Abstract
The number of chemicals in the anthroposphere is increasing and some of them end up in humans. A literature search was made to assess which anthropogenic organic contaminants (OCs) that have been analysed in blood from the general population. The reviewed articles were used to create a database of studies [human blood database (HBDB), containing 559 OCs] reporting blood analyses made worldwide. All studies analysing blood from the Swedish population were compiled into a second database [Swedish exposure database (SEDB), containing 166 OCs] listing blood concentrations of OCs. Data from the SEDB showed decreasing levels of regulated chemicals in blood over time, indicating that regulation had made an impact. The Hazard Index (HI) approach was used as a qualitative mixture risk assessment of the OCs with established human biomonitoring guidance values (HBM-GVs) and blood levels in the SEDB. Nine HBM-GVs were found and the HI of the corresponding OCs/groups of OCs showed that a risk of adverse effects in the general population could not be excluded, which is a cause for concern considering that only a fraction of the analysed OCs in the SEDB were included. This study presents the OCs identified in human blood and concentration time trends. The study highlights the lack of HBM-GVs needed for mixture risk assessments to assess the combined risk of chemical exposure to the general population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production and use of chemicals have increased worldwide for many decades as the anthroposphere is getting more complex (Eurostat 2021). Diseases of civilisation (such as diabetes type 2, obesity etc.) and autoimmune diseases have increased over the last decades (Huhn et al. 2021). Genetic factors can only account for 30% of the risk for such diseases, whereas the rest of the risk might be ascribed to differences in the environment (Rappaport and Smith 2010; Vojdani et al. 2014). This difference in environment is described as the exposome, i.e. the totality of both simultaneous and sequential environmental exposure (Rappaport and Smith 2010). A contributing part of the exposome is chemical exposure, which the European Commission recently stated as a threat to human health (European Commission 2020).
There are a range of different sample types which can be used when assessing human chemical exposure. Commonly, exposure to chemicals is assessed by analysis of matrices of human intake (e.g. food, air, etc.), reflecting the external exposure. Human biomonitoring uses human body fluids and tissues to measure the exposure of target chemicals. Blood is an ideal matrix for anthropogenic organic contaminants (OCs) due to bloods equilibrium with organs and tissues of the whole organism (Esteban and Castano 2009). Thus, blood reflects the internal OC levels and includes uptake and metabolism of chemicals. Also, blood is the matrix that reflects the long-term OC exposure in the general population, whereas urine mainly reflects the short-term exposure. Breast milk is another relevant monitoring matrix, although it only reflects chemical exposure of women and infants.
Biomonitoring programmes are used to capture temporal changes in chemical exposure, to investigate when regulation is needed and to monitor if the regulation has the desired outcome. Health-related Environmental Monitoring (HÄMI) is Sweden’s governmentally funded biomonitoring program, run by the Swedish Environmental Protection Agency (SEPA). In 1993, HÄMI was initiated to monitor inorganic and organic contaminants in humans in different matrices on a long-term basis (Swedish Environmental Protections Agency 2021). To date, HÄMI covers over 380 anthropogenic OCs analysed in human matrices (Swedish Environmental Protections Agency 2021). The Division of Laboratory Sciences at the US Centers for Disease Control and Prevention (US-CDC) runs the US National Biomonitoring Program (NHANES), which monitors over 400 environmental chemicals, including OCs and metals in human blood and urine (US Centers for Disease Control and Prevention 2020). In 2017, the EU initiated the Human Biomonitoring for EU (HBM4EU) project, a collaboration between 24 member states, Norway, Iceland, Israel and Switzerland, along with the European Commission and the European Environment Agency. HBM4EU has prioritised 19 organic and inorganic chemicals or groups of chemicals with risk-related concerns, with the aim of harmonising the human biomonitoring efforts in Europe and increasing the understanding of the temporal changes of the chemical exposure, as well as providing evidence for chemical policy making to protect human health (HBM4EU 2020). The European Commission’s Information platform for Chemical monitoring (IPCHEM) provides more information on different biomonitoring programmes within the EU (European Commission 2021). Additionally, assessing chemical exposure by including peer-reviewed literature data is of importance since biomonitoring programmes only cover a limited number of chemicals (Bopp et al. 2019). Chemicals with properties of persistence, bioaccumulation, a potential for long-range environmental transport and adverse effects in humans or in the environment are classified as persistent organic pollutants (POPs) (Secretariat of the Stockholm Convention 2020). The Stockholm Convention, which came into force in 2004, is a collaborative strategy to remove these types of chemicals from the market (Secretariat of the Stockholm Convention 2020). Since then, a range of biomonitoring programmes have monitored POPs in humans and in the environment, as part of the agreement. HÄMI reports time trends that are used to follow up on the Stockholm Convention on a national level.
Classical risk assessments of single chemicals are usually based on external exposure dose, derived from in vivo experimental data. When blood concentrations are used as exposure data, however, internal exposure dose levels are needed for the risk assessments. The internal dose is rarely measured after external exposure in animal experiments. Human biomonitoring guidance values (HBM-GVs) or biomonitoring equivalent (BE) values express the acceptable internal exposure dose levels. The German Human Biomonitoring Commission’s HBM-GV I and BE values are concentration limits in human matrices below which there is no risk of adverse effects (Hays et al. 2007, HBM-Communication 2020). The German Human Biomonitoring Commission has defined an additional higher HBM-GV (HBM-GV II). When blood levels exceed the HBM-GV II, actions to decrease the exposure need to be taken (HBM-Communication 2020). Herein, HBM-GV I and BE values are treated as equals and denominated as HBM-GVs. The usage of HBM-GVs is limited by a lack of established values and the amount of time taken to produce new HBM-GVs (Apel et al. 2020). Furthermore, these values should not be considered static and when new toxicity data are available the values need to be updated. HBM-GVs can be derived either from epidemiological data, in situ models, or in vivo data by using assessment factors (AF), depending on available data (Apel et al. 2020). HBM4EU aims to increase the number of HBM-GVs and has recently produced urinary HBM-GVs for cadmium, bisphenol A, N-methyl-2-pyrrolidone (NMP), N-ethyl-2-pyrrolidone (NEP) and a number of phthalates (David et al. 2021; Lamkarkach et al. 2021; Lange et al. 2021; Ougier et al. 2021).
When both exposure and inherent toxicity are known for a set of chemicals, there are different ways of calculating the combined risk of chemicals. Mixture risk assessments (MRAs) estimate the effect of the combined exposure. One MRA method is to use the Hazard Index (HI) approach, derived by the US EPA in 1986 and supplemented in 2000 (US Environmental Protection Agency 1986). The HI approach assumes concentration addition, meaning that each component of the mixture adds to the toxicity through the same mode of action. When using HBM-GVs, only a qualitative MRA can be achieved when calculating the HI, since the critical effects they are based on are different for different OCs. All HBM-GVs have an added margin of safety. Different critical effects can be combined in an MRA when HBM-GVs are used, as the margin of safety generates a conservative HI (Bopp et al. 2019). However, OCs contributing to the same adverse outcome may act synergistically and could cause a larger effect compared to when they act additively (Kortenkamp and Faust 2018).
