Abstract
With the increasing demand for aquatic products, the requirement for the safety detection of aquatic products is also increasing. In the past decade, graphene oxide (GO) and reduced graphene oxide (r-GO) have become hot topics in many fields due to their special physical and chemical properties. With their excellent conductivity, a variety of electrochemical sensors have been developed in the fields of biology, food and chemistry. However, the unique optical properties of GO/r-GO have not yet been widely utilized. With the deepening of research, the fluorescence quenching performance of GO/r-GO has been proven to have excellent potential for building fluorescent sensors, and GO/r-GO fluorescent sensors have thus become an inevitable trend in sensor development. This review summarizes the main preparation methods of GO/r-GO and the principles of GO/r-GO fluorescent sensors comprehensively. Additionally, recent advances in utilizing GO/r-GO fluorescent sensors to detect aquatic food are discussed, including the application for the detection of harmful chemicals, microorganisms, and endogenous substances in aquatic products, such as pesticides, antibiotics and heavy metals. It is hoped that this review will help accelerate the progress in the field of analysis, and promote the establishment of an aquatic food supervision system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Driven by the growth of global aquaculture, especially in Asia, world aquatic production is increasing continuously [1]. In 2020, the total global aquatic production was 214 million tons with 36 million tons of algae and 178 million tons of aquatic animals [2]. Aquatic animal products have gradually become the main source of animal protein intake for consumers [3,4,5]. However, aquatic products are vulnerable to various physical, chemical and microbiological hazards [6,7,8], such as allergens, harmful chemicals (antibiotics and pesticides), and pathogens (Salmonella spp, Escherichia coli, and Staphylococcus aureus) [9]. They are usually accumulated in humans through the food chain, affecting the safety of aquatic food products, and posing a significant threat to human health [10, 11]. Therefore, to ensure the safety of aquatic products, it is important to explore effective detection tools during processing, transportation and storage.
Traditional detection methods are generally based on chromatography, capillary electrophoresis (CE), polymerase chain reaction (PCR), enzyme-linked immunosorbent assay (ELISA), lateral flow test (LFT) and mass spectrometry (MS). For example, there have been several studies on the detection of tropomyosin based on PCR [12], LFT [13], ELISA [14] and MS [15]. However, these methods still suffer from some defects, limiting their wide applications, including complex processes, time-consuming, expensive instruments and professional skills [16]. Therefore, the development of practical, economical and efficient detection methods is significant to global food safety [17].
Graphene oxide (GO) is a common derivative of graphene, and reduced graphene oxide (r-GO) can be obtained by reducing it [18]. As a kind of novel carbon material with a single-atom-thick two-dimensional (2D) structure, GO and r-GO have the advantages of high intrinsic surface, large electron mobility, functional capability and others. Therefore, they have been widely used in the field of detection and sensing applications, including the detection of organic small molecules, inorganic ions, and biomolecules [19,20,21,22,23,24,25,26]. Because of their excellent electrical properties (high conductivity and low resistivity), they have been mainly applied in the field of electrochemical food detection sensing [27,28,29]. Although the electrochemical sensor has the advantages of good stability, repeatability and high sensitivity, drawbacks also exist. For example, the signal is easy to interfere with, the electrode surface modification is difficult, and it is not suitable for the simultaneous detection of multiple substances [30]. In recent years, food fluorescence detection sensors have been extensively developed, which take fluorescence as an output signal to reflect the content of a substance [31], and possess the advantages of high sensitivity, simple operation, and fewer samples needed for detection. Two main methods are available for improving the accuracy and sensitivity of these fluorescent sensors, including enlarging the detection signal and reducing the background signal. These can be achieved by enhancing the efficiency of fluorescent groups, prolonging the lifespan of fluorescent clusters, reducing the intensity of sample irradiation, and inhibiting the spontaneous fluorescence in the sample [32,33,34,35]. GO and r-GO have been proven to be significant fluorescence resonance energy transfer (FRET) receptors for a variety of fluorescent dyes, whose quenching effect are better than that of many other carbon materials, and can significantly reduce the detection background signal [36,37,38]. r-GO is considered a more effective quencher because of its higher visible light absorption capacity than GO [39, 40]. Therefore, as a quenching basis, the application of GO/r-GO in the field of fluorescence sensing is also very promising. Compared with other traditional detection methods (such as PCR, ELISA, MS) and new-type sensors (such as electrochemical sensors), the GO/r-GO fluorescent biosensor detection method has many advantages in terms of detection performance and application. Figure 1 summarizes the advantages of GO/r-GO fluorescent sensors.
Currently, a number of reviews on the synthesis, characteristic, and functional modification of GO have been reported [41,42,43]. Meanwhile, several relevant reviews on the application of GO and r-GO in fluorescent sensors have been published, such as the detection of proteins, DNA, and viruses [44,45,46]. More specifically, Gao et al. [47] reviewed the construction and application of GO-DNA-based fluorescent sensors and electrochemical sensors. Similarly, Zhang et al. [48] discussed the development of graphene and GO, and the fluorescent sensors and bioanalytical systems with graphene or GO as a major component for in vivo and in vitro biological detection, suggesting that follow-up studies should address platform stability, probe release, and non-specific adsorption of other substrates, as well as the cytotoxicity of GO and r-GO to facilitate the future development of such sensors. However, despite these reviews, there is a lack of reviews on the application of GO/r-GO fluorescent sensors in food safety analysis, especially in the field of aquatic food safety.
Therefore, different from the previous reviews, this review focuses on the use of GO/r-GO fluorescent sensors in three aspects of aquatic product safety detection: hazardous chemical substances, hazardous microorganisms and their toxins, and hazardous endogenous substances, emphasizing the detection application advances of GO/r-GO fluorescent sensors. In addition, the review also compares the advantages and disadvantages of different preparation methods of GO and r-GO and points out the challenging issues and suggests future possible research directions in the GO/r-GO fluorescence sensing area. It is hoped that this review can provide a comprehensive summary of the application of GO/r-GO fluorescent sensors in aquatic product safety detection and enlighten the future development direction [49].
GO/r-GO Preparation and Principles of GO/r-GO Fluorescent Sensors
Common preparation methods of GO and r-GO include Brodie’s method, Staudenmaier’s method, Hummers’ method, chemical method, thermal method, electrochemical method, microwave-assisted method, and plasma method. Table 1 compares the advantages and disadvantages of these methods. Based on the principle of FRET between the quenched substrate and fluorescent labelling, GO/r-GO fluorescent sensors can be developed for safety detection in food and other relevant fields.
GO Preparation Methods
The polar oxygen-containing functional groups of GO are typically produced by oxidizing graphite with strong acids, such as hydroxyls (-OH), epoxides (C-O-C), ketone (C=O), and carboxylic (-COOH) [72]. Three common methods for preparing GO exist including Brodie’s method, Staudenmaier’s method and Hummers’ method, among them, Hummers’ method is the most widely used one [73].
The earliest reports of the GO preparation can be dated back to 1859 when Brodie used a fuming nitric acid system and potassium chlorate as an oxidant [50]. In order to further improve Brodie’s method, multiple additions of potassium chlorate and concentrated sulfuric acid were incorporated by Staudenmaier to improve the acidification process [53]. Different from the former, potassium permanganate was added as an oxidant to a mixture of graphite, sulfuric acid, and NaNO3 by Hummers to produce GO, which exhibited a higher degree of oxidation with a more regular structure, which was prone to swelling and layering in water [56]. Hummers’ method offered several advantages, such as the in-situ generation of nitric acid, avoiding the use of highly corrosive fuming nitric acid, replacing potassium chlorate with potassium permanganate to reduce toxic gas emissions as the release of NO2 and N2O4 gas in the reaction cannot be completely avoided, and improving the safety and environmental protection of preparation. Most of the current GO preparation experiments are improved based on Hummers’ method.
r-GO Preparation Methods
Factors such as high temperature during GO preparation often lead to the destruction of its surface structure [74]. Therefore, the reduction process should focus on reducing the oxygen content and repairing the surface structure. Currently, there are several reduction methods available for reducing GO, including chemical reduction, thermal reduction, electrochemical reduction, microwave-assisted reduction, and plasma reduction [75]. As the two most commonly used methods, chemical reduction methods are usually carried out in liquid or gas environments using strong reducing agents, such as hydrazine hydrate and hydrohalic acid [57,58,59], and thermal methods mainly include solvothermal and high-temperature annealing [60,61,62].
Besides, plasma reduction has gained increasing prominence in recent years. Various discharge methods are employed to generate plasma, including direct current discharge (DCD), glow discharge (GD), arc discharge, radiofrequency discharge (RF), and dielectric barrier discharge (DBD). Additionally, plasma can also be categorized into two types based on electron temperature: high-temperature plasma and low-temperature plasma [76]. As the fourth form of matter, plasma contains a range of active particles such as ions, electrons, free radicals and neutrals [77]. The active species in plasma can effectively sever oxygen-containing bonds present on the surface and edges of GO sheets, thus reducing the amount of oxygen functional groups in GO [78]. For example, the early report on the reduction of GO by plasma treatment was carried out by Hafiz using hydrogen as a gas medium [79]. The gas equivalent plasma reduction method commonly employs inert gases, hydrogen, nitrogen, ammonia, methane, and acetylene [76, 80, 81]. The liquid phase plasma reduction method involves the addition of alcohol, reducing sugar, and ammonia water to the solution system [82,83,84].
Principles of GO/r-GO-based Fluorescent Sensors
Many fluorescent sensors are developed based on the principle of FRET, which is a process of photoexcitation energy transfer from the donor fluorophore to the acceptor molecule [85]. The energy transfer occurs through dipole-dipole interactions between the excited state of the donor fluorophore and the ground state of the acceptor fluorophore [86].
In their study, Zhou et al. [87] highlighted the phenomenon that graphene was able to quench dye molecules adsorbed on its surface, owing to the presence of sp2 domains. This quenching property is also exhibited by GO and r-GO, making them promising candidates for developing fluorescent sensors. The use of fluorescent-labelled aptamers further enhances the functionality of GO/r-GO sensors [88]. When there is no target present, the fluorescent-labeled aptamers tend to adsorb onto the GO/r-GO surface. They are in close proximity to the GO/r-GO, allowing for efficient FRET between the aptamers (donor) and the GO/r-GO (acceptor), resulting in the transfer of energy, thus leading to either no fluorescence or weak fluorescence signals. However, due to the high specificity and affinity of aptamers, their binding with targets becomes favoured when the targets are introduced. This competition for binding sites between targets and GO/r-GO causes the aptamers to detach from the surface and form new complexes with targets. Consequently, the FRET phenomenon is abolished, allowing the fluorescence to reappear. By monitoring the change in fluorescence intensity, the concentration of the targets can be conveniently determined. It is worth noting that r-GO, compared to GO, exhibits a higher number of sp2 hybridization regions after reduction, rendering it more effective as a fluorescence quencher [89]. This property enhances the sensitivity and accuracy of r-GO-based fluorescent sensors in detecting and quantifying targets [90]. Figure 2 summarizes the detection principles of GO/r-GO fluorescent sensors.
Detection principles of GO/r-GO fluorescent sensors. A Fluorescent-labeled aptamers are added to the system containing GO/r-GO as the quenching substrate. B The aptamers are adsorbed on the GO/r-GO surface, and the fluorescence signals are quenched. C Add a solution containing the target detection substance (such as target molecule, target protein, and target heavy metal) to the system, desorption aptamer attached to the GO/r-GO surface, specific binding with the target detection object, and fluorescence signal recovery
Applications in Aquatic Food Safety Detection
As shown in Table 2, aquatic foods are prone to multiple hazards during production, packaging, transportation and storage, that can have adverse effects on human health, including chemicals (such as antibiotics, pesticides and heavy metals), microorganisms, mycotoxins, and harmful endogenous substances (for example, allergens and biotoxins) [91, 92]. In order to conduct sensitive and accurate detection of aquatic product safety, GO/r-GO fluorescent sensors have been extensively studied in detecting these hazards [93].
Hazardous Chemical Substances
Antibiotic Veterinary Drug
Antibiotics are extensively applied in the process of agriculture production and animal breeding. However, antibiotic abuse can result in unwanted residues in food, including sulfamethazine (SMZ), kanamycin, chloramphenicol (CAP) and ampicillin [118, 119]. They usually lead to serious side effects after consumption, posing a great threat to human health [96]. Sensor platforms based on GO/r-GO fluorescence quenching ability have been used to achieve qualitative and quantitative antibiotic detection.
Kou et al. [95] explored a fluorescent biosensor for detecting SMZ residues in animal-derived foods using carboxyfluorescein (FAM) labelled aptamers and GO, which achieved a low detection limit (as low as 0.35 ng/mL) and a wide dynamic range (from 2 to 100 ng/mL) under optimal conditions. Furthermore, it was effective in detecting SMZ residues in real samples. Li et al. [120] constructed a DNA probe consisting of an aptamer region for tobramycin binding and a template for amplification for detecting tobramycin, achieving a low detection limit (0.06 nM). Ye et al. [121] achieved sensitive monitoring of kanamycin by employing split aptamer (with a detection limit of 0.36 nM). Wen et al. [122] screened the truncated aptamer and built a GO-based fluorescent sensor to specifically detect nitrofurazone, achieving a detection limit of 1.13 ng/mL, which was lower than most of the other common detection methods. Liu et al. [123] realized the sensitive detection of fluoroquinolones by constructing fluorescent sensors using cadmium selenide quantum dots and GO (with a detection limit of 0.42 nM). Similarly, in order to achieve multiple antibiotics detection simultaneously (SMZ, kanamycin, and ampicillin), Youn et al. [124] applied different fluorescent modifiers for each antibiotic aptamer, namely cyanine 3 (Cy3), FAM, and cyanine 5 (Cy5), resulting in high efficiencies (94.36%, 93.94%, and 96.97% respectively). The sensor rapidly and synchronously screened multiple antibiotics with a low detection limit (1.997, 2.664, and 2.337 ng/mL respectively).
In addition to traditional fluorescent groups like FAM and rhodamine B (RhB), aggregation-induced emission (AIE) groups are also widely utilized in the detection system [125, 126]. In dilute solutions, AIE-active molecules remain unresponsive, while in aggregated states, they emit strong fluorescent signals. Zhang et al. [127] and Ning et al. [128] realized sensitive detection of CAP by introducing AIE DSA short alkyl chain derivative and hairpin structure respectively (1.26 pg/mL and 0.875 fM).
Pesticides
Pesticides are extensively used in agricultural production, resulting in the release of toxic compounds into the natural aquatic environment [129]. This contamination poses a health risk for consumers of aquatic products [130, 131]. As one of the main pest control chemicals, organophosphorus pesticides (OPs) are widely used in agriculture, having the ability to inhibit phosphatidylcholine biosynthesis in the central and peripheral nervous system [98]. Common OPs include Edifenphos (EDI), diazinon, acephate, and chlorpyrifos (CPF). Fluorescent sensors based on GO/r-GO have been developed to detect OPs residues.
Singh et al. [132] screened acephate aptamer and constructed a GO-based fluorescence sensor that exhibited excellent characteristics in real sample detection (detection limit as low as 4 ng/mL). Gaviria et al. [133] established a CPF fluorescence detection sensor on the basis of AChE (bio-mediator), GO (quenching agent) and carbon dots (fluorescent transductor). The sensor demonstrated high efficacy, achieving limits of detection as low as 0.14 ppb and 2.05 ppb for pure and commercial pesticides, respectively. The study held great significance as one of the few works that showed promising results in commercial pesticide formulation detection. Arvand et al. [134] synthesized ZnS and CdS [135] QDs capped with L-cysteine and have been widely used in various tests for their excellent luminescence properties, such as GO based fluorescent sensors for detecting EDI and diazinon with low detection limit (1.3 × 10−4 mg/L and 0.13 nM respectively), providing an effective solution for practical applications in the field of environmental and agricultural analysis. In comparison, Rong et al. [136] used the up-conversion nanoparticles (UCNPs) modified by aptamers as a fluorescence signal to detect diazinon, which improved detection accuracy (the detection limit was down to 0.023 ng/mL). The experiment also confirmed the applicability of this method not only to environmental samples but also to agricultural samples.
Paraquat (PQ) is broadly used in weeding practices and agricultural production [137, 138]. However, it poses a significant danger to human beings and animals as no effective treatment or specific antidote exists for paraquat poisoning currently. Over the last decades, numerous fatalities occurred because of ingesting PQ. Qian et al. [139] reported a 2D graphene nanosheet synthesis method by the water-soluble phosphate pillar[6] arene (PP6) in water phase exfoliated, which simultaneously combined fluorescence quenching capacity with the molecular identification property of graphene and PP6. Based on it, they reported a simple and sensitive fluorescence quenching sensor to detect PQ and showed good recovery in the actual water sample evaluation. Fipronil is a commercially developed pesticide that is widely around the world. Zhang et al. [140] achieved fipronil detection by using nitrogen-doped carbon quantum dots (NCQDs) modified aptamer (with a detection limit of 3.58 nM).
Heavy Metals
With continuous development of global industrialization, heavy metals (HMs) have become one of the main factors causing water pollution, such as cadmium (Cd2+), lead (Pb2+), chromium (Cr6+), arsenic (As3+), and mercury (Hg2+) [141,142,143,144,145,146]. HMs can accumulate via the food chain causing many health risks, and have been considered as human carcinogens even at low concentrations [102]. The detection limits for HMs in aquatic products are clearly stipulated in the food regulations of many countries.
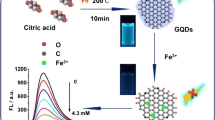
Mo et al. [104] designed a unique fluorescence nano platform for evaluating Cd2+ sensitively using AuNCs and holey-reduced graphene oxide (HRGO) with a large specific surface area and porous structure. This study marked the first application of HRGO in the construction of fluorescent sensors, expanding its use beyond electrochemical sensors. Figure 3 describes the principle of this fluorescent biosensor for the detection of Cd2+. To further enhance Cd2+ detection accuracy, Wang et al. [147] explored a colourimetric fluorescent sensor based on AIE and integrated paper strips with a smartphone platform. Through mixing GO functionalized by blue emission ethylenediamine (EDA) with orange emission glutathione-stabilized AuNCs, the author prepared a colourimetric fluorescent sensor and also introduced Cu2+ to form fluorescent colour-changing switches. The reliability and accuracy of the detection sensor were confirmed in the detection of rice samples by a smartphone platform integrated with fluorescent paper strips, and the limit of detection was 0.1 mM eventually. This sensor not only offered high sensitivity but also demonstrated portability and convenience, providing a reliable and convenient method for evaluating aquatic food safety.
Fluorescent biosensors for the detection of Cd2+. A Preparation of HRGO: HRGO was obtained via a hydrothermal method on the basis of GO with H2O2 as an etchant and ascorbic acid as a reducing agent. B Detection principle: fluorescence was quenched by HRGO at first, and then the fluorescence was recovered due to the formation of the Cd-GSH complex [104]
Li et al. [148] and Wang et al. [149] detected Pb2+ with GO and r-GO-based fluorescence sensors, respectively. The results indicate that the latter had a lower detection line (0.17 nM), and there are two pivotal reasons for the higher sensitivity. On the one hand, in the experiment of Wang, the fluorescence recovery ability of FAM labelled probes could be enhanced effectively with the help of the construction of a strong DNA branching junction (formed by Pb2+ dependent DNAzyme and cascade CHA). On the other hand, r-GO itself shows stronger fluorescence quenching ability and aptamer adsorption capacity than GO, and can significantly reduce the background signal of detection. Ebrahim et al. [150] developed a highly selective Cr6+ sensor using a fluorescent nanocomposite based on doped polyaniline, GO QDs, and 2-acrylamido-2-methylpropanesulfonic acid capped Ag nanoparticles synthesized via in situ reaction. The detection limit of the sensor (0.0065 mg/L) was significantly lower than the WHO-permitted limit standard for Cr6+ in drinking water (50 µg/L).
Pathan et al. [151] prepared a “Turn On” As3+ biosensor with high selectivity and sensitivity by using GQDs functionalized with Fe3O4 nanoparticles (Fe-GQDs), whose detection limit (5 ppb) lower than the WHO permissible limit for As3+ in drinking water (10 µg/L) remarkably. The complexation between As3+ and Fe-GQDs induced AIE, which was not affected by any other interfering ions. The application of Fe-GQDs provided a large number of binding sites and enabled the magnetic separation of materials for reuse in continuous cycles. Sharma et al. [152] prepared a complex of GO and fluorescence dye nanoparticles with sizes ranging from 50 to 100 nm to assess the content of As3+. Meanwhile, femtosecond laser synthesis technology has also proven to have great potential in improving the sensitivity and selectivity of HMs detection sensors. Molybdenum disulfide (MoS2) and nitrogen-doped GO nanoparticles synthesized and functionalized by this technique had been used to detect several metals (Hg2+, As3+, Pb2+, and Cd2+) successfully with each detection limit lower than 15 nM [153]. Zhang et al. [154] and Li et al. [155] achieved rapid and sensitive detection of Hg2+ (the detection limit was down to 0.5 nM and 0.92 nM), with the latter having a faster response time of 5 min.
Fe3O4 magnetized GO nanoparticles and silver nanoparticle-decorated r-GO nanocomposites were also applied in the detection of Hg2+ [156, 157]. Differently, to detect Hg2+, Abdelhamid et al. [158] quenched the fluorescence of r-GO by forming a complex of Hg2+ on its surface.
Microorganisms and Toxins
Bacteria
Aquatic products are susceptible to contamination from pathogenic bacteria, which can be occurred in aquatic animals naturally, or due to mishandling during processing [9]. Common pathogenic bacteria found in aquatic products include Campylobacter jejuni (C. jejuni), Acinetobacter baumannii (A. baumannii), Staphylococcus aureus (S. aureus), Salmonella typhimurium (S. typhimurium), Helicobacter pylori (H. pylori), and Salmonella enteritidis (S. enteritidis), which would bring potential health risks to human beings [105, 159,160,161,162]. Given the higher cost of treating illnesses caused by contaminated food, it is more cost-effective to develop effective identification and accurate quantification of pathogenic bacteria [163,164,165]. Therefore, it is important to have reliable and sensitive methods to detect these bacterial contaminants in aquatic products.
Chattopadhyay et al. [166] prepared H. pylori antibody fluorescent probe (FCDs-Ab) to detect H. pylori effectively. Dehghani et al. [167] built a sensor which could be bound with C. jejuni membrane surface proteins, leading to specific interactions between the probe and the target bacteria. The limit detection of the sensor was 10 CFU/mL.
The detection of S. aureus contamination can be achieved in two ways: indirect detection and direct detection [168, 169]. Ravikumar et al. [170] developed an “On–Off–On” fluorescent probe for the detection of micrococcus nuclease (MNase), which was the gold standard for identifying the presence and content of S. aureus. The detection limits for fluorescence-based strips and assay were 0.5 ng/mL and 0.3 ng/mL, respectively. For the detection of S. aureus enterotoxin A, Ma et al. [171] obtained lower background signal interference and lower detection limit (0.899 ng/mL) by centrifuging ssDNA attached GO.
Duan et al. [172] and Chinnappan et al. [173] used full-length and truncated fluorescent labelled aptamers to detect S. Typhimurium, respectively, and the results showed that the detection limit of the latter (25 CFU/mL) was much lower than that of the former (100 CFU/mL). Compared with these two studies, Renuka et al. [174] utilized QDs-modified aptamers, which had a lower detection limit (10 CFU/mL). Satisfactorily, this sensing platform had no cross-reaction with other similar bacteria. Through the comparison of the above three studies, it can be found that the length of aptamer and the type of fluorescent substance used for labelling will affect the evaluation accuracy.
In order to detect A. baumannii, Xie et al. [175] developed a sensor based on a DNA-catalyzed amplification mechanism (with a detection limit of 1.1 CFU/mL). A. baumannii could be bound with aptamer, causing the release of the template strand, which triggered an entropy-driven catalysis (EDC) reaction. Then, one product of EDC was used as the catalyst for catalytic hairpin assembly (CHA) on a GO nanosheet, leading to lots of FAM-labeled DNA duplicates released from GO, which could be detected with fluorescence intensity change. Besides normal fluorescent sensors, the ratiometric fluorescent sensor and the multicolour time-resolved fluorescence (TRF) sensor have also been applied in the detection system. Bahari et al. [176] reported an ingenious ratiometric fluorescent sensor based on nitrogen-doped carbon nanodots together with ortho-phenylenediamines carbon dots and GO. The sensor had a detection limit as low as 3.0 × 10−2 CFU/mL and was confirmed to detect A. baumannii in urine samples with satisfactory results.
The time-resolved fluorescent sensor is a promising tool for food safety control and inspection [177]. Huang et al. [178] established a TRF sensor for the evaluation of multiple S. aureus enterotoxins (SEs) simultaneously, including SEA, SEB, and SEC1. Each aptamer was labelled with a specific combination, which emitted unique fluorescence signals when bound to their respective SE toxin targets. Time-resolved detection reduced background noise and enhanced detection sensitivity. The sensor successfully performed the detection of milk samples with a detection limit of 0.020 to 0.068 ng/mL. The TRF sensor is a promising tool for food safety control and inspection [179].
Mycotoxin
Aflatoxin (AF) is a bifuran ring toxins produced by some strains of Aspergillus parasiticus and Aspergillus flavus, which is carcinogenic, immunogenic and teratogenic to human beings and animals [180]. There are a wide variety of aflatoxins, with about 20 structurally related compounds. Among them, aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1) and aflatoxin G2 (AFG2) are the major aflatoxin metabolites associated with human and animal disease [181]. Besides, lactating animals can metabolize AFB1 to AFM1 and AFM2 by taking contaminated feed, presenting a risk to humans [182]. Zhang et al. [183] developed a novel method for simultaneous separation and measurement of AFM1 by synthesizing r-GO decorated by monoclonal antibody functionalized Fe3O4. Under optimized conditions, the quantitative and visual detection limits of AFM1 were 3.8 ng/L and 50 ng/L, respectively. These results demonstrated the high sensitivity and selectivity of this newly developed method for detecting the presence of AFM1.
In several AFB1 detecting studies [31, 106, 107, 184, 185] achieved lower detection by eliciting two fluorophore-labeled hairpin probes (HP1/HP2) and specific apt sensor with internal complementary sequence, respectively(0.03 pg/mL and 0.03 pg/mL). Although these methods exhibit sensitivity and specificity, there is still room for improvement in terms of speed, simplicity, and cost-effectiveness [186]. Future research may focus on developing portable, real-time, and multiplexed detection systems for aflatoxins, which will enhance food safety control and public health protection [187].
Ochratoxin is another mycotoxin that has attracted worldwide attention after aflatoxin, including 7 compounds with similar structures. Among them, ochratoxin A (OTA) has the highest content and the strongest toxicity [188]. Zhang et al. [109] and Wu et al. [189] realized sensitive detection of OTA (0.02 ng/mL and 11 pg/mL respectively). Besides, the platform built by Wu could also be used for assessing fumonisin B1 with a low detection limit (0.1 ng/mL).
Hazardous Endogenous Substances
Allergen
To date, effective medical treatment has not been found for food allergies [190]. The only possible method of prevention may be to rely heavily on avoiding foods containing specific allergens altogether [191]. Figure 4 describes the schematic diagram of the food anaphylactic reaction. The major allergens of aquatic species include tropomyosin (TM) and arginine kinase (AK) [192]. In general, allergenic components of aquatic products can cause allergic reactions ranging from mild to life-threatening when they come into contact with the mucosal immune system in the gut [111].
TM is a major allergenic protein found in invertebrates such as shrimps and crabs, whose structure remains highly stable even in high-temperature environments [193]. Zhang et al. [194] designed a labelling-free TM fluorescent biosensor based on GO and OliGreen labelled aptamers. The aptamers obtained by SELECT showed high affinity for TM, and could selectively recognize TM across a broad concentration range. The detection limit for TM evaluation was 4.2 nM, and the range of detection was from 0.5 to 50 µg/mL in binding buffer solution. For the purpose of further improving detection accuracy, Chinnappan et al. [110] used a minimal-length aptamer sequence with fluorescein at the 5’end to build an evaluation platform based on GO. The result eventually confirmed that the detection limit was as low as 2.5 nM, which was significantly lower than that of the full-length aptamer sequence. Meanwhile, the reaction could be finished within 30 min. The detecting platform proved that truncated aptamers could form unique secondary structures to capture TM with higher affinity. After mixing shrimp TM into chicken noodle soup purchased at a local market, the authors detected the sample and verified the feasibility of the detection sensor.
AK is a kind of guanidine phosphate compound kinase found in invertebrates, which is regarded as one of the main shellfish allergens [114]. To achieve sensitive and swift quantitative detection of AK, Zhou et al. [195] used carboxyl functionalized CQDs to modified aptamers, and constructed an “On-Off-On” fluorescent aptamer biosensor based on GO, providing a detection limit as low as 0.14 ng/mL.
Biotoxins
In the area of biotoxin detection, several studies have been conducted to develop innovative methods for detecting various toxins [196]. Microcystin (MC) is a potentially harmful toxin found in aquatic systems and has been identified as a potent hepatotoxin and tumour promotor [116]. Shi et al. [108] established a reliable MC biosensor by using gold nanoparticles (AuNPs), which met the WHO guidelines for detecting MC-RR and MC-LR (0.3 µg/L and 0.5 µg/L separately). Gu et al. [197] conducted the first study that used GQD-labeled aptamers and DNase I-catalyzed amplified target circulation signals to detect marine organisms. To achieve sensitive and rapid detection of saxitoxin (STX), the authors employed magnetic r-GO as an energy receptor, STX-41 aptamers as a recognition element, GQDs as fluorescence signal, and DNase I for enzymatic signal amplification. They successfully detected STX with a broad detection range of 0.1 to 100 ng/mL and a detection limit as low as 0.0035 ng/mL under optimized conditions. Kweon et al. [115] constructed an okadaic acid fluorescent biosensor with satisfactory specificity recognition compared to other marine toxins, whose detection limit was 6.35 ppb.
Advantages, Challenges and Future Works
With the continuous improvement of global consumption demand for aquatic products, aquatic product safety control is becoming a tough problem gradually. The requirement for efficient, rapid and low-cost detection methods is increasing as well [198]. At present, GO/r-GO fluorescent sensors have made some progress in aquatic food safety detection, especially compared with traditional detection methods, they have several outstanding advantages as shown in Fig. 1, which are mainly reflected in four aspects. However, there are certain challenging issues that should be addressed to facilitate future developments, indicating that more efforts should be made in future.
-
Currently, graphene and its derivatives are predominantly used in applications such as display screens, conductive devices, and thermal conductive devices. Although graphene’s excellent electrical conductivity has made it useful for biosensing, its unique optical properties have yet to be extensively explored in this regard. Additionally, most research on fluorescent sensors has focused on GO rather than r-GO. However, the sensitivity of fluorescent sensors is closely related to the substrate’s fluorescence quenching ability. As r-GO has shown better quenching ability than GO, further exploration is needed for the development of r-GO-based fluorescence sensing systems.
-
The preparation of r-GO typically involves chemical reduction, and consuming hydrazine and other toxic chemicals. Not only can this be harmful to human health, but it also poses a significant threat to the environment. Therefore, future work can focus on exploring green and efficient GO reduction methods. One potential approach is to improve plasma treatment technology, which can reduce GO under specific gas (methane, argon, NH3, etc.) or liquid (alcohol) environmental conditions. This method offers advantages such as high speed, controllability, high yield, and low cost [70, 83, 199].
-
The large-scale commercial production of aptamers is currently not feasible, and their relatively weak affinity and selectivity in real samples greatly limit their application. As a result, only a few aptamers have been fully validated and widely used. To address this limitation, greater efforts should be directed towards improving the sensitivity and specificity of aptamers. Promising approaches include structure optimization, function improvement, truncation of non-essential parts, and combined use of aptamers [200].
-
There are still certain gaps in aquatic food safety detection using GO/r-GO fluorescent sensors. The range of detectable substances is not sufficiently diverse, which prevents the establishment of a comprehensive detection system. Future research should focus on expanding the detection capabilities to include more harmful substances and improving the overall detection system for aquatic products.
-
Looking ahead, the attempts to explore potential synergies between GO/r-GO sensors and other emerging technologies, such as microfluidics and surface-enhanced Raman scattering sensors, could lead to the development of integrated systems for comprehensive real-time monitoring of food safety parameters, facilitating their development in commercial applications [201].
-
All in all, while challenges remain in the development and adoption of GO/r-GO fluorescence sensors for aquatic food safety testing, the field holds great promise in revolutionizing food safety practices and ensuring the well-being of consumers worldwide. By addressing technology and commercialization challenges in a collaborative and interdisciplinary way, researchers and industry stakeholders can pave the way for a safer and more secure food supply chain.
Conclusions
The construction of fluorescent biosensors based on GO/r-GO with highly efficient fluorescence quenching performance is presented. Its application in aquatic food safety detection is also discussed, mainly focusing on three aspects, including the detection of harmful chemicals, harmful microorganisms and their toxins, and endogenous harmful substances. The sensitivity of aptamers, fluorescence labelling performance and fluorescence quenching ability of substrate are the main factors affecting the detection sensitivity of GO/r-GO fluorescent sensors. Future research should focus on further exploration in the above three aspects, such as improving green and safe graphene oxide reduction technology, exploring and using more sensitive aptamers or truncated aptamers, and laying a research foundation for the industrial production of such detection sensors. In conclusion, the construction of fluorescent sensors will be the mainstream trend of future detection development. Hopefully, this review will encourage further research, and explore more applications of r-GO optical properties in food detection.
Data Availability
The authors are unable or have chosen not to specify which data has been used.
References
Costa-Pierce BA (2022) The Anthropology of Aquaculture. Front Sustain Food Syst 6:843743
Wenning R (2020) The state of world fisheries and aquaculture (Sofia) 2020 report. Integr Environ Assess Manag 16:800–801
Qiu L, Zhang M, Chitrakar B, Bhandari B (2020) Application of power ultrasound in freezing and thawing processes: effect on process efficiency and product quality. Ultrason Sonochem 68:105230
Xiao RC, Wei YG, An D, Li DL, Ta XX, Wu YH, Ren Q (2019) A review on the research status and development trend of equipment in water treatment processes of recirculating aquaculture systems. Rev Aquac 11:863–895
Zhang J, Zhang ZG, Liu Y (2020) Collection and application of intelligent technical information data of cold chain logistics of aquatic products. J Phys Conf Ser 1648:42038
Hellberg RS, DeWitt CAM, Morrissey MT (2012) Risk-benefit analysis of seafood consumption: a review. Compr Rev Food Sci Food Saf 11:490–517
Panebianco F, Giusti A, Giarratana F, Armani A (2019) Ethnic seafood products sold on the Italian market: labelling assessment and biological, chemical and physical risk characterization. Food Control 105:198–208
Yemmen C, Gargouri M (2022) Potential hazards associated with the consumption of scombridae fish: infection and toxicity from raw material and processing. J Appl Microbiol 132:4077–4096
Lehel J, Yaucat-Guendi R, Darnay L, Palotas P, Laczay P (2021) Possible food safety hazards of ready-to-eat raw fish containing product (sushi, sashimi). Crit Rev Food Sci Nutr 61:867–888
Lloret J, Raetz H-J, Lleonart J, Demestre M (2016) Challenging the links between seafood and human health in the context of global change. J Mar Biol Association United Kingd 96:29–42
Wang B, Huang D, Weng Z (2023) Recent advances in polymer-based biosensors for food safety detection. Polymers 15:3253
Ho CW, Hsu JL, Chen SH, Liaw ET, Liu SS, Huang ES, Chen YK, Huang CCJ, Yu HS (2021) Development and validation of Mass Spectrometry-based method for detecting shrimp Allergen Tropomyosin. LWT Food Sci Technol 152:112367
Wang YQ, Li ZX, Lin H, Siddanakoppalu RN, Zhou J, Chen GZ, Yu ZW (2019) Quantum-dot-based lateral flow immunoassay for the rapid detection of crustacean major allergen tropomyosin. Food Control 106:106714
Zhao JL, Li YH, Li RR, Timira V, Dasanayaka BP, Zhang ZY, Zhang JK, Lin H, Li ZX (2022) Evaluation of poly- and monoclonal antibody-based sandwich enzyme-linked immunosorbent assay (ELISA) for their performance to detect crustacean residues in Processed Foods. Food Control 138:108983
Fan SF, Ma JM, Li CS, Wang YB, Zeng W, Li Q, Zhou JR, Wang LM, Wang Y, Zhang Y (2022) Determination of tropomyosin in shrimp and crab by liquid chromatography-tandem mass spectrometry based on immunoaffinity purification. Front Nutr 9:848294
Torre R, Freitas M, Costa-Rama E, Nouws HPA, Delerue-Matos C (2022) Food allergen control: tropomyosin analysis through electrochemical immunosensing. Food Chem 396:133659
Hitabatuma A, Wang PL, Su XO, Ma MM (2022) Metal-organic frameworks-based sensors for food safety. Foods 11:382
Lee CW, Eom TH, Cho SH, Jang HW (2023) Chemical sensors based on graphene and 2D graphene analogs. Adv Sens Res. https://doi.org/10.1002/adsr.202200057
Dinu LA, Kurbanoglu S (2023) Enhancing electrochemical sensing through the use of functionalized graphene composites as nanozymes. Nanoscale 15:16514–16538
Gao J, Chakraborthy A, He S, Yang S, Afsarimanesh N, Nag A, Deng S (2023) Graphene-based sensors for the detection of microorganisms in food: a review. Biosensors-Basel 13(6):579
Mandal TK, Hou Y, Gao Z, Ning H, Yang W, Gao M (2016) Graphene oxide-based sensor for ultrasensitive visual detection of fluoride. Adv Sci 3:1600217
Mishra S, Mishra S, Patel SS, Singh SP, Kumar P, Khan MA, Awasthi H, Singh S (2022) Carbon nanomaterials for the detection of pesticide residues in food: a review. Environ Pollut 310:119804
Pei J, Ren T, Huang Y, Chen R, Jin W, Shang S, Wang J, Liu Z, Liang Y, El-Aty A (2022) Application of graphene and its derivatives in detecting hazardous substances in food: a comprehensive review. Front Chem 10:894759
Siddiqui AS, Hayat A, Nawaz MH, Ahmad MA, Nasir M (2020) Effect of sulfur doping on graphene oxide towards amplified fluorescence quenching based ultrasensitive detection of hydrogen peroxide. Appl Surf Sci 509:114695
Smitha PK, Bathula C, Chandrashekara KN, Das M (2020) Usage of graphene oxide in fluorescence quenching-linked immunosorbent assay for the detection of Cry2Ab protein present in transgenic plants. J Agric Food Chem 68:3656–3662
Sundramoorthy AK, Gunasekaran S (2014) Applications of graphene in quality assurance and safety of food. Trac-Trends Anal Chem 60:36–53
Bose R, Alanazi AK, Bhowmik S, Garai S, Roy M, Pakhira B, Pramanik T (2023) Applications of graphene and graphene oxide as versatile sensors: a brief review. Biointerface Res Appl Chem. https://doi.org/10.33263/BRIAC135.457
Chekin F, Singh SK, Vasilescu A, Dhavale VM, Kurungot S, Boukherroub R, Szunerits S (2016) Reduced graphene oxide modified electrodes for sensitive sensing of gliadin in food samples. Acs Sens 1:1462–1470
Yu H, Guo W, Lu X, Xu H, Yang Q, Tan J, Zhang W (2021) Reduced graphene oxide nanocomposite based electrochemical biosensors for monitoring foodborne pathogenic bacteria: a review. Food Control 127:108117
Wu Z, Sun D-W, Pu H, Wei Q (2023) A Dual signal-on biosensor based on dual-gated locked mesoporous silica nanoparticles for the detection ofaflatoxin B1. Talanta 253:124027
Jia YM, Wu F, Liu PL, Zhou GH, Yu B, Lou XD, Xia F (2019) A label-free fluorescent aptasensor for the detection of aflatoxin B1 in food samples using AIEgens and graphene oxide. Talanta 198:71–77
Fu B, Chen JK, Cao YT, Li HH, Gao F, Guo DY, Wang FX, Pan QH (2022) Post-modified metal-organic framework as ratiometric fluorescence-scattering probe for trace ciprofloxacin residue based on competitive coordination. Sens Actuators B Chem 369:132261
Lv JZ, Liu SY, Miao YM (2021) Synthesis of biological quantum dots based on single-strand DNA and its application in melamine detection. Spectrochimica Acta A Mol Biomol Spectrosc 248:119254
Wu TF, Hu B, Lv J, Li YH, Shao J, Ma YQ, Cui Y (2022) Enhanced ratiometric fluorescence molecularly imprinted nanosensor based on CDs for selective and visual detection of NOR in water samples. Opt Mater 132:112784
Yang LM, Liu B, Wang MM, Li J, Pan W, Gao XN, Li N, Tang B (2018) A highly sensitive strategy for fluorescence imaging of MicroRNA in living cells and in vivo based on graphene oxide-enhanced signal molecules quenching of molecular beacon. ACS Appl Mater Interfaces 10:6982–6990
Liang L, Jiang YJ, Zhang LC, Liu H, Li YF, Li CM, Huang CZ (2022) Rational fabrication of a DNA walking nanomachine on graphene oxide surface for fluorescent bioassay. Biosens Bioelectron 211:114349
Neema PM, Tomy AM, Cyriac J (2020) Chemical sensor platforms based on fluorescence resonance energy transfer (FRET) and 2D materials. Trac-Trends Anal Chem 124:115797
Salama AM, Yasin G, Zourob M, Lu J (2022) Fluorescent biosensors for the detection of viruses using graphene and two-dimensional carbon nanomaterials. Biosensors-Basel 12:460
Richter L, Szalai AM, Manzanares-Palenzuela CL, Kaminska I, Tinnefeld P (2023) Exploring the synergies of single-molecule fluorescence and 2D materials coupled by DNA. Adv Mater. https://doi.org/10.1002/adma.202303152
Xi GN, Wang X, Chen T (2016) A reduced graphene oxide-based fluorescence resonance energy transfer sensor for highly sensitive detection of matrix metalloproteinase 2. Int J Nanomed 11:1537–1547
Kim YJ, Jeong B (2018) Graphene-based nanomaterials and their applications in biosensors. In: Noh I (ed) Biomimetic medical materials: from nanotechnology to 3D bioprinting, vol 1064. pp 61–71
Li L, Zhang D, Deng J, Kang Q, Liu Z, Fang J, Gou Y (2020) Review-progress of research on the preparation of graphene oxide via electrochemical approaches. J Electrochem Soc 167:155519
Yu W, Sisi L, Haiyan Y, Jie L (2020) Progress in the functional modification of graphene/graphene oxide: a review. RSC Adv 10:15328–15345
Battisti A, Samal SK, Puppi D (2023) Biosensing systems based on graphene oxide fluorescence quenching effect. Micromachines 14:1522
Wang S-Y, Wang C-F, Lv Y-K, Shen S-G (2018) Fabrication of fluorescent biosensing platform based on graphene oxide-DNA and their application in biomolecule detection. Trac-Trends Anal Chem 106:53–61
Yim Y, Shin H, Ahn SM, Min D-H (2021) Graphene oxide-based fluorescent biosensors and their biomedical applications in diagnosis and drug discovery. Chem Commun 57:9820–9833
Gao L, Lian CQ, Zhou Y, Yan LR, Li Q, Zhang CX, Chen L, Chen KP (2014) Graphene oxide-DNA based sensors. Biosens Bioelectron 60:22–29
Zhang H, Zhang HL, Aldalbahi A, Zuo XL, Fan CH, Mi XQ (2017) Fluorescent biosensors enabled by graphene and graphene oxide. Biosens Bioelectron 89:96–106
Liu Y, Pu H, Li Q, Sun D-W (2023) Discrimination of pericarpium citri reticulatae in different years using terahertz time-domain spectroscopycombined with convolutional neural network. Spectrochim Acta A: Mol Biomol Spectrosc 286:122035
Feicht P, Biskupek J, Gorelik TE, Renner J, Halbig CE, Maranska M, Puchtler F, Kaiser U, Eigler S (2019) Brodie’s or hummers’ method: oxidation conditions determine the structure of graphene oxide. Chem A Eur J 25:8955–8959
Talyzin AV, Mercier G, Klechikov A, Hedenstrom M, Johnels D, Wei D, Cotton D, Opitz A, Moons E (2017) Brodie vs hummers graphite oxides for preparation of multi-layered materials. Carbon 115:430–440
Poh HL, Sanek F, Ambrosi A, Zhao GJ, Sofer Z, Pumera M (2012) Graphenes prepared by Staudenmaier, Hofmann and hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties. Nanoscale 4:3515–3522
Sali S, Mackey HR, Abdala AA (2019) Effect of graphene oxide synthesis method on properties and performance of polysulfone-graphene oxide mixed matrix membranes. Nanomaterials 9:769
Samadi S, Nouroozshad M, Zakaria SA (2021) ZnO@SiO2/rGO core/shell nanocomposite: a superior sensitive, selective and reproducible performance for 1-propanol gas sensor at room temperature. Mater Chem Phys 271:124884
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun ZZ, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of graphene oxide. ACS Nano 4:4806–4814
Yu HT, Zhang BW, Bulin CK, Li RH, Xing RG (2016) High-efficient synthesis of graphene oxide based on improved hummers method. Sci Rep 6:108117
Agarwal V, Zetterlund PB (2021) Strategies for reduction of graphene oxide - a comprehensive review. Chem Eng J 405:127018
Nishina Y, Eigler S (2020) Chemical and electrochemical synthesis of graphene oxide - a generalized view. Nanoscale 12:12731–12740
Pei SF, Zhao JP, Du JH, Ren WC, Cheng HM (2010) Direct reduction of graphene oxide films into highly conductive and flexible graphene films by hydrohalic acids. Carbon 48:4466–4474
Chin SJ, Doherty M, Vempati S, Dawson P, Byrne C, Meenan BJ, Guerra V, McNally T (2019) Solvothermal synthesis of graphene oxide and its composites with poly(epsilon-caprolactone). Nanoscale 11:18672–18682
Klemeyer L, Park H, Huang JX (2021) Geometry-dependent thermal reduction of graphene oxide solid. Acs Mater Lett 3:511–515
Meng HB, Zhang XF, Pu YL, Chen XL, Feng JJ, Han DM, Wang AJ (2019) One-pot solvothermal synthesis of reduced graphene oxide-supported uniform PtCo nanocrystals for efficient and robust electrocatalysis. J Colloid Interface Sci 543:17–24
Aadil M, Zulfiqar S, Sabeeh H, Warsi MF, Shahid M, Alsafari IA, Shakir I (2020) Enhanced electrochemical energy storage properties of carbon coated Co3O4 nanoparticles-reduced graphene oxide ternary nano-hybrids. Ceram Int 46:17836–17845
Molina J, Fernandez J, Garcia C, del Rio AI, Bonastre J, Cases F (2015) Electrochemical characterization of electrochemically reduced graphene coatings on platinum. Electrochemical study of dye adsorption. Electrochim Acta 166:54–63
Ray SC (2015) Application and uses of graphene oxide and reduced graphene oxide. Appl Graphene Graphene Oxide Based Nanomater 41:39–55
Lee WJ, Jang HR, Kim MJ, Kim HM, Oh JM, Paek SM (2019) Microwave-irradiated reduced graphene oxide nanosheets for highly reversible and ultrafast sodium storage. J Alloys Compd 778:382–390
Xie X, Zhou Y, Huang K (2019) Advances in microwave-assisted production of reduced graphene oxide. Front Chem 7:355
Zhao YJ, He JH (2019) Novel template-assisted microwave conversion of graphene oxide to graphene patterns: a reduction transfer mechanism. Carbon 148:159–163
Miao YL, Ma YL, Wang Q (2019) Plasma-assisted simultaneous reduction and nitrogen/sulfur codoping of graphene oxide for high-performance supercapacitors. ACS Sustain Chem Eng 7:7597–7608
Yang C, Gong JY, Zeng P, Yang XL, Liang RQ, Ou QR, Zhang SY (2018) Fast room-temperature reduction of graphene oxide by methane/argon plasma for flexible electronics. Appl Surf Sci 452:481–486
Zhou HP, Ye X, Huang W, Wu MQ, Mao LN, Yu B, Xu S, Levchenko I, Bazaka K (2019) Wearable, flexible, disposable plasma-reduced graphene oxide stress sensors for monitoring activities in austere environments. ACS Appl Mater Interfaces 11:15122–15132
Xiao XZ, Zhang YM, Zhou L, Li B, Gu L (2022) Photoluminescence and fluorescence quenching of graphene oxide: a review. Nanomaterials 12:2444
Szabo T, Maroni P, Szilagyi I (2020) Size-dependent aggregation of graphene oxide. Carbon 160:145–155
He H, Sun D-W, Pu H, Wu Z (2023) A SERS-fluorescence dual-signal aptasensor for sensitive and robust determination of AFB1 in nut samplesbased on Apt-Cy5 and MNP@Ag-PEI. Talanta 253:123962
Baraket M, Walton SG, Wei Z, Lock EH, Robinson JT, Sheehan P (2010) Reduction of graphene oxide by electron beam generated plasmas produced in methane/argon mixtures. Carbon 48:3382–3390
von Keudell A, Gathen VSV (2017) Foundations of low-temperature plasma physics-an introduction. Plasma Sources Sci Technol 26:113001
Ekezie FGC, Cheng JH, Sun D-W (2018) Effects of Nonthermal Food Processing Technologies on Food Allergens: a review of recent research advances. Trends Food Sci Technol 74:12–25
Zhang D, Huang L, Sun D-W, Pu H, Wei Q (2023) Bio-interface engineering of MXene nanosheets with immobilized lysozyme for light enhancedenzymatic inactivation of methicillin-resistant Staphylococcus aureus. Chem Eng J 452:139078
Hafiz SM, Ritikos R, Whitcher TJ, Razib NM, Bien DCS, Chanlek N, Nakajima H, Saisopa T, Songsiriritthigul P, Huang NM, Rahman SA (2014) A practical carbon dioxide gas sensor using room-temperature hydrogen plasma reduced graphene oxide. Sens Actuators B Chem 193:692–700
Li J, Chen CL, Wei J, Li JX, Wang XK (2014) Enhanced electrochemical performance of reduced graphene oxides by H2/Ar plasma treatment. J Phys Chem C 118:28440–28447
Zhou Q, Zhao Z, Chen Y, Hu H, Qiu J (2012) Low temperature plasma-mediated synthesis of graphene nanosheets for supercapacitor electrodes. J Mater Chem 22:6061–6066
Kim SH, Dao VD, Larina LL, Jung KD, Choi HS (2016) Solution-processable rGO-Pt nanohybrids synthesized in an aqueous fructose solution for transparent and efficient dye-sensitized solar cells. Chem Eng J 283:1285–1294
Wang CG, Sun XH, Zhu XM, Sun B (2022) Alcohol addition improves the liquid-phase plasma process for green reduction of graphene oxide. Vacuum 205:111373
Huang L, Sun D-W, Pu H, Zhang C, Zhang, D (2023) Nanocellulose-based polymeric nanozyme as bioinspired spray coating for fruit preservation. Food Hydrocolloids 135:108138
Liu HY, Li CY, Li J, Cheng YQ, Zhao JF, Chen JN, Sun MT (2023) Plasmon-enhanced fluorescence resonance energy transfer in different nanostructures and nanomaterials. Appl Mater Today 30:101731
Jayan H, Sun D-W, Pu H, Wei Q (2023) Mesoporous silica coated core-shell nanoparticles substrate for size-selective SERS detection ofchloramphenicol. Spectrochim Acta A: Mol Biomol Spectrosc 284:121817
Zhou JX, Wang R, Su WW, Zhang LX, Li AD, Jiao TF (2022) Efficient detection of glucose by graphene-based non-enzymatic sensing material based on carbon dot. Colloids Surf A Physicochem Eng Asp 647:129122
Wu Z, Sun D-W, Pu H, Wei Q (2023) A novel fluorescence biosensor based on CRISPR/Cas12a integrated MXenes for detecting aflatoxin B1. Talanta 252:123773
Loh KP, Bao QL, Eda G, Chhowalla M (2010) Graphene oxide as a chemically tunable platform for optical applications. Nat Chem 2:1015–1024
Jayan H, Pu H, Sun D-W (2022) Detection of bioactive metabolite in Escherichia Coli culture using surface-enhanced Raman spectroscopy. AppliedSpectroscopy 76:812
Gao PR, Noor N, Shaarani SM (2022) Current status of food safety hazards and health risks connected with aquatic food products from Southeast Asian region. Crit Rev Food Sci Nutr 62:3471–3489
Jiang XY, Zhao YQ, Tang CY, Appelbaum M, Rao QC (2022) Aquatic food animals in the United States: status quo and challenges. Compr Rev Food Sci Food Saf 21:1336–1382
Pu H, Zhu H, Xu F, Sun D-W (2022) Development of core-satellite-shell structured MNP@Au@MIL-100(Fe) substrates for surface-enhanced Raman spectroscopy and their applications in trace level determination of malachite green in prawn. J Raman Spectrosc 53:682–693
Ben YJ, Fu CX, Hu M, Liu L, Wong MH, Zheng CM (2019) Human health risk assessment of antibiotic resistance associated with antibiotic residues in the environment: a review. Environ Res 169:483–493
Kou QM, Wu P, Sun Q, Li CX, Zhang L, Shi HX, Wu J, Wang YR, Yan XL, Le T (2021) Selection and truncation of aptamers for ultrasensitive detection of sulfamethazine using a fluorescent biosensor based on graphene oxide. Anal Bioanal Chem 413:901–909
Liu Y, Deng Y, Li S, Chow FWN, Liu M, He NY (2022) Monitoring and detection of antibiotic residues in animal derived foods: solutions using aptamers. Trends Food Sci Technol 125:200–235
Oliver JP, Gooch CA, Lansing S, Schueler J, Hurst JJ, Sassoubre L, Crossette EM, Aga DS (2020) Invited review: fate of antibiotic residues, antibiotic-resistant bacteria, and antibiotic resistance genes in US dairy manure management systems. J Dairy Sci 103:1051–1071
Ding LJ, Jiang D, Wen ZR, Xu YH, Guo YS, Ding CF, Wang K (2020) Ultrasensitive and visible light-responsive photoelectrochemical aptasensor for edifenphos based on zinc phthalocyanine sensitized MoS2 nanosheets. Biosens Bioelectron 150:111867
Filep SC, Black KR, Smith BRE, Block DS, Kuklinska-Pijanka A, Bermingham M, Oliver MA, Thorpe CM, Schuhmacher ZP, Agah S, Wuenschmann S, Chapman MD (2022) Simultaneous quantification of specific food allergen proteins using a fluorescent multiplex array. Food Chem 389:132986
Tudi M, Li HR, Li HY, Wang L, Lyu J, Yang LS, Tong SM, Yu QJ, Ruan HD, Atabila A, Phung DT, Sadler R, Connell D (2022) Exposure routes and health risks associated with pesticide application. Toxics 10(6):335
Chowdhury S, Mazumder MAJ, Al-Attas O, Husain T (2016) Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci Total Environ 569:476–488
Gontrani L, Pulci O, Carbone M, Pizzoferrato R, Prosposito P (2021) Detection of heavy metals in water using graphene oxide quantum dots: an experimental and theoretical study. Molecules 26:5519
Lindenmayer R, Lu L, Eivazi F, Afrasiabi Z (2023) Atomic spectroscopy-based analysis of heavy metals in seaweed species. Appl Sci-Basel 13(8):4764
Mo FY, Ma ZY, Wu TT, Liu ML, Zhang YY, Li HT, Yao SZ (2019) Holey reduced graphene oxide inducing sensitivity enhanced detection nanoplatform for cadmium ions based on glutathione-gold nanocluster. Sens Actuators B Chem 281:486–492
Bianchi DM, Maurella C, Lenzi C, Fornasiero M, Barbaro A, Decastelli L (2022) Influence of season and food type on bacterial and entero-toxigenic prevalence of Staphylococcus aureus. Toxins 14:671
Dadmehr M, Shahi SC, Malekkiani M, Korouzhdehi B, Tavassoli A (2023) A stem-loop like aptasensor for sensitive detection of aflatoxin based on graphene oxide/AuNPs nanocomposite platform. Food Chem 402:134212
Li ZB, Xue N, Ma HY, Cheng ZY, Miao XM (2018) An ultrasensitive and switch-on platform for aflatoxin B1 detection in peanut based on the fluorescence quenching of graphene oxide-gold nanocomposites. Talanta 181:346–351
Shi Y, Wu JZ, Sun YJ, Zhang Y, Wen ZW, Dai HC, Wang HD, Li Z (2012) A graphene oxide based biosensor for microcystins detection by fluorescence resonance energy transfer. Biosens Bioelectron 38:31–36
Zhang Q, Kang LZ, Yue PF, Shi LC, Wang M, Zhou LD, Zhao HP, Kong WJ (2022) Development of a graphene oxide nanosheet and double-stranded DNA structure based fluorescent “signal off” aptasensor for ochratoxin A detection in malt. Food Chem X 14:100308
Chinnappan R, Rahamn AA, AlZabn R, Kamath S, Lopata AL, Abu-Salah KM, Zourob M (2020) Aptameric biosensor for the sensitive detection of major shrimp allergen, tropomyosin. Food Chem 314:126133
Dong X, Raghavan V (2022) A comprehensive overview of emerging processing techniques and detection methods for seafood allergens. Compr Rev Food Sci Food Saf 21:3540–3557
Gendel SM (2013) The regulatory challenge of food allergens. J Agric Food Chem 61:5634–5637
Monaci L, De Angelis E, Montemurro N, Pilolli R (2018) Comprehensive overview and recent advances in proteomics MS based methods for food allergens analysis. Trac-Trends Anal Chem 106:21–36
Wang YB, Ma JJ, Li H, Zhou JR, Zhang H, Fu LL (2021) A sensitive Immunosensor based on FRET between gold nanoparticles and InP/ZnS quantum dots for arginine kinase detection. Food Chem 354:129536
Kweon SY, Park JP, Park CY, Park TJ (2022) Graphene oxide-mediated fluorometric aptasensor for okadaic acid detection. Biochip J 16:207–213
Yang F, Huang FY, Feng H, Wei J, Massey IY, Liang GY, Zhang F, Yin LH, Kacew S, Zhang X, Pu YP (2020) A complete route for biodegradation of potentially carcinogenic cyanotoxin microcystin-LR in a novel indigenous bacterium. Water Res 174:115638
Zhang X, Ye X, Chen L, Zhao H, Shi Q, Xiao Y, Ma L, Hou X, Chen Y, Yang F (2020) Functional role of bloom-forming cyanobacterium Planktothrix in ecologically shaping aquatic environments. Sci Total Environ 710:136314
Ghimpeteanu OM, Pogurschi EN, Popa DC, Dragomir N, Dragotoiu T, Mihai OD, Petcu CD (2022) Antibiotic use in livestock and residues in food-A public health threat: a review. Foods 11:1430
Zhang W, Sun D-W, Ma J, Cheng J, Wang Z, Tang BZ (2022) A volatile basic nitrogens-responsive tag based on aggregation-induced emission luminogen for real-time monitoring and in situ visualization of salmon freshness. Anal Chim Acta 122:340122
Li DW, Ling S, Meng DD, Zhou B, Liang PD, Lv B (2022) Sensitive fluorescent aptasensing of tobramycin on graphene oxide coupling strand displacement amplification and hybridization chain reaction. Int J Biol Macromol 220:1287–1293
Ye H, Wan TX, Li XF, Li C, He K, Guo YX (2023) Rapid detection of kanamycin using cooperative recognition split aptamer and graphene oxide nanosheets. J Food Meas Charact 17:2144–2151
Wen X, Yan XL, Zheng XL, Kou QM, Yang LL, Tang JM, Chen XY, Xie Y, Le T (2023) Selection and truncation of aptamers as fluorescence sensing platforms for selective and sensitive detection of nitrofurazone. Anal Chim Acta 1252:341044
Liu Z, Zhou J, Wang X, Zhao J, Zhao P, Ma Y, Zhang S, Huo D, Hou C, Ren K (2023) Graphene oxide mediated CdSe quantum dots fluorescent aptasensor for high sensitivity detection of fluoroquinolones. Spectrochim Acta A 305:123497
Youn H, Lee K, Her J, Jeon J, Mok J, So J-I, Shin S, Ban C (2019) Aptasensor for multiplex detection of antibiotics based on FRET strategy combined with aptamer/graphene oxide complex. Sci Rep 9:7659
Jayan H, Sun D-W, Pu H, Wei Q (2022) Surface-enhanced Raman spectroscopy combined with stable isotope probing to assess the metabolic activity of Escherichia Coli cells in chicken carcass wash water. Spectrochim Acta A: Mol Biomol Spectrosc 280:121549
Huang L, Sun D-W, Pu H (2022) Photosensitized peroxidase mimicry at the hierarchical 0D/2D heterojunction-like quasi metal-organic framework interface for boosting biocatalytic disinfection. Small 18:2200178
Zhang S, Ma L, Ma K, Xu B, Liu LJ, Tian WJ (2018) Label-free aptamer-based biosensor for specific detection of chloramphenicol using AIE probe and graphene oxide. Acs Omega 3:12886–12892
Ning Y, Wang XQ, Liu SW, Li L, Lu FG (2023) A graphene-oxide-based aptasensor for fluorometric determination of chloramphenicol in milk and honey samples utilizing exonuclease III-assisted target recycling and Nb.BbvCI-powered DNA walker cascade amplification. Ecotoxicol Environ Saf 249:114449
Narayanan N, Mandal A, Kaushik P, Singh S (2022) Fluorescence turn off azastilbene Sensor for detection of pesticides in vegetables: an experimental and computational investigation. Microchem J 175:107205
Dawood MAO, Abdel-Tawwab M, Abdel-Latif HMR (2020) Lycopene reduces the impacts of aquatic environmental pollutants and physical stressors in Fish. Rev Aquac 12:2511–2526
Liu ZZ, Zhao HY, Wang J, Wang ZW, Di SS, Xu H, Wang Q, Wang XH, Wang XQ, Qi PP (2022) Magnetic polymer particles as a highly efficient and facile cleanup adsorbent for multi-pesticide residues analysis in aquatic products. Ecotoxicol Environ Saf 241:113830
Singh P, Kumar S, Verma SK (2023) Development of fluorescent aptasensor for detection of acephate by utilizing graphene oxide platform. Talanta 252:123843
Gaviria MI, Barrientos K, Arango JP, Cano JB, Penuela GA (2022) Highly sensitive fluorescent biosensor based on acetylcholinesterase and carbon dots-graphene oxide quenching test for analytical and commercial organophosphate pesticide detection. Front Environ Sci 10:825112
Arvand M, Mirroshandel AA (2017) Highly-sensitive aptasensor based on fluorescence resonance energy transfer between L-cysteine capped ZnS quantum dots and graphene oxide sheets for the determination of edifenphos fungicide. Biosens Bioelectron 96:324–331
Rong YW, Li HH, Ouyang Q, Ali S, Chen QS (2020) Rapid and sensitive detection of diazinon in food based on the FRET between rare-earth doped upconversion nanoparticles and graphene oxide. Spectrochim Acta A Mol Biomol Spectrosc 239:118500
Lin MH, Sun L, Kong FB, Lin MS (2021) Rapid detection of paraquat residues in green tea using surface-enhanced raman spectroscopy (SERS) coupled with gold nanostars. Food Control 130:108280
Qian XC, Zhou XJ, Gao W, Li J, Ran X, Du GB, Yang L (2019) One-step and green strategy for exfoliation and stabilization of graphene by phosphate pillar 6 arene and its application for fluorescence sensing of paraquat. Microchem J 150:104203
Jayan H, Pu H, Sun D-W (2022) Recent developments in Raman spectral analysis of microbial single cells: techniques and applications. Crit Rev Food Sci Nutr 62:4294–4308
Arvand M, Mirroshandel AA (2019) An efficient fluorescence resonance energy transfer system from quantum dots to graphene oxide nano sheets: application in a photoluminescence aptasensing probe for the sensitive detection of diazinon. Food Chem 280:115–122
Zhang YH, Bai XL, Lv CZ, Fang YZ, Tang YL, Jiang H, Huang GR (2023) An aptasensor based on the fluorescence resonance energy transfer of nitrogen-doped carbon quantum dots and graphene oxide to detect fipronil in eggs. Eur Food Res Technol 249:2887–2895
Chi HF, Hou YW, Li GF, Zhang YC, Coulon F, Cai C (2020) In vitro model insights into the role of human gut microbiota on arsenic bioaccessibility and its speciation in soils. Environ Pollut 263:114580
Coetzee JJ, Bansal N, Chirwa EMN (2020) Chromium in environment, its toxic effect from chromite-mining and ferrochrome industries, and its possible bioremediation. Exposure Health 12:51–62
Sun T, Li X, Jin X, Wu Z, Chen X, Qiu J (2022) Function of graphene oxide as the nanoquencher for Hg2+ detection using an exonuclease I-assisted biosensor. Int J Mol Sci 23:6326
Yue JY, Ding XL, Wang YT, Wen YX, Yang P, Ma Y, Tang B (2021) Dual functional sp2 carbon-conjugated covalent organic frameworks for fluorescence sensing and effective removal and recovery of Pd2+ ions. J Mater Chem A 9:26861–26866
Zhang JZ, Ma XF, Yuan L, Zhou DX (2020) Comparison of adsorption behavior studies of Cd2+ by vermicompost biochar and KMnO4-modified vermicompost biochar. J Environ Manage 256:109959
Lv M, Sun D-W, Huang L, Pu H (2022) Precision release systems of food bioactive compounds based on metal-organic frameworks: synthesis, mechanisms and recent applications. Crit Rev Food Sci Nutr 62:3991–4009
Wang HQ, Da LG, Yang L, Chu SY, Yang F, Yu SM, Jiang CL (2020) Colorimetric fluorescent paper strip with smartphone platform for quantitative detection of cadmium ions in real samples. J Hazard Mater 392:122506
Li M, Zhou XJ, Guo SW, Wu NQ (2013) Detection of lead (II) with a turn-on fluorescent biosensor based on energy transfer from CdSe/ZnS quantum dots to graphene oxide. Biosens Bioelectron 43:69–74
Wang JL, Chen SH, Yuan R, Hu FX (2020) DNA branched junctions Induced the enhanced fluorescence recovery of FAM-labeled probes on r-GO for detecting Pb2+. Anal Bioanal Chem 412:2455–2463
Ebrahim S, Shokry A, Khalil MMA, Ibrahim H, Soliman M (2020) Polyaniline/Ag nanoparticles/graphene oxide nanocomposite fluorescent sensor for recognition of chromium (VI) ions. Sci Rep 10:13617
Pathan S, Jalal M, Prasad S, Bose S (2019) Aggregation-Induced enhanced photoluminescence in magnetic graphene oxide quantum dots as a fluorescence probe for as(III) sensing. J Mater Chem A 7:8510–8520
Sharma MD, Rayalu SS, Kolev SD, Krupadam RJ (2021) Graphene/fluorescein dye-based sensor for detecting As (III) in drinking water. Sci Rep 11:17321
Shahin A, Ibrahim K, Ye F, Karimi R, Sanderson J, Musselman KP (2022) Selective sensing of heavy metal ions via fluorescence quenching of femtosecond-laser-synthesized 2D nanoparticles. Sens Actuators B Chem 359:131576
Zhang JR, Huang WT, Xie WY, Wen T, Luo HQ, Li NB (2012) Highly sensitive, selective, and rapid fluorescence Hg2+ sensor based on DNA duplexes of poly(dT) and graphene oxide. Analyst 137:3300–3305
Li M, Zhou XJ, Ding WQ, Guo SW, Wu NQ (2013) Fluorescent aptamer-functionalized graphene oxide biosensor for label-free detection of mercury(II). Biosens Bioelectron 41:889–893
Li MK, Hu LY, Niu CG, Huang DW, Zeng GM (2018) A fluorescent DNA based probe for Hg(II) based on Thymine-Hg(II)-Thymine Interaction and Enrichment via Magnetized Graphene Oxide. Microchim Acta 185:207
Sahu D, Sarkar N, Mohapatra P, Swain SK (2020) Rhodamine B associated Ag/r-GO nanocomposites as ultrasensitive fluorescent sensor for Hg2+. Microchem J 154:104577
Abdelhamid HN, Wu HF (2015) Reduced graphene oxide conjugate thymine as a new probe for ultrasensitive and selective fluorometric determination of mercury(II) ions. Microchim Acta 182:1609–1617
Babu US, Raybourne RB (2008) Impact of dietary components on chicken immune system and Salmonella infection. Expert Rev Anti Infect Ther 6:121–135
Lu YH, Yuan ZL, Bai JR, Lin Q, Deng RJ, Luo AM, Chi YL, Deng S, He Q (2020) Directly profiling intact Staphylococcus aureus in water and foods via enzymatic cleavage aptasensor. Anal Chim Acta 1132:28–35
Vinayaka AC, Ngo TA, Kant K, Engelsmann P, Dave VP, Shahbazi MA, Wolff A, Bang DD (2019) Rapid detection of Salmonella enterica in food samples by a novel approach with combination of sample concentration and direct PCR. Biosens Bioelectron 129:224–230
Xie RQ, Shao NY, Zheng J (2020) Integrated co-functional network analysis on the resistance and virulence features in Acinetobacter baumannii. Front Microbiol 11:598380
Jayan H, Pu H, Sun D-W (2022) Detection of bioactive metabolite in Escherichia coli culture using surface-enhanced Raman spectroscopy. Appl Spectrosc 76:812–822
Jayan H, Pu H, Sun D-W (2022) Analyzing macromolecular composition of E. Coli O157:H7 using Raman-stable isotope probing. Spectrochim Acta A: Mol Biomol Spectrosc 276:121217
Li D, Zhu Z, Sun D-W (2022) Visualization and quantification of content and hydrogen bonding state of water in apple and potato cells by confocal Raman microscopy: a comparison study. Food Chemistry 385:132679
Chattopadhyay S, Choudhary M, Singh H (2022) Carbon dots and graphene oxide based FRET immunosensor for sensitive detection of Helicobacter pylori. Anal Biochem 654:114801
Dehghani Z, Mohammadnejad J, Hosseini M, Bakhshi B, Rezayan AH (2020) Whole cell FRET immunosensor based on graphene oxide and graphene dot for Campylobacter jejuni detection. Food Chem 309:125690
Chen M, Song YQ, Han L, Zhou DD, Wang Y, Pan LQ, Tu K (2022) An ultrasensitive upconversion fluorescence aptasensor based on graphene oxide release and magnetic separation for Staphylococcus aureus detection. Food Anal Methods 15:2791–2800
Zhang Y, Gao L, Han J, Miao XM (2023) Dual-signal and one-step monitoring of Staphylococcus aureus in milk using hybridization chain reaction based fluorescent sensor. Spectrochim Acta A Mol Biomol Spectrosc 303:123191
Ravikumar CH, Gowda MI, Balakrishna RG (2019) An “off-on” quantum dot-graphene oxide bioprobe for sensitive detection of micrococcal nuclease of Staphylococcus aureus. Analyst 144:3999–4005
Ma XY, Meng RZ, Yu MM, Guo N, Wang H, Zheng HR, Sun CY (2024) Label-free and low-background fluorescent structure-switching aptasensor for sensitive detection of staphylococcal enterotoxin A based on graphene oxide-assisted separation of ssDNA. Food Control 155:110105
Duan YF, Ning Y, Song Y, Deng L (2014) Fluorescent aptasensor for the determination of Salmonella typhimurium based on a graphene oxide platform. Microchim Acta 181:647–653
Chinnappan R, AlAmer S, Eissa S, Rahamn AA, Abu Salah KM, Zourob M (2018) Fluorometric graphene oxide-based detection of Salmonella enteritis using a truncated DNA aptamer. Microchim Acta 185:61
Renuka RM, Maroli N, Achuth J, Ponmalai K, Kadirvelu K (2020) Highly adaptable and sensitive FRET-based aptamer assay for the detection of Salmonella paratyphi A. Spectrochim Acta A Mol Biomol Spectrosc 243:118662
Xie JL, Jiang H, Zhao YQ, Zhong M, Jin XR, Zhu ZX, Li BL, Guo JL, Zhang LM, Liu JB (2023) Aptamer-based DNA-catalyzed amplification strategy for sensitive fluorescence resonance energy transfer detection of Acinetobacter baumannii. Talanta 255:124212
Bahari D, Babamiri B, Salimi A, Salimizand H (2021) Ratiometric fluorescence resonance energy transfer aptasensor for highly sensitive and selective detection of acinetobacter Baumannii bacteria in urine sample using carbon dots as optical nanoprobes. Talanta 221:121619
He H, Sun D-W, Wu Z, Pu H, Wei Q (2022) On-off-on fluorescent nanosensing: materials, detection strategies and recent food applications. Trends Food Sci Technol 119:243–256
Huang Y, Zhang H, Chen X, Wang X, Duan N, Wu S, Xu B, Wang Z (2015) A multicolor time-resolved fluorescence aptasensor for the simultaneous detection of multiplex Staphylococcus aureus enterotoxins in the milk. Biosens Bioelectron 74:170–176
Wu Z, Sun D-W, Pu H, Wei Q, Lin X (2022) Ti3C2Tx MXenes loaded with Au nanoparticle dimers as a surface-enhanced Raman scattering aptasensor for AFB1 detection. Food Chem 372:131293
Marshall H, Meneely JP, Quinn B, Zhao Y, Bourke P, Gilmore BF, Zhang G, Elliott CT (2020) Novel decontamination approaches and their potential application for post-harvest aflatoxin control. Trends Food Sci Technol 106:489–496
Xu L, Zhu Z, Sun D-W (2021) Bioinspired nanomodification strategies: moving from chemical based agro-systems to sustainable agriculture. ACS Nano 15:12655–12686
Meneely JP, Kolawole O, Haughey SA, Miller SJ, Krska R, Elliott CT (2022) The challenge of global aflatoxins legislation with a focus on peanuts and peanut products: a systematic review. Exposure Health 15:467–487
Zhang XY, Zhang XJ, Song LJ, Huang XQ, Li Y, Qiao MW, Liu WJ, Zhang TT, Qi YC, Wang WZ, Yu XZ, Dou LN, Yang HJ, Wang LY, Mao YX, Wang ZH (2021) An ultrasensitive, homogeneous fluorescence quenching immunoassay integrating separation and detection of aflatoxin M-1 based on magnetic graphene composites. Microchim Acta 188:59
Joo M, Baek SH, Cheon SA, Chun HS, Choi S-W, Park TJ (2017) Development of aflatoxin B1 aptasensor based on wide-range fluorescence detection using graphene oxide quencher. Colloids Surf B Biointerfaces 154:27–32
Lu Z, Chen X, Wang Y, Zheng X, Li CM (2015) Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim Acta 182:571–578
Li D, Zhu Z, Sun D-W (2021) Quantification of hydrogen bonding strength of water in saccharide aqueous solutions by confocal Raman microscopy. J Mol Liq 342:117498
Zhang W, Ma J, Sun D-W (2021) Raman spectroscopic techniques for detecting structure and quality of frozen foods: principles and applications. Crit Rev Food Sci Nutr 61:2623–2639
Wang L, Hua X, Shi J, Jing NH, Ji T, Lv B, Liu LJ, Chen Y (2022) Ochratoxin A: occurrence and recent advances in detoxification. Toxicon 210:11–18
Wu SJ, Duan N, Ma XY, Xia Y, Wang HG, Wang ZP, Zhang Q (2012) Multiplexed fluorescence resonance energy transfer aptasensor between upconversion nanoparticles and graphene oxide for the simultaneous determination of mycotoxins. Anal Chem 84:6263–6270
Hussain N, Pu H, Sun D-W (2021) Synthesis of bimetallic core-shelled nanoparticles modified by 2-mercaptoethanol as SERS substrates for detecting ferbam and thiabendazole in apple puree. Food Addit Contam 38:1386–1399
Alves RC, Barroso F, Gonzalez-Garcia MB, Oliveira MBPP, Delerue-Matos C (2016) New trends in food allergens detection: toward biosensing strategies. Crit Rev Food Sci Nutr 56:2304–2319
Zhang C, Huang L, Pu H, Sun D-W (2021) Magnetic surface-enhanced Raman scattering (MagSERS) biosensors for microbial food safety: fundamentals and applications. Trends Food Sci Technol 113:366–381
Zhou JR, Wang YB, Qian YF, Zhang T, Zheng L, Fu LL (2020) Quantification of shellfish major allergen tropomyosin by SPR biosensor with gold patterned biochips. Food Control 107:106547
Zhang Y, Wu Q, Wei X, Zhang J, Mo S (2017) DNA aptamer for use in a fluorescent assay for the shrimp allergen tropomyosin. Microchim Acta 184:633–639
Zhou J, Ai R, Weng J, Li L, Zhou C, Ