive analysis, medians and geometric mean (GM) with 95% confidence intervals (CI) of bisphenols were used. Range and percentiles 25, 75 and 95 were also reported in the tables. The distributions of the concentrations are shown using boxplots. Association between concentrations was evaluated using the Pearson correlation coefficient, after log-transformation of the concentrations. For DI comparisons between boys and girls, non-parametric Wilcoxon test was used and Bonferroni adjusted values were calculated. Significance was set at 0.05.
Results
We quantified the bisphenols BPA, BPF, and BPS in 150 urine samples from Polish children from the Polish Mother and Child Cohort. The distribution of BPA, BPF and BPS concentrations in these urine samples are shown in Table 1 and Fig. 2. For BPA, one urine sample was excluded, as analysis without enzymatic deconjugation revealed pre-analytical contamination (35.6 µg/L free BPA vs. 37.4 µg/L total BPA). In three other urine samples, urinary BPF concentrations could not be determined due to strong signal suppression. BPA was the most abundant bisphenol, found in all the analyzed samples, with a median concentration of 2.5 μg/L, followed by BPF (found above the LOQ in 95% of the samples) with a median concentration of 1.0 μg/L. BPS was the compound showing the lowest percentage of quantifiable samples (only 20% of the samples were above the LOQ), accordingly with a median below the LOQ (Table 1). The maximum concentrations of bisphenols were found for BPF (90 μg/L), followed by BPA (27.3 μg/L) and BPS (7.0 μg/L). The correlations of the studied bisphenols were: r = 0.30 (BPA vs. BPF), r = 0.16 (BPA vs. BPS) and r = 0.13 (BPF vs. BPS) (Fig. 3).
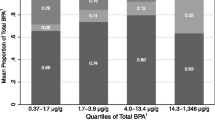
Figure 4 shows the distribution of individual and socio-demographic characteristics of 7-year-old children from Poland for each bisphenol compound. There were no statistically significant differences between the variables studied and the concentrations reported for each group. In general, ETS at age 7, maternal education and, to a lesser extent, socio-economic status, were associated with bisphenols concentrations in children, with a positive relationship except in the case of BPA, in which SES was negatively associated.
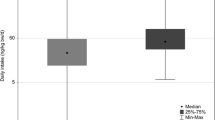
Figure 5 shows the bisphenols estimated daily intakes by sex, and using different Fue values. Boys had higher median DIs than girls, although no statistically significant differences were found.
Boxplots of estimated daily intakes (in ng/kg bw/day) in 7-year-old children for BPA, BPF and BPS, by sex, and taking into account different case scenarios depending on Fue values (see Table 1)
Discussion
There are more than 10 bisphenol analogues available in the market for industrial applications (Chen et al. 2016), but among the most commonly used BPA substitutes, BPS, BPF and rarely BPAF are usually analyzed in human biomonitoring studies (Yang et al. 2014; Asimakopoulos et al. 2015; Rocha et al. 2016; Sakhi et al. 2018; Lehmler et al. 2018; Dualde et al. 2019). Urine analysis is the most common, simplest and least intrusive method for assessing human exposure to bisphenols (Ougier et al. 2021). Some studies have detected bisphenols in other human matrices, such as blood and breast milk (Owczarek et al. 2018; Dualde et al. 2019). Although Lucarini et al. (2020) showed that the bisphenols BPC and BPM were more frequently detected than BPA (at comparable detection limits in urine samples from infants and toddlers from Switzerland), BPA and BPS are among the most frequently detected bisphenols in the above-mentioned studies.
Table 2 shows a comparison of urinary bisphenol concentrations in children worldwide. Urinary BPA levels in 7-year-old children from Poland (median concentration 2.5 μg/L) were comparatively higher than those found in children from the USA recruited within the NHANES study (age 6–11 years; Lehmler et al. 2018) and from Brazil (age 6–14 years; Rocha et al. 2018), but lower than those found in children’s urine from Norway (3.7 μg/L; Sakhi et al. 2018), India (5.1 μg/L; Xue et al. 2015); China (4.1 μg/L; Li et al. 2013) and another previous study performed in the USA (3.6 μg/L; Calafat et al. 2008). These differences could be related to the different years of study, as a general decrease in the concentrations of environmental phenols, parabens and BPA has been observed throughout the recent years (Frederiksen et al. 2013; Gys et al. 2020). Therefore, different sampling periods and increasing substitution of BPA towards other bisphenols could have contributed to the aforementioned differences between studies. Our previous assessment of BPA in urine of children from Poland (n = 250 samples) found lower concentrations (median of 1.8 μg/L) than the current report, but in a similar range (Garí et al. 2021). This might be related to differences in sampling batches and children’s location, since in the first report, only three places out of five were represented (data not shown for data privacy reasons). On the other hand, the median daily intake for BPA calculated in the previous study (46.3 ng/kg bw/d) was virtually identical to that from the present study (45.9 ng/kg bw/d; see below), so that differences in the compositions of the analyzed subpopulations and/or differences in diuresis-related urinary dilution between these sample sets could at least partially explain this difference in urinary BPA concentrations.
For BPF, the present study is the first showing such concentrations in children (median of 1.0 μg/L), which is at least about four times higher than the median concentrations reported thus far in children’s urine (see Table 2). In any case, the number of available studies does not allow for generalization. Another study performed in Japanese children found maximum concentrations of BPF more than twice those of BPA, alike our report (Gys et al. 2020). In the Norwegian and Brazilian studies, concentrations of BPF were below the respective limits of quantification (0.07 µg/L and 0.6 µg/L) (Sakhi et al. 2018; Rocha et al. 2018). It should be noted that, in addition to industrial use, there are natural exposure sources of 4,4’-BPF, such as mustard and certain plant materials used in traditional Chinese medicine (Zoller et al. 2016; Huang et al. 2019). Therefore, differences in nutritional behaviour might contribute to the observed differences in exposure towards BPF (Lietzou 2021).
Unlike BPF, BPS is rather frequently detected in urine, at concentrations ranging from 0.040 μg/l in Indian children (Xue et al. 2015) to 0.27 μg/l in the USA report (Lehmler et al. 2018). In our study, the median BPS concentration was below the LOQ (< 0.25 μg/l). A comparison with other studies is hampered by the fact that most reported median or GM values were below this LOQ.
Daily intakes for the three bisphenols determined in the present study are reported in Table 1. Median DIs were 45.9 ng/kg bw/day for BPA, between 18.5 ng/kg bw/day and 33.1 ng/kg bw/day for BPF, depending on the Fue value used (1, 0.82 and 0.56, respectively), and between 3.0 ng/kg bw/day and 4.4 ng/kg bw/day for BPS (for Fue values of 0.82 and 0.56, respectively). DIs were higher in boys than girls for the three bisphenols, although no statistically significant results were found. Previous reports performed in children from China found similar trends, with higher median DIs in boys than in girls (Liu et al. 2019). Further health implications for these sexual differences must be examined.
For risk assessment, hazard quotients (HQ) were calculated individually for BPA and BPF, based on the calculated daily intakes in conjunction with the newly established TDI of 0.2 ng/kg bw/day for BPA and the TDI of 11,000 ng/kg bw/day suggested for BPF by Dietrich and Hengstler (2016), respectively. Since no TDI is available for BPS thus far, the calculation of HQs was performed based on urinary BPS concentrations in conjunction with the HBM-GV for the general population of 1 µg/L BPS in urine. Median values (and 95th percentiles) for the HQs for BPA, BPF, and BPS were 1,145 (969), 0.0030 (0.011; DI calculated with an assumed Fue of 0.56 as worst-case assumption), and 0.13 (0.63), respectively. Most strikingly, the HQ for BPA was above 1 in 100% of the analyzed samples. Therefore, the hazard index (HI) calculated for the sum of the three bisphenols was above 1 in 100% of the samples as well. Accordingly, whereas BPF did not exceed safe levels, and BPS only in three cases, BPA exposures (and thus mixture exposures) cannot be regarded as safe in relation to the TDI newly established by EFSA.
Regarding socio-demographic and life-style characteristics, our study was not able to find any significant differences for each of the three bisphenols analysed. Some studies have found a relationship between BPA and obesity or overweight (Li et al. 2013; Trasande et al. 2012). We observed that underweighted children had lower BPA concentrations than recommended weight and overweighted/obese children, a trend that was already reported in our previous research on Polish children (Garí et al. 2021). Although food has been identified as the main source of human exposure to BPA, unfortunately we could not explore different nutritional behaviours that contribute to the differences of bisphenols in the current population study. Bisphenols migrate from plastics and food contact materials into food items, contributing to the overall exposure together with other routes, such as inhalation and dermal contact (Geens et al. 2012; Liao and Kannan 2013).
Other studies have reported differences in bisphenol concentrations and place of residence. For instance, Dualde et al. (2019) found higher BPA concentrations in children living in rural areas from Spain, while Snoj Tratnik et al. (2019) reported the opposite trend in Slovenia.
This is the first assessment of BPA replacement compounds in children from Poland, and one of the first in Europe. It needs to be underlined that the study is based on a well-defined child cohort in Europe. However, the sample size was small, with only 150 urine samples analyzed. Although the collection of 24-h urine samples in general is more favourable, the collection of spot urine samples in the present study has been appropriate for bisphenols general exposure and risk assessment. It has been shown specifically for urinary BPA concentrations that GMs of spot urine samples do not tend to overestimate the GM of the 24-h composite samples (Aylward et al. 2017). Further considering similar terminal half-lives for BPA and BPS in urine (Völkel et al. 2002; Oh et al. 2018), it seems unlikely that collecting spot urines resulted in an overestimation of the population medians for bisphenol exposures. The results from the present study do not only provide valuable insight into children’s exposure to this important class of chemicals, but considering the available HBM data for this population (Polańska et al. 2011; 2016; Garí et al. 2019), it also enables further in-depth investigation of potential exposure associations and possible impacts of lifestyle factors in future studies.
Conclusion
This study provides current biomonitoring data of bisphenol A and its replacement compounds bisphenol F and bisphenol S in the urine of 150 children, 7-year-old, from Poland. BPA could be quantified in all children, followed by BPF (quantifiable in 95%) and BPS (20%, i.e., less than 30 children). Median bisphenol concentrations were in the same order of magnitude as those observed in previous studies on children’s bisphenol exposures, with the median BPF concentration being the highest observed thus far in children. In contrast to BPF and BPS exposures, BPA exposures were above safe levels in 100% of the samples when comparing to the TDI newly established by EFSA. We were not able to find any statistically significant association between bisphenol concentrations and socio-demographic and life-style characteristics, although a trend was found with ETS, maternal educational level and socio-economic status. Further studies are required in order to recognize other BPA replacement exposures among children.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Asimakopoulos AG, Xue J, Pereira De Carvalho B, Iyer A, Abualnaja KO, Yaghmoor SS, Jumosani TA, Kannan K (2015) Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah. Saudi Arabia Env Res 150:573–581
Aylward LL, Hays SM, Zidek A (2017) Variation in urinary spot sample, 24 h samples, and longer-term average urinary concentrations of short-lived environmental chemicals: implications for exposure assessment and reverse dosimetry. J Expo Sci Environ Epidemiol 27:582–590
Bader M, Barr D, Göen T, Schaller KH, Scherer G, Angerer J. Reliability criteria for analytical methods [Biomonitoring Methods, in: The MAK Collection for Occupational Health and Safety], 2010, pp. 55E101
Bury D, Belov VN, Yulin Q, Hayen H, Volmer DA, Brüning T, Koch HM (2018) Determination of urinary metabolites of the emerging UV filter octocrylene by online-SPE-LC-MS/MS. Anal Chem 90(1):944–951
Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL (2008) Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect 116(1):39–44
Chen D, Kannan K, Tan H, Zheng Z, Feng Y-L, Wu Y, Widelka M (2016) Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity—a review. Environ Sci Technol 50:5438–5453
Covaci A, Den Hond E, Geens T, Govarts E et al (2015) Urinary BPA measurements in children and mothers from six European member states: overall results and determinants of exposure. Environ Res 141:77–85
Dietrich DR, Hengstler JG (2016) From bisphenol A to bisphenol F and a ban of mustard due to chronic low-dose exposures? Arch Toxicol 90(2):489–491
Dualde P, Pardo O, Corpas-Burgos F, Kuligowski J, Gormaz M, Vento M, Pastor A, Yusà V (2019) Biomonitoring of bisphenols A, F, S in human milk and probabilistic risk assessment for breastfed children. Sci Total Environ 668:797–805
ECHA (2018) Market survey: use of bisphenol A and its alternatives in thermal paper in the EU from 2014 to 2017. https://echa.europa.eu/-/bpa-being-replaced-by-bps-in-thermal-paper-echa-survey-finds. Accessed 2 July 2020
European Food Safety Authority (2015) Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs: Part II - Toxicological assessment and risk characterisation. EFSA J 13:3978
European Food Safety Authority (2023) Re-evaluation of the risks to public health related to thepresence of bisphenol A (BPA) in foodstuffs. EFSA J 21(4):6857
Frederiksen H, Nielsen JK, Morck TA, Hansen PW, Jensen JF, Nielsen O, Andersson AM, Knudsen LE (2013) Urinary excretion of phthalate metabolites, phenols and parabens in rural and urban Danish mother-child pairs. Int J Hyg Environ Health 216(6):772–783
Garí M, Koch HM, Pälmke C, Jankowska A, Wesolowska E, Hanke W, Nowak D, Bose-O’Reilly S, Polańska K (2019) Determinants of phthalate exposure and risk assessment in children from Poland. Environ Int 127:742–753
Garí M, Moos R, Bury D, Kasper-Sonnenberg M, Jankowska A, Andysz A, Hanke W, Nowad D, Bose-O’Reilly S, Koch HM, Polanska K (2021) Human-biomonitoring derived exposure and daily intakes of bisphenol a and their associations with neurodevelopmental outcomes among children of the polish mother and child cohort study. Environ Health 20(1):95
Geens T, Goeyens L, Covaci A (2011) Are potential sources for human exposure to bisphenol—a overlooked? Int J Hygiene Environ Health 214(5):339–347
Geens T, Aerts D, Berthot C, Bourguignon J-P, Goeyens L, Lecomte P et al (2012) A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol 50:3725–3740
Gys C, Ait Bamai Y, Araki A, Bastiaensen M, Caballero-Casero N, Kishi R, Covaci A (2020) Biomonitoring and temporal trends of bisphenols exposure in Japanese school children. Environ Res 191:110172
Huang T, Danaher L-A, Brüschweiler BJ, Kass GEN, Merten C (2019) Naturally occurring bisphenol F in plants used in traditional medicine. Arch Toxicol 93:1385–1490
Khmiri I, Cöté J, Mantha M, Khemiri R, Lacroix M, Gely C, Toutain P-L, Picard-Hagen N, Gayrard V, Bouchard M (2020) Toxicokinetics of bisphenol-S and its glucuronide in plasma and urine following oral and dermal exposure in volunteers for the interpretation of biomonitoring data. Environ Int 138:105644
Koch HM, Wittassek M, Brüning T, Angerer J, Heudorf U (2011) Exposure to phthalates in 5–6 years old primary school starters in Germany—a human biomonitoring study and cumulative risk assessment. Int J Hyg Environ Health 214:188–195
Koch HM, Kolossa-Gehring M, Schröter-Kermani C, Angerer J, Brüning T (2012) Bisphenol A in 24 h urine and plasma samples of the german environmental specimen bank from 1995 to 2009: a retrospective exposure evaluation. J Expo Sci Environ Epidemiol 22:610–616
Kulaga Z, Rózdzynska-Swiatkowska A, Grajda A, Gurzkowska B, Wojtylo M, Gçozdz M et al (2015) Percentile charts for growth and nutritional status assessment in Polish children and adolescents from birth to 18 years of age. Standardy Medyczne/Pediatria 12:119–135
Lehmler H-J, Liu B, Gadogbe M, Bao W (2018) Exposure to bisphenol A, bisphenol F, and bisphenol S in U.S. Adults and Children: The national health and nutrition examination survey 2013–2014. ACS Omega 3:6523–6532
Li D-K, Miao M, Zhou Z, Wu C, Shi H, Liu X, Wang S, Yuan W (2013) Urine bisphenol-A level in relation to obesity and overweight in school-age children. PLoS ONE 8(6):e65399
Liao C, Kannan K (2013) Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem 61:4655–4662
Liao C, Liu F, Moon H-B, Yamashita N, Yun S, Kannan K (2012a) Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: spatial and temporal distributions. Environ Sci Technol 46(21):11558–11565
Liao C, Liu F, Aalomirah H, Duc Loi V, Mohd MA, Moon H-B, Nakata H, Kannan K (2012) Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposure. Environ Sci Technol 46(12):6860–6866
Lietzou J (2021) Biologically active compounds in mustard seeds: a toxicological perspective. Foods 10(9):2089
Liu Y, Yan Z, Zhang Q, Song N, Cheng J, Lopez Torres O, Chen J, Zhang S, Guo R (2019) Urinary levels, composition profile and cumulative risk of bisphenols in preschool-aged children from Nanjing suburb, China. Ecotoxicol & Environ Safety 172:444–450
Lucarini F, Krasniqi T, Bailat Rosset G, Roth N, Hopf NB, Broillet M-C, Staedler D (2020) Exposure to new emerging bisphenols among young children in Switzerland. Int J Environ Res Public Health 17:4793
Lupsa IR, Nunes B, Ligocka D, Gurzau AE, Jakubowski M, Casteleyn L et al (2015) Urinary cotinine levels and environmental tobacco smoke in mothers and children of Romania, Portugal and Poland within the European human biomonitoring pilot study. Environ Res 141:106–117
Meer TP, Faassen M, Frederiksen H, Beek AP, Wolffenbuttel BHR, Kema IP, Vliet-Ostaptchouk JV (2019) Development and interlaboratory validation of two fast UPLC-MS-MS methods determining urinary bisphenols. Parabens and phthalates. J Anal Toxicol 43(6):452–464
Meslin M, Beausoleil C, Zeman FA, Antignac JP, Kolossa-Gehring M, Rousselle C, Apel P (2022) Human biomonitoring guidance values (HBM-GVs) for bisphenol S and assessment of the risk due to the exposure to bisphenols A and S. Eur Toxicls 10(5):228
Oh J, Choi JW, Ahn YA, Kim S (2018) Pharmacokinetics of bisphenol S in humans after single oral administration. Environ Int 112:127–133
Ougier E, Zeman F, Antignac J-P, Rousselle C, Lange R, Kolossa-Gehring M, Apel P (2021) Human biomonitoring initiative (HBM4EU): human biomonitoring guidance values (HBM-GVs) derived for bisphenol A. Environ Int 154:106563
Owczarek K, Kubica P, Kudlak B, Rutkowska A, Konieczna A, Rachon D, Namiesnik J, Wasik A (2018) Determination of trace levels of eleven bisphenol A analogues in human blood serum by high performance liquid chromatography-Tandem mass spectrometry. Sci Total Environ 628–629:1362–1368
Polańska K, Hanke W, Gromadzińska J, Ligocka D, Gulczyńska E, Sobala W, Wásowicz W (2009) Polish mother and child cohort study—defining the problem, the aim of the study and methodological assumption. Int J Occup Med Environ Health 22(4):383–391
Polańska K, Hanke W, Jurewicz J, Sobala W, Madsen C, Nafstad P, Magnus P (2011) Polish mother and child cohort study (REPRO_PL)—methodology of follow-up of the children. Int J Occup Med Environ Health 24(4):391–398
Polańska K, Hanke W, Król A, Potocka A, Waszkowska M, Jacukowicz A, Gromadzińska J, Wąsowicz W, Jerzyńska J, Stelmach W, Stelmach I (2016) Polish mother and child cohort study (REPRO_PL)—methodology of the follow-up of the children at the age of 7. Int J Occup Med Environ Health 29(6):883–893
R Development Core Team. R: a language and environment for statistical computing. Vienna. R Foundation for Statistical computing; 2021. https://www.R-project.org/
Remer T, Neubert A, Maser-Gluth C (2002) Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr 75(3):561–569
Rocha BA, da Costa BR, de Albuquerque NC, de Oliveira AR, Souza JM, Al-Tameemi M, Campiglia AD, Barbosa F Jr (2016) A fast method for bisphenol A and six analogues (S, F, Z, P, AF, AP) determination in urine samples based on dispersive liquid-liquid microextraction and liquid chromatography-tandem mass spectrometry. Talanta 154:511–519
Rocha BA, Asimakopoulos AG, Honda M, da Costa NL, Barbosa RM, Barbosa F, Kurunthachalam K (2018) Advanced data mining approaches in the assessment of urinary concentrations of bisphenols, chlorophenols, parabens and benzophenones in Brazilian children and their association to DNA damage. Environ Int 116:269–277
Rochester JR (2013) Bisphenol A and human health: a review of the literature. Reprod Toxicol 42:132–155
Rochester JR, Bolden AL (2015) Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol A substitutes. Environ Health Perspect 123(7):643–650
Sakhi KA, Sabaredozovic A, Papadopoulou E, Cequier E, Thomsen C (2018) Levels, variability and determinants of environmental phenols in pairs of Norwegian mothers and children. Environ Int 114:242–251
Snoj Tratnik J, Kosjek T, Heath E, Mazej D, Cehic S, Karakitsios SP, Sarigiannis DA, Tratnik JS, Kosjek T, Heath E, Mazej D, Cehic S, Karakitsios SP, Sarigiannis DA, Horvat M (2019) Urinary bisphenol A in children, mothers and fathers from Slovenia: overall results and determinants of exposure. Environ Res 168:32–40
Stragierowicz J, Mikołajewska K, Zawadzka-Stolarz M, Polańska K, Ligocka D (2013) Estimation of cutoff values of cotinine in urine and saliva for pregnant women in Poland. Biomed Res Int 2013:386784
Trasande L, Attina TM, Blustein J (2012) Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA 308(11):1113–21
Usman A, Ahmad M (2016) From BPA to its analogues: is it a safe journey? Chemosphere 158:131–142
Vaccher Vincent, Esteban Lopez M, Castaño A, Mol H, Haji-Abbas-Zarrabi K, Bury D, Koch HM, Dvorakova D, Hajslova J, Nübler S, Kaur Sakhi A, Thomsen C, Vorkamp K, Göen T, Antignac JP (2022) European Interlaboratory comparison investigations (ICI) and external quality assurance schemes (EQUAS) for the analysis of bisphenol A, S and F in human urine: Results from the HBM4EU project. Environmental Research 112933.
Völkel W, Colnot T, Csanády GA, Filser JG, Dekant W (2002) Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem Res Toxicol 15(10):1281–1287
WHO & UNEP (2012) State of the science of endocrine disrupting chemicals—2012. Geneva: World Health Organization, United Nations Environment Programme 2013
Wickham H (2016) ggplot2: elegant graphics for data analysis. New York http://ggplot2.org Springer https://doi.org/10.1007/978-3-319-24277-4
Xue J, Wu Q, Sakthivel S, Pavithran PV, Vasukutty JR, Kannan K (2015) Urinary levels of endocrine-disrupting chemicals, including bisphenols, bisphenol A diglycidyl ethers, benzophenones, parabens, and triclosan in obese and non-obese Indian children. Environ Res 137:120–128
Yang Y, Guan J, Yin J, Shao B, Li H (2014) Urinary levels of bisphenol analogues in residents living near a manufacturing plant in south China. Chemosphere 112:481–486
Zoller O, Brüschweiler BJ, Magnin R, Reinhard H, Rhyn P, Rupp H et al (2016) Natural occurrence of bisphenol F in mustard. Food Addit Contamin 33(1):137–146
Acknowledgements
Mercè Garí acknowledges the support from the IDAEA-CSIC Severo Ochoa Excellence Programme through the Grant CEX 2018-000894-S funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and the office of the Secretary of Universities and Research and the Ministry of Research and Universities of the Government of Catalonia through the Beatriu de Pinós Programme (Grant No. 2021BP00152). She also acknowledges the support from the FGCSIC's ComFuturo programme which has received funding from the EU H2020 research and innovation programme under the MSCA No. 101034263.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This research is funded by the National Science Centre, Poland (Grant No. 2014/15/B/NZ7/00998 and No. 2021/41/B/NZ7/04341); Internal grant (Grant No. PNP/IMP/7/2022) and the Medical Faculty of the Ludwig Maximilians Universität Munich, Germany (Grant No. WIFOMED-80926024). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Mercè Garí: conceptualization, methodology, formal analysis (exposure and risk assessment, correlation analyses), investigation, data curation, visualization, funding acquisition, writing—original draft, review and editing; Daniel Bury: methodology, validation, formal analysis (exposure and risk assessment), investigation (HBM analyses), writing—review and editing; Rebecca K. Moos: methodology, validation, formal analysis (exposure and risk assessment), investigation (HBM analyses), writing—review and editing; Monika Wolniewicz: methodology, investigation (field work and child examination), resources, data curation, writing—review and editing; Agnieszka Jankowska: methodology, validation, investigation (field work and child examination), resources, data curation, writing—original draft, review and editing; Agnieszka Brzozowska: methodology, investigation (field work and child examination), resources, data curation, writing—review and editing; Joanna Jerzynska: conceptualization; methodology, investigation (field work and child examination), resources, data curation, writing—review and editing; Stephan Bose-O’Reilly: conceptualization, supervision, resources, funding acquisition, validation, writing—review and editing; Holger M. Koch: supervision, resources, funding acquisition, validation, writing—review and editing; Kinga Polanska: project administration, conceptualization, supervision, resources, funding acquisition, validation, writing—original draft, review and editing.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethical Approval
Written informed consent was obtained from the parents of each child before the study, which was approved by the Ethical Committee of the Nofer Institute of Occupational Medicine, Lodz, Poland (Decision No. 22/2014).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Garí, M., Bury, D., Moos, R.K. et al. Urinary Concentrations of BPA and Analogous Bisphenols (BPF and BPS) among School Children from Poland: Exposure and Risk Assessment in the REPRO_PL Cohort. Expo Health (2024). https://doi.org/10.1007/s12403-024-00654-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12403-024-00654-4