To be able to assess the combined risk of a mixture of chemicals, the toxicity evaluation needs to be performed and reported in a consistent manner, e.g. regarding species, endpoints and reported toxicological dose descriptors. Finding this information for a larger set of chemicals of concern can be a challenge (Engelhardt et al. 2021).
The first aim of this study was to make an exposure assessment of anthropogenic OCs in human blood from the Swedish general population. In addition, the identity of OCs analysed in human blood worldwide was also collected. It was expected that the majority of the chemicals found in this study are POPs, as these are commonly analysed in blood. Where possible, time trends of OCs in blood were established to follow regulatory implementations. A second aim of this study was to assess the combined risk of exposure to the mixture of OCs reported in blood in the Swedish general population, by applying existing HBM-GVs derived for blood.
Method
OCs in Human Blood
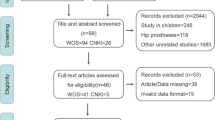
The data were collected through a review of scientific literature and inclusion of OCs from selected, accessible biomonitoring programs. The literature search was conducted between September 2019 and December 2020 with the keywords “blood”, “serum”, “human”, “contaminant”, “chemical”, “substance”, “pollutant” and “molecule”. The collection was limited to articles published between 2000 and 2020. Articles in other languages than English or Swedish were excluded in the selection process, as well as studies focusing on pharmaceuticals or inorganics. Only articles analysing blood from the general population were used, i.e. studies analysing patients with chronic illnesses were excluded as well as studies on people living in the vicinity of a contaminated site. A detailed description of the literature search can be found in the supporting information (Online Resource 2, Table S1-2). The collected OCs were compiled into the human blood database (HBDB). The blood concentrations from studies within the HBDB that had analysed Swedish blood were included into the Swedish exposure database (SEDB). For that purpose, “Sweden” or “Swedish” were added to the search terms. OCs analysed in human blood, reported at the international symposium on Halogenated Persistent Organic Pollutants, and available in the Dioxin database (International Symposium on Halogenated Persistent Organic Pollutants 2020) containing summaries and extended peer-reviewed abstracts, published between 2000 and 2020 were included in the HBDB. OCs analysed in blood and reported in the National report on Human Exposure to Environmental Chemicals (NHANES) from United States Centers for Disease Control and Prevention (US-CDC) were added to the HBDB (US Centers for Disease Control and Prevention 2019). The HBDB consists of 245 references (10,829 entries) and two biomonitoring programmes (Fig. 1).
All analyses reported in SEPA’s HÄMI database were added to the SEDB (Swedish Environmental Protections Agency 2021). The SEDB consists of 53 references (6476 entries) and one biomonitoring programme (Fig. 1). In the SEDB, the cohorts covered in peer-reviewed literature were European Prospective Investigation into Cancer and nutrition (EPIC) (Slimani et al. 2007), Prospective Investigation of Vasculature in Uppsala Seniors (PIVUS) (Lind et al. 2005), Persistent Organic Pollutants in Uppsala Primiparas (POPUP) (Glynn et al. 2007), Riksmaten Adults 2010–2011 (Swedish Environmental Protections Agency 2021), Riksmaten Adolescents 2016–2017 (Swedish Food Agency et al. 2020), Västerbotten Intervention Programme (VIP) (controls) (Norberg et al. 2010), Fishermen and their wives (Rylander and Hagmar 1995; Svensson et al. 1995), Malmö Maternity Unit Serum Biobank (MMUSB) (Ode et al. 2014), Swedish Environmental Longitudinal, Mother and child, Asthma and allergy study (SELMA) (Bornehag et al. 2012), Adolescents in Scania (Norén et al. 2020) and small groups of people used for one study only (Fig. 1). The chemical identity, country of origin of the blood samples and year of sampling were extracted manually from all articles and entered into the HBDB. Additionally, for the OCs analysed in Swedish blood, the concentration range (min–max), average concentration, cohort, sex, LOD/LOQ values and number of individuals analysed were included in the SEDB. The entries were divided based on concentration determined in human blood from women, men, “both” (when the sex of the individuals was undefined or concentrations reported as pooled averages), and children and adolescents (0–20 years old).
In the HBDB, all OCs that have been analysed in blood were included and the OCs reported below detection level (< LOD) were indicated in the HBDB (Online Resource 1; HBDB overview). In the SEDB, only OCs that have been reported above quantification level (> LOQ) were included (Online Resource 1, SEDB), i.e. OCs analysed but not detected above the quantification level (< LOQ) at any timepoint analysed in Swedish blood were not included in the SEDB. If more than one article reported levels of a specific OC from the same cohort and sex with very similar concentrations, it was assumed that the results were coming from the same analysis. Chemical identifiers (such as CAS, SMILES, InChIKey) were taken from CompTox Chemical Dashboard, ChemSpider, PubChem and SciFinder. Each chemical was given a unique ID number (chemical ID) and classified according to chemical group, structural identifier (aromatic, halogenated, phenolic and/or just OCs), halogen type (brominated, chlorinated, fluorinated or non-halogenated) and POP/POP metabolite status according to the Stockholm Convention. OCs reported together (co-eluents and sum of congeners such as ∑PCBs) were not included in the database, except for seven PFASs which were reported as linear, branched isomers or undefined when not specified.
To enable the comparison of different studies, the concentrations were converted to both wet weight and lipid-adjusted weight. When the lipid concentration in human serum was not reported, a default value of 0.006656 g/mL was used, based on a lipid percentage of 0.65% and a density of human serum of 1.024 g/mL (Sniegoski and Moody 1979; Weiss et al. 2006). For plasma, a lipid percentage of 0.42% was used and the density was estimated to be the same as in serum (Hovander et al. 2000; Sniegoski and Moody 1979). When the matrix was whole blood or not specified further than “blood”, the lipid concentration in serum was used. For comparison, all results in the graphs are presented in wet weight (pg/mL), even though some lipophilic chemicals normally are presented as lipid-adjusted weight (available in SEDB, Online Resource 1, SEDB). For the time trends of the OCs that had been analysed for more than five years, a linear regression model was applied on the logarithmic values of the concentrations, in order to check for a significant temporal trend (p-value < 0.05). A linear regression line with the logarithmic values was added to the antilogarithmic graph when there was a significant relationship between time and concentration. A sensitivity test was carried out to check the influence of the values below LOQ on the temporal trend of each graph. For each dataset, the values below LOQ were either set to 0, LOQ/2, or LOQ and the slope of the trends and the p-values of the trends were noted (Table S3). Concentrations below LOQ levels were not included (corresponding to LOQ = 0) when making the trendlines in the graphs. Over time, LOQ levels have decreased as a result of refined analytical methods. Some LOQ values were above or in the same range as reported concentrations from other studies. The LOQ values have been included in the graphs to illustrate the performance of the laboratories and the number of analyses. The sensitivity tests showed that the slope did not drastically change in the different scenarios (Table S3).
Risk Assessments
HI, a component-based approach, was used to assess the combined risk of the chemical mixture. The hazard quotient (HQ) for each OC (Eq. 1) was calculated by dividing the exposure level (Exp) of the ith compound by the acceptable level (AL) of the ith compound. The HQs were summed into an HI (Eq. 1) for the mixture. If the HI exceeded one, the mixture risk of adverse effects in the general population could not be excluded and thus further refinements were needed (US Environmental Protection Agency 1986). Herein, the average blood concentrations for each year from the whole population were used as exposure levels (Online Resource 1, MRA). By using blood concentrations as the exposure, we were limited to use AL reported as internal concentrations rather than administered dose commonly reported from in vivo studies. Therefore, blood HBM-GVs were used. The HBM-GVs were taken from peer-reviewed literature and the German Human Biomonitoring Commission (Aylward et al. 2010; Aylward et al. 2008; HBM-Communication 2020; Kirman et al. 2011; Krishnan et al. 2011). A summarising table with all HBM-GVs, their respective critical endpoint and other information regarding the derivation process can be found in the supplementary information (Online Resource 2, Table S4). All the HBM-GVs, except for dioxin-like compounds (PCDD/Fs and dl-PCBs), were converted into wet weight (pg/mL), using the same lipid percentage and density as previously described for blood concentrations. The HBM-GV for PCDD/Fs and dl-PCBs was a lipid-adjusted TEQ value. Therefore, the exposure concentrations were converted to the same unit using the TEF values from (Van den Berg et al. 2006). The TEQ sum was calculated based on the lipid-adjusted blood concentrations of seven dioxins, ten furans and twelve dl-PCBs.
Results
OCs in Human Blood
The two databases are available in the online repository (Online Resource 1, HBDB and SEDB). The HBDB includes 559 OCs analysed in human blood, out of which 440 OCs were reported to have been detected (> LOD/LOQ). For the OCs in the HBDB, the majority (83%) are halogenated, and 49 OCs (11%) are simultaneously halogenated, phenolic and aromatic (Fig. 2). In the HBDB, 54% of the OCs are POPs, and 9.1% are metabolites of POPs (Online Resource 2, Table S5). The HBDB contains OCs analysed in blood from 38 countries, mostly from Western Europe, North America and Asia (Online Resource 2, Table S6).
The SEDB includes 166 OCs, out of which 91% are halogenated (Fig. 2). In the SEDB, 57% of the OCs are POPs and 10% are metabolites of POPs (Online Resource 2, Table S5). The OCs analysed in blood in Sweden are mainly regulated OCs. The OCs analysed in Sweden are flame retardants, polychlorinated dibenzo-p-dioxins and furans (PCDD/F), chlorinated pesticides and their metabolites, polychlorinated biphenyls (PCBs) and their metabolites, poly- and perfluoroalkyl substances (PFASs) and phthalates (Fig. 3). Beyond the chemical groups in the SEDB, the HBDB consists of alkylphenols, chlorinated paraffins, flame-retardant metabolites, polycyclic aromatic hydrocarbons (PAHs), parabens, personal care product ingredients, solvents and UV filters (Fig. 3). In Sweden, alkylphenols, bisphenols, PAHs, parabens, personal care product ingredients and UV filters are analysed in urine (Swedish Environmental Protections Agency 2021). A list with all chemical groups in the HBDB and the SEDB and the time frame of blood sampling is presented in Online Resource 2, Table S7.
For a few selected OCs, the number of studies and number of individuals analysed are listed to illustrate the focus on sex and age groups (Online Resource 2, Table S8). It appears that blood from women and children are most frequently analysed, followed by studies reporting levels for all adults combined. The least number of analyses were reported from men.
Swedish Blood Levels
Chemical groups commonly monitored in blood are PCBs, pesticides, PFASs and flame retardants (Fig. 4). The OCs with the highest average levels reported in human blood are the PFASs, some as high as 14,000 pg/mL, with perfluorooctane sulfonate (PFOS) at the highest level followed by perfluorohexane sulfonate (PFHxS) and perfluorooctanoic acid (PFOA) (Fig. 4a, b). Among the PFASs, PFOA and PFOS are regulated by the Stockholm Convention (Annex A and B, respectively) and in 2019 PFHxS was proposed for listing under the Convention (Secretariat of the Stockholm Convention 2019). Perfluorobutanesulfonic acid (PFBS) shows increasing time trends for women and children (Online Resource 2, Fig. S1) but most of the PFASs show decreasing trends (Online Resource 2, Fig. S1-11). PCBs have been restricted by the Stockholm Convention (Annex A) since 2004 and occur in blood in levels around 100 pg/mL, depending on congener (Fig. 4c, d). The brominated flame retardants occur in lower levels in blood compared to other chemical groups and appear in blood at average levels around 10 pg/mL (Fig. 4e; Online Resource 2, Fig. S2). Most of the pesticides analysed in human blood are POPs (Fig. 4f), which are regulated by the Stockholm Convention (Annex A and B). Pentachlorophenol (PCP) average levels in Swedish blood can reach up to 7000 pg/mL (Fig. 4g), reflecting a high use of this preservative in the forest industry (Palm et al. 2002). The analysed organophosphorous flame-retardant di-n-butylphosphate is not regulated and occurs in higher blood levels than the brominated flame retardants, with an average blood concentration of around 1000 pg/mL (Online Resource 1, SEDB). The phthalates and BPA have only been analysed in blood between 1999 and 2009 (Table S7) and there are too few reported analyses to discuss time trends. Generally, polar substances are more easily conjugated and excreted via urine than the non-polar OCs (typically the POPs). Therefore, polar OCs and their metabolites are more commonly analysed in urine and not reported in the same degree as other OCs in the SEDB (Swedish Environmental Protections Agency 2019).
The chemical profile in blood from the Swedish general population. The width of the violin plot corresponds to the frequency of a certain concentration, the broader the violin, the more frequent the concentration is within the dataset. The colour in the graphs correspond to the magnitude of each OC concentration, high (red) to low (blue). Each OC’s concentration range is presented in the graph with a black line as the average concentration and two white lines on each side as the 25th and 75th percentiles. The range correspond to min–max values after outliers have been identified (ROUT test with Q = 1) a–b PFASs c–d PCBs e PBDEs f–g Pesticides
Risk Assessment
Nine HBM-GVs were found for OCs or groups of OCs reported in the SEDB (Online Resource 1, MRA). An HBM-GV for PCBs has been set to 3500 pg/mL by the German HBM Commission, for the sum of the concentrations of CB138, CB153 and CB180 (HBM-Communication 2020; Rauchfuss et al. 2013). The total concentration in blood of the three PCBs in the Swedish population is below the HBM-GV. The group has been reported below this value since year 2004 (Fig. 5a), when it was first regulated by the Stockholm Convention (Secretariat of the Stockholm Convention 2020; Swedish Chemicals Agency 2021). The concentration of PCBs in blood is decreasing in the Swedish general population (Fig. 5a–d). The most recent HQ of PCBs was 0.032 (Fig. 9a).
Time trends for some PCBs. Trendlines have been added when p-value < 0.05. a Blood concentration of Σ3 PCBs (CB138, CB153, CB180); Σ7 PCBs (CB28, CB52, CB101. CB118, CB153, CB138, CB180) and Σall PCBs. The horizontal line corresponds to the set HBM-GV for Σ3 PCBs (HBM Commission 2020; Rauchfuss et al. 2013). Time trends of b CB138 c CB153 d CB180
Few studies reported blood levels of PCDD/Fs and dioxin-like PCBs (dl-PCBs) (Fig. 6; Online Resource 2, Table S8). PCDD/Fs are regulated by the Stockholm Convention (Annex C) since 2004 (Secretariat of the Stockholm Convention 2020; Swedish Chemicals Agency 2021). The four latest analyses (after 2003) only reported dl-PCBs (Fig. 6) and therefore calculated TEQ levels do not include the PCDD/Fs. The dl-PCB TEQ levels are below the HBM-GV of 15 ppt (Aylward et al. 2008). The PCDD/Fs have not been analysed to the same extent as the dl-PCBs, and the lack of data makes it difficult to conclude if the total level is associated with risk of adverse effects or not. Since the number of congeners differs throughout the time period, no interpretation of a trend can be made (Online Resource 1, MRA). In the two instances when all PCDD/Fs were analysed (1987 and 2002), the total blood level of the group exceeded the HBM-GV. The most recent HQ of dl-PCBs was 0.0000037 (Fig. 9a).
Time trend of the TEQ levels of the sum (green) of seven dioxins, ten furans (PCDD/Fs, yellow) and twelve dl-PCBs (blue). The dashed line at 15 ppt is the set HBM-GV for PCDD/Fs and dl-PCBs (Aylward et al. 2008). As the number of congeners analysed differed throughout the time period, no interpretation of a trend can be made
Hexachlorobenzene (HCB) is regulated by the Stockholm Convention (Annex A) since 2004 (Secretariat of the Stockholm Convention 2020; Swedish Chemicals Agency 2021). HCB shows decreasing concentrations in men from 1990 to 2012 (Fig. 7a). During the last year of samples analysed (2017), the average exposure for values were below the set HBM-GV of 170 pg/mL (Aylward et al. 2010). The blood concentration of HCB in women has decreased since 2004. The most recent HQ for HCB was 0.31, which is the third highest HQ value that year (2017) (Fig. 9a). The pesticide p,pʹ-DDT was added to the Stockholm Convention (Annex B) in 2004 (Secretariat of the Stockholm Convention 2020; Swedish Chemicals Agency 2021). The major metabolite of p,pʹ-DDT is p,pʹ-DDE and it is analysed more frequently than the parent compound. The lower level of p,pʹ-DDT, compared to p,pʹ-DDE, indicates a historical use. The average reported levels of both p,pʹ-DDT and p,pʹ-DDE show decreasing trends. The decrease in blood levels of p,pʹ-DDE in men and children is statistically significant. In 2013, at the most recent reporting of blood samples from women, the concentration in women was among the lowest (Fig. 7b). No significant trend can be observed for p,pʹ-DDT. All reported levels are below the set HBM-GV of 33,000 pg/mL for the sum of p,pʹ-DDT and p,pʹ-DDE (Kirman et al. 2011). The most recent HQ of p,pʹ-DDT and p,pʹ-DDE was 0.0077 (Fig. 9a).
a The blood concentration of hexachlorobenzene (HCB) in the general population in Sweden. The black horizontal dashed line corresponds to the set HBM-GV in blood (Aylward et al. 2010). b The blood concentration of p,p’-DDE in the general population in Sweden. The HBM-GV for p,pʹ-DDT and p,pʹ-DDE of 33,000 pg/mL. Trendlines have been added when p-value < 0.05
PFASs are the group with the highest blood levels of all OCs in the SEDB (Fig. 4a, b). PFOS has been regulated by the Stockholm Convention (Annex B) since 2009 and PFOA has been regulated (Annex A) since 2019 (Swedish Chemicals Agency 2021). The blood concentrations of PFOS and PFOA have been decreasing in both men and women during the studied time frame. The most recent analysis of PFOS reported levels close to the HBM-GV of 5000 pg/mL (Hölzer et al. 2021) with an HQ of 0.58 (Figs. 8a, 9a). The HBM-GV for PFOA is 2000 pg/mL and the latest analysis showed levels below this value (Fig. 8a) (Hölzer et al. 2021). The most recent HQ for PFOA was 0.58 (Fig. 9a). Before 2010, the blood concentrations of PFOS were above the higher set HBM-GV II of 20,000 pg/mL and 10,000 pg/mL for the general population and women in child-bearing age, respectively (Fig. 8a) (HBM-Communication 2020). When the concentration exceeds the HBM-GV II, actions need to be taken to avoid adverse effects to the general population (HBM-Communication 2020).
Time trends of blood levels of a PFOS and b PFOA in the general population in Sweden. The black horizontal dashed lines correspond to the German Human Biomonitoring Commission’s HBM-GV I, and the green dashed lines corresponds to the HBM-GV II, where (w) is the HBM-GV II set for women in child-bearing age (light green) and (g) is the HBM-GV II for the general population (dark green) (HBM Commission 2020; Hölzer et al. 2021). Trendlines have been added when p-value < 0.05
a Hazard quotients (HQ) for the different OCs calculated by dividing average blood concentration of each OC by the HBM-GV b ΣHQ, the hazard index (HI). When HI is above 1, a risk of adverse effects in the general population cannot be excluded (orange horizontal line). The green dots correspond to the number of OCs included in the HI each year
A calculation of the HQ for the nine OCs/groups of OCs each year shows that some OCs exceed their risk threshold of 1 individually in at least one year (ΣPCBs, HCB, PFOS and PFOA) (Fig. 9a). The OCs with HBM-GVs are all regulated by the Stockholm Convention and the majority of the HQs have been decreasing over the reported time period, as would be expected (Swedish Chemicals Agency 2021). When considering the combined risk, the highest HI occurred in 2004 when the risk was eight times higher than accepted (Fig. 9b). PFOS and PFOA are driving the risk during the studied time period. The peak of PCDD/Fs and dl-PCBs can be explained by the monitoring of many individual congeners in year 2002 (Fig. 9a, b). Since 1988, the HI has been above 1 each year, with PFOS and PFOA as the drivers. Assuming single chemical risk assessment, no OC was individually posing a risk of adverse effects after 2015. However, the HI was 1.5, which is still above 1 in 2017, highlighting the importance to evaluate to combined risk of chemical exposure (Fig. 9a, b).
Discussion
The overall aims of this study were to create an overview of the current chemical exposure of OCs in blood from the general population in Sweden, and to list the OCs analysed in blood worldwide by manually reviewing scientific literature and human biomonitoring data. In another study, the exposome was estimated by compiling chemicals found in human blood using text-mining and a database fusion approach (Barupal and Fiehn 2019). The study generated a database of over 41,000 chemicals found in human blood, including organic and inorganic, endogenous metabolites, exogenous pharmaceuticals and so on. Manual curation would be needed to extract the target OCs of this study, i.e. anthropogenic organic contaminants, from that study. To the best of our knowledge the HBDB is the most comprehensive list of OCs in blood to date. Most likely more OCs could be added to the HBDB as we do not claim to have performed an exhaustive review of OCs analysed in human blood worldwide.
Generally, most of the OCs reported in the HBDB and the SEDB were POPs regulated by the Stockholm Convention. This is in line with what could be expected due to the choice of matrix and because the Stockholm Convention requires monitoring of the chemicals included in Annex A, B and C (Article 11) (Esteban and Castano 2009; Secretariat of the Stockholm Convention 2020).
There are too few analyses to be able to see a time trend for PCDD/F levels in blood since the target matrix for PCDD/F analysis has changed to breast milk (for most congeners in 2004). A decreasing time trend in concentrations of PCDD/F and dl-PCBs was reported in both breast milk and in fish, our primary source of these contaminants (Gyllenhammar et al. 2021; Miller et al. 2012). Also, the decreasing trends of the OCs in both blood and milk demonstrate that the regulatory work is necessary and efficient. According to the data in the SEDB, some OCs have not been analysed in blood for the past ten years, especially the phenolic OCs, such as the OH-PCBs (metabolites of PCBs), bisphenols and phthalates. Instead, non-halogenated, polar OCs are commonly analysed in urine, such as alkylphenols, bisphenols, parabens, personal care product ingredients and UV filters (Esteban and Castano 2009; Swedish Environmental Protections Agency 2021). Chlorinated paraffin is the only chemical group present in the HBDB that is not currently covered in HÄMI. The short-chained CPs were added to Annex A of the Stockholm Convention in 2017, and the medium-chained CPs are currently being proposed for listing. A first screening of CPs in human blood levels of the Swedish population is being planned by HÄMI (personal information from co-author K. Norström).
By looking at the data present in the SEDB, the OCs with the highest concentrations in blood were PFASs (PFOS > PFHxS > PFOA). Several PFASs (n = 50) are analysed worldwide (Online Resource 1, HBDB), which could reflect the increased public awareness after the evident exposure taking place all around the world, raising concern for human health (Sunderland et al. 2019). The blood levels of PFOS and PFOA show similar decreasing trends (Fig. 8), even though PFOA was added to the POP list ten years later than PFOS. Other PFASs have replaced the previously used PFOS and PFOA, some of which show increasing levels (PFBS and PFHxS, found in Online Resource 2, S1 and S6), or increasing levels in women but decreasing levels in children (PFNA PFDA, PFUnDA, PFTrDA, found in Online Resource 2, S8-11).
The European HBM Dashboard provides summary statistics from existing HBM data collections within the HBM4EU project. A comparison with the SEDB show similar levels of PFASs reported in the European HBM Dashboard for Norway, Germany, Belgium, Spain, Denmark and Czechia, while lower levels have been reported for Slovakia and Austria (Flemish Institute for Technological Research et al. 2021). PBDEs were also reported in the European HBM Dashboard and the levels were similar to those in the SEDB for countries like Belgium and Denmark and higher in Slovakia (Flemish Institute for Technological Research et al. 2021).
It takes time and funds to develop and validate new analytical methods for emerging chemical groups, and therefore harmonised methods developed within international monitoring programmes are important (HBM4EU 2020). HBM4EU has recently published a review addressing different analytical methods to be used for analysis of a selected range of chemicals (Vorkamp et al. 2021). Reporting the analytical method LOD and LOQ values is a valuable quality control to better understand the performance of the laboratories and quality of analysis. As analytical instrumentation and performance develop, the LOD/LOQ levels are decreasing. In the SEDB, the LOD and LOQ values were not always available, or laboratories reported a range of values for the same analytes. As a consequence of different analytical performance, some laboratories reported LOD/LOQ values in the same range as others reported quantifiable concentrations. In this study, < LOQ/LOD values were therefore excluded in the statistical analysis, although visually included in the figures (Online Resource 2; Fig. S1-12) so as not to discard any analyses. To be able to generate time trends for chemicals reaching trace levels, analytical methods with low LOD/LOQ values are needed.
A challenge posed in the analysis of new chemicals is the lack of analytical standards. Even though non-target analytical methods could provide us with some chemical structural information, an analytical standard is needed for accurate confirmation and quantification (Schymanski et al. 2015). However, new strategies to semi-quantify chemicals without accurate analytical standards are currently being developed, e.g. based on chemical ionisation efficiency (Malm et al. 2021). Analytical standards of high purity are also needed for newly identified risk chemicals in order to evaluate the potential toxicity. A suggestion is that previously untested pure chemicals or technical mixtures should be provided upon registration by the producers or importers intending to use them. That would facilitate the monitoring of “tomorrow’s” chemicals and the setup of early warning systems.
In a first attempt to investigate whether or not a simplified risk assessment of the OCs in the SEDB could be performed, the US EPA’s CompTox Chemicals Dashboard was used to extract available toxicity data (Engelhardt et al. 2021). Out of the 166 OCs in the SEDB, 64% of the OCs did not have any toxicity data in the CompTox Chemicals Dashboard (Engelhardt et al. 2021). Furthermore, the data acquired for the remaining 36% of OCs in the SEDB did not provide sufficient detail to enable a risk assessment, at least from a search for easily available toxicity data. Two databases (EFSA’s OpenFoodTox and US EPA’s ECOTOX) provided more information regarding the study design, but the data were reported in externally administered dose and could not be used (Engelhardt et al. 2021). Comparing the toxicity data to human blood levels would only be possible using generic PBTK modelling, which is still in development. An MRA can be used for the identification of the OC(s) (or OC groups) which are the strongest contributors to the risk. The HBM-GVs were compared with the average concentrations in blood for each year in the Swedish general population. The concentrations of ΣPCBs, HCB, PFOA and PFOS in blood of the Swedish population were all above the HBM-GVs at some point during the last 30 years (Fig. 9). The blood concentrations have been decreasing and all are now below the individual threshold for adverse effects. When using HBM-GVs, a qualitative MRA can be conducted by summing up the individual HQ to the mixture HI, even though there are different target endpoints involved (Bopp et al. 2019). Each OC can exert multiple adverse effects, and thereby contribute to mixture effects even if the adverse outcome is not the critical endpoint. The estimated combined risk of OCs with different outcomes will most likely be conservative and overestimate the risk since only chemicals with the same adverse outcome can act additively. One important finding is that the combined risk of the nine OCs/groups of OCs included in the MRA suggested that a risk of adverse effects to the general population cannot be excluded, as the HI was 1.5 for 2017. This suggests that a further refinement of the MRA is needed, e.g. by considering grouping similar structures and investigating the variation of blood levels within the Swedish general population. The variation can be studied by considering, e.g. sex or age-specific groups, which years to consider for the concentrations and certain percentiles of the concentration range (EFSA et al. 2021). According to the Pareto principle, it can be expected that only a few OCs are responsible for the largest part of the mixture effect; in this study the drivers are PFOA and PFOS (Bopp et al. 2019). The HBM-GVs for PFOA and PFOS are based on epidemiological studies supported by in vivo and in vitro studies with the following critical endpoints: decreased fertility, foetal growth, lipid metabolism as well as effects on the immune system, sex hormone system and thyroid metabolism (Apel et al. 2017). The third highest HQ was HCB, with a relatively low HBM-GV (Aylward et al. 2010). The HBM-GV is derived based on the critical endpoint of hepatic toxicity from rat and pig studies (Aylward et al. 2010). Note that the HQs in this paper were based on the average concentrations from all analysed samples during a specific year, and that there are subpopulations with higher blood levels that should be considered. Due to the lack of HBM-GVs, an MRA could only be assessed for a small proportion of the OCs and groups of OCs in the SEDB. Considering the numerous OCs we are exposed to, the results highlight the importance of deriving more HBM-GVs and/or providing a general guidance on how to estimate mixture risks from the chemical cocktail analysed in blood.
Several studies have attempted to evaluate the risk of specific effects in a selected population. The SELMA study used epidemiological data and statistical models to find probable correlations (Bornehag et al. 2019). They identified OCs called “bad actors” measured in prenatal blood/urine, which were associated with a shorter anogenital distance in boys. A mixture of these bad actors was compiled and tested in vivo to find a point of departure. It could be seen that 13% of the women in the SELMA cohort had concentrations associated with a shorter anogenital distance. Using the HI approach, only 3% of the women were at risk of having a child with a shorter anogenital distance. The conclusion was that with nothing but a component-based MRA using HI, the risk could be underestimated (Bornehag et al. 2019).
Considering the extent of chemical exposure, lack of toxicity data and the infinite variety of mixtures present, assessing mixture risks remains challenging (Bopp et al. 2019). As there is a general lack of exposure data for the bulk of new and emerging chemicals, and the concentrations vary over time and space, we do not have a comprehensive overview of the chemical exposome. Based on what we have gathered in this study, we could postulate that the chemical exposure consists of (at least) 440 OCs in human blood. Assuming equal HQ distribution between the human blood contaminants and the nine OCs/groups of OCs with a total HI of 1.5, the 440 OCs would correspond to a HI of > 70. Thus, the estimated chemical exposure level could be 70 times above a theoretical safe level. This is in line with the conclusion by Gennings et al., that a chemical-by-chemical assessment underestimates the risk of chemical mixtures by a factor of up to 100 (Gennings et al. 2018).
The databases created in this study will be used in the project RiskMix (FORMAS) with the higher aim to further evaluate the combined risk of exposure to the OCs relevant to humans. Complex chemical mixtures with levels relevant to humans based on the SEDB have been created and will be tested in vivo and in vitro for mixture toxicity. The OCs listed in the SEDB and the HBDB have been used in in silico predictions towards thyroid disrupting properties (Dracheva et al. 2022).
Conclusion
In this study, we have shown that regulation of OCs successfully leads to decreases in human exposure. To the best of our knowledge, the HBDB is currently the most comprehensive database of anthropogenic OCs analysed in human blood. The exposure assessment of the general Swedish population presented in the SEDB showed that the group of OCs with the highest concentrations in blood is the PFASs, which also are the strongest contributors to the combined risk calculated with the HI approach. We hope that these databases will contribute to a better understanding of OC exposure. Furthermore, the databases reveal knowledge gaps regarding certain OCs studied. The lack of derived HBM-GVs limited the MRA to cover only nine OCs/groups of OCs in the SEDB. There is an urgent need to derive HBM-GVs for relevant OCs determined in blood to make better use of HBM data. A cause for concern is that the HI of 1.5 suggests that a risk of adverse effects cannot be excluded even when only considering these nine OCs and groups of OCs and that a further refinement of the assessment is needed. To add to the urgency, it is worth bearing in mind that in this study we have not considered the effects on sensitive groups or high-exposure subpopulations.
Data Availability
The datasets generated during the current study are available in the Stockholm University repository Figshare ( https://doi.org/10.17045/sthlmuni.19173965). Online Resource 1 is a spreadsheet containing the two compiled databases. Online Resource 2 provides additional concentration trends and tables.
Code Availability
Not applicable.
References
Apel P, Angerer J, Wilhelm M, Kolossa-Gehring M (2017) New HBM values for emerging substances, inventory of reference and HBM values in force, and working principles of the German Human Biomonitoring Commission. Int J Hyg Environ Health. https://doi.org/10.1016/j.ijheh.2016.09.007
Apel P, Rousselle C, Lange R, Sissoko F, Kolossa-Gehring M, Ougier E (2020) Human biomonitoring initiative (HBM4EU): strategy to derive human biomonitoring guidance values (HBM-GVs) for health risk assessment. Int J Hyg Environ Health. https://doi.org/10.1016/j.ijheh.2020.113622
Aylward LL, Hays SM, Gagné M, Nong A, Krishnan K (2010) Biomonitoring equivalents for hexachlorobenzene. Regul Toxicol Pharmacol. https://doi.org/10.1016/j.yrtph.2010.06.003
Aylward LL, Lakind JS, Hays SM (2008) Derivation of biomonitoring equivalent (BE) values for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds: a screening tool for interpretation of biomonitoring data in a risk assessment context. J Toxicol Environ Health A. https://doi.org/10.1080/15287390802361755
Barupal DK, Fiehn O (2019) Generating the blood exposome database using a comprehensive text mining and database fusion approach. Environ Health Perspect. https://doi.org/10.1289/EHP4713
Bopp SK, Kienzler A, Richarz A-N, van der Linden SC, Paini A, Parissis N, Worth AP (2019) Regulatory assessment and risk management of chemical mixtures: challenges and ways forward. Crit Rev Toxicol. https://doi.org/10.1080/10408444.2019.1579169
Bornehag C-G, Kitraki E, Stamatakis A, Panagiotidou E, Rudén C, Shu H, Lindh C, Ruegg J, Gennings C (2019) A novel approach to chemical mixture risk assessment: linking data from population-based epidemiology and experimental animal tests. Risk Anal. https://doi.org/10.1111/risa.13323
Bornehag C-G, Moniruzzaman S, Larsson M, Lindström CB, Hasselgren M, Bodin A, von Kobyletzkic LB, Carlstedt F, Lundin F, Nånberg E, Jönsson BAG, Sigsgaard T, Janson S (2012) The SELMA study: a birth cohort study in Sweden following more than 2000 mother-child pairs. Paediatr Perinat Epidemiol. https://doi.org/10.1111/j.1365-3016.2012.01314.x
David M, Gerofke A, Lange R, Kolossa-Gehring M, Apel P (2021) The European Human Biomonitoring Initiative (HBM4EU): human biomonitoring guidance values (HBM-GVs) for the aprotic solvents N-methyl-2-pyrrolidone (NMP) and N-ethyl-2-pyrrolidone (NEP). Int J Hyg Environ Health. https://doi.org/10.1016/j.ijheh.2021.113856
Dracheva E, Norinder U, Rydén P, Engelhardt J, Weiss J, Andersson P (2022) In silico identification of potential thyroid hormone system disruptors among chemicals in human serum and chemicals with high exposure index. Environ Sci Technol. https://doi.org/10.1021/acs.est.1c07762
EFSA ESC, More SJ, Bampidis V, Benford D, Bragard C, Hernandez-Jerez A, Bennekou SH, Halldorsson TI, Koutsoumanis KP, Lambré C (2021) Guidance Document on Scientific criteria for grouping chemicals into assessment groups for human risk assessment of combined exposure to multiple chemicals. EFSA J. https://doi.org/10.2903/j.efsa.2021.7033
Engelhardt AJ, Dracheva E, Nordström K , Weiss JM (2021) Chemical exposure profile in human blood, are we at risk? In: 41st International Symposium on Halogenated Persistent Organic Pollutants, Tianjin, China, vol 82, pp 350–356
Esteban M, Castaño A (2009) Non-invasive matrices in human biomonitoring: a review. Environ Int. https://doi.org/10.1016/j.envint.2008.09.003
European Commission (2020) Chemicals strategy for sustainability towards a toxic-free environment. https://ec.europa.eu/environment/pdf/chemicals/2020/10/Strategy.pdf
European Commission (2021) Information platform for chemical monitoring (IPCHEM). https://ipchem.jrc.ec.europa.eu/. Accessed 25 Nov 2021
Eurostat (2021) Production and consumption of chemicals went up in 2020. https://ec.europa.eu/eurostat/en/web/products-eurostat-news/-/ddn-20211130-3. Accessed 1 Dec 2021
Flemish Institute for Technological Research , HBM4EU (2021) Dashboard European Human Biomonitoring Data for visualisation of aggregated data. https://www.hbm4eu.eu/eu-hbm-dashboard/. Accessed 1 July 2021
Gennings C, Shu H, Rudén C, Öberg M, Lindh C, Kiviranta H, Bornehag C-G (2018) Incorporating regulatory guideline values in analysis of epidemiology data. Environ Int. https://doi.org/10.1016/j.envint.2018.08.039
Glynn A, Aune M, Darnerud PO, Cnattingius S, Bjerselius R, Becker W, Lignell S (2007) Determinants of serum concentrations of organochlorine compounds in Swedish pregnant women: a cross-sectional study. Environ Health. https://doi.org/10.1186/1476-069X-6-2
Gyllenhammar I, Aune M, Fridén U, Cantillana T, Bignert A, Lignell S, Glynn A (2021) Are temporal trends of some persistent organochlorine and organobromine compounds in Swedish breast milk slowing down? Environ Res. https://doi.org/10.1016/j.envres.2021.111117
Hays SM, Becker RA, Leung HW, Aylward LL, Pyatt DW (2007) Biomonitoring equivalents: a screening approach for interpreting biomonitoring results from a public health risk perspective. Regul Toxicol Pharmacol. https://doi.org/10.1016/j.yrtph.2006.08.004
HBM4EU (2020) Reporting for first and second set of substances. https://www.hbm4eu.eu/wp-content/uploads/2021/03/HBM4EU_AD5.4_Reporting_first_and_second_set_substances_v1.1.pdf. Accessed 10 Jan 2021
HBM-Commission GHBC (2020) Current Human Biomonitoring (HBM) values for blood and/or urine. https://www.umweltbundesamt.de/en/image/current-human-biomonitoring-hbm-values-for-blood. Accessed 22 Apr 2021
Hölzer J, Lilienthal H, Schümann M (2021) Human Biomonitoring (HBM)-I values for perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS): description, derivation and discussion. Regul Toxicol Pharmacol. https://doi.org/10.1016/j.yrtph.2021.104862
Hovander L, Athanasiadou M, Asplund L, Jensen S, Wehler EK (2000) Extraction and cleanup methods for analysis of phenolic and neutral organohalogens in plasma. J Anal Toxicol. https://doi.org/10.1093/jat/24.8.696
Huhn S, Escher BI, Krauss M, Scholz S, Hackermüller J, Altenburger R (2021) Unravelling the chemical exposome in cohort studies: routes explored and steps to become comprehensive. Environ Sci Eur. https://doi.org/10.1186/s12302-020-00444-0
International Symposium on Halogenated Persistent Organic Pollutants (2020) The Organohalogen Compounds (OHC) database. http://dioxin20xx.org/organohalogen-compounds-database-search/
Kirman CR, Aylward LL, Hays SM, Krishnan K, Nong A (2011) Biomonitoring equivalents for DDT/DDE. Regul Toxicol Pharmacol. https://doi.org/10.1016/j.yrtph.2011.03.012
Kortenkamp A, Faust M (2018) Regulate to reduce chemical mixture risk. Science. https://doi.org/10.1126/science.aat9219
Krishnan K, Adamou T, Aylward LL, Hays SM, Kirman CR, Nong A (2011) Biomonitoring equivalents for 2,2′,4,4′,5-pentabromodiphenylether (PBDE-99). Regul Toxicol Pharmacol. https://doi.org/10.1016/j.yrtph.2011.03.011
Lamkarkach F, Ougier E, Garnier R, Viau C, Kolossa-Gehring M, Lange R, Apel P (2021) Human biomonitoring initiative (HBM4EU): Human biomonitoring guidance values (HBM-GVs) derived for cadmium and its compounds. Environ Int. https://doi.org/10.1016/j.envint.2020.106337
Lange R, Apel P, Rousselle C, Charles S, Sissoko F, Kolossa-Gehring M, Ougier E (2021) The European Human Biomonitoring Initiative (HBM4EU): Human biomonitoring guidance values for selected phthalates and a substitute plasticizer. Int J Hyg Environ Health. https://doi.org/10.1016/j.ijheh.2021.113722
Lind L, Fors N, Hall J, Marttala K, Stenborg A (2005) A comparison of three different methods to evaluate endothelium-dependent vasodilation in the elderly. Arterioscler Thromb Vasc Biol. https://doi.org/10.1161/01.ATV.0000184769.22061.da
Malm L, Palm E, Souihi A, Plassmann M, Liigand J, Kruve A (2021) Guide to semi-quantitative non-targeted screening using LC/ESI/HRMS. Molecules. https://doi.org/10.3390/molecules26123524
Miller A, Hedman J , Bignert A (2012) Temporal, seasonal and spatial variation in dioxins and dioxin-like PCBs from Baltic herring (Clupea harengus) in the Baltic Sea. https://www.nrm.se/download/18.5d34f45c13c8cffc9a0cf7/15_2012+Dioxins+in+herring+in+the+Baltic+Sea.pdf. Accessed 05 Oct 2021
Norberg M, Wall S, Boman K, Weinehall L (2010) The Västerbotten intervention programme: background, design and implications. Glob Health Action. https://doi.org/10.3402/gha.v3i0.4643
Norén E, Lindh C, Rylander L, Glynn A, Axelsson J, Littorin M, Faniband M, Larsson E, Nielsen C (2020) Concentrations and temporal trends in pesticide biomarkers in urine of Swedish adolescents, 2000–2017. J Eposure Sci Environ Epidemiol. https://doi.org/10.1038/s41370-020-0212-8
Ode A, Källén K, Gustafsson P, Rylander L, Jönsson BAG, Olofsson P, Ivarsson SA, Lindh CH, Rignell-Hydbom A (2014) Fetal exposure to perfluorinated compounds and attention deficit hyperactivity disorder in childhood. PLoS ONE. https://doi.org/10.1371/journal.pone.0095891
Ougier E, Zeman F, Antignac J-P, Rousselle C, Lange R, Kolossa-Gehring M, Apel P (2021) Human biomonitoring initiative (HBM4EU): Human biomonitoring guidance values (HBM-GVs) derived for bisphenol A. Environ Int. https://doi.org/10.1016/j.envint.2021.106563
Palm A, Sternbeck J, Remberger M, Kaj L , Brorström-Lundén E (2002) Screening av pentaklorfenol (PCP) i miljön (in Swedish). https://www.ivl.se/download/18.694ca0617a1de98f4739d8/1628417408967/FULLTEXT01.pdf. Accessed 5 Nov 2021
Rappaport SM, Smith MT (2010) Environment and disease risks. Science. https://doi.org/10.1126/science.1192603
Rauchfuss K, Kraft M , Michael W (2013) Derivation of a health based guidance value for PCB in human blood samples. https://doi.org/10.1289/isee.2013.P-2-13-08. Accessed 12 Jan 2022
Rylander L, Hagmar L (1995) Mortality and cancer incidence among women with a high consumption of fatty fish contaminated with persistent organochlorine compounds. Scand J Work Environ Health. https://doi.org/10.5271/sjweh.57
Schymanski EL, Singer HP, Slobodnik J, Ipolyi IM, Oswald P, Krauss M, Schulze T, Haglund P, Letzel T, Grosse S, Thomaidis NS, Bletsou A, Zwiener C, Ibáñez M, Portolés T, de Boer R, Reid MJ, Onghena M, Kunkel U, Schulz W, Guillon A, Noyon N, Leroy G, Bados P, Bogialli S, Stipaničev D, Rostkowski P, Hollender J (2015) Non-target screening with high-resolution mass spectrometry: critical review using a collaborative trial on water analysis. Anal Bioanal Chem. https://doi.org/10.1007/s00216-015-8681-7
Secretariat of the Stockholm Convention (2019) Chemicals proposed for listing under the Convention. http://chm.pops.int/TheConvention/ThePOPs/ChemicalsProposedforListing/tabid/2510/Default.aspx. Accessed 5 May 2021
Secretariat of the Stockholm Convention (2020). Stockholm Convention of Persistent Organic Pollutants (POPs) -Text and annexes, Revised in 2019. S. Convention and U. e. program, Secretariat of the Stockholm Convention
Slimani N, Deharveng G, Unwin I, Southgate DAT, Vignat J, Skeie G, Salvini S, Parpinel M, Møller A, Ireland J, Becker W, Farran A, Westenbrink S, Vasilopoulou E, Unwin J, Borgejordet Å, Rohrmann S, Church S, Gnagnarella P, Casagrande C, van Bakel M, Niravong M, Boutron-Ruault MC, Stripp C, Tjønneland A, Trichopoulou A, Georga K, Nilsson S, Mattisson I, Ray J, Boeing H, Ocké M, Peeters PHM, Jakszyn P, Amiano P, Engeset D, Lund E, Santucci de Magistris M, Sacerdote C, Welch A, Bingham S, Subar AF, Riboli E (2007) The EPIC nutrient database project (ENDB): a first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur J Clin Nutr. https://doi.org/10.1038/sj.ejcn.1602679
Sniegoski LT, Moody JR (1979) Determination of serum and blood densities. Anal Chem. https://doi.org/10.1021/ac50045a052
Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG (2019) A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol. https://doi.org/10.1038/s41370-018-0094-1
Svensson B-G, Nilsson A, Jonsson E, Schütz A, Åkesson B, Hagmar L (1995) Fish consumption and exposure to persistent organochlorine compounds, mercury, selenium and methylamines among Swedish fishermen. Scand J Work Environ Health. https://doi.org/10.5271/sjweh.16
Swedish Chemicals Agency (2021) Stockholm Convention - Substances and implementation plan. https://www.kemi.se/en/international-cooperation/international-conventions-and-agreements/stockholm-convention---substances-and-implementation-plan. Accessed 31 Mar 2021
Swedish Environmental Protections Agency (2019) Hälsorelaterad miljöövervakning (HÄMI). https://ki.se/imm/halsorelaterad-miljoovervakning. Accessed 25 Oct 2019
Swedish Environmental Protections Agency (2021) Environmental monitoring program area: Health-related environmental monitoring (HÄMI). https://www.swedishepa.se/Environmental-objectives-and-cooperation/Swedish-environmental-work/Environmental-monitoring-describes-the-state-of-the-environment/Environmental-monitoring-program-areas/Health-related-environmental-monitoring/. Accessed 16 June 2021
Swedish Food Agency , Swedish Environmental protection agency (2020) Contaminants in blood and urine from adolescents in Sweden. https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2020/s-2020-nr-01-contaminants-in-blood-and-urine-from-adolescents-in-sweden.pdf. Accessed 29 June 2021
US Centers for Disease Control and Prevention (2019) Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, January 2019 -Volume 2
US Centers for Disease Control and Prevention (2020) National Biomonitoring Program. https://www.cdc.gov/biomonitoring/index.html. Accessed 16 June 2021
US Environmental Protection Agency (1986) Guidelines for the Health Risk Assessment of Chemical Mixtures. https://www.epa.gov/sites/default/files/2014-11/documents/chem_mix_1986.pdf
Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, Fiedler H, Hakansson H, Hanberg A, Haws L, Rose M, Safe S, Schrenk D, Tohyama C, Tritscher A, Tuomisto J, Tysklind M, Walker N, Peterson RE (2006) The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. https://doi.org/10.1093/toxsci/kfl055
Vojdani A, Pollard KM, Campbell AW (2014) Environmental triggers and autoimmunity. Autoimmune Dis. https://doi.org/10.1155/2014/798029
Vorkamp K, Castaño A, Antignac J-P, Boada LD, Cequier E, Covaci A, Esteban López M, Haug LS, Kasper-Sonnenberg M, Koch HM, Pérez Luzardo O, Osīte A, Rambaud L, Pinorini M-T, Sabbioni G, Thomsen C (2021) Biomarkers, matrices and analytical methods targeting human exposure to chemicals selected for a European human biomonitoring initiative. Environ Int. https://doi.org/10.1016/j.envint.2020.106082
Weiss J, Wallin E, Axmon A, Jönsson BAG, Åkesson H, Janák K, Hagmar L, Bergman Å (2006) Hydroxy-PCBs, PBDEs, and HBCDDs in serum from an elderly population of Swedish fishermen’s wives and associations with bone density. Environ Sci Technol. https://doi.org/10.1021/es0610941
Funding
Open access funding provided by Stockholm University. This study is part of the RiskMix project (www.aces.su.se/riskmix) funded by the Swedish Research Council FORMAS (No 2018-02264), with additional funding from the Swedish Environmental Protection Agency and the Health-Related Environmental Monitoring (HÄMI) programme (Nr 215-20-010).
Author information
Authors and Affiliations
Contributions
JMW contributed to conceptualisation. AJE contributed to literature search, data compilation and data analysis. KN involved in collecting information from HÄMI database. The first draft of the manuscript was written by AJE and all authors commented on the previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Engelhardt, A.J., Norström, K. & Weiss, J.M. Anthropogenic Organic Contaminants Analysed in Human Blood and Combined Risk. Expo Health 15, 551–565 (2023). https://doi.org/10.1007/s12403-022-00507-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-022-00507-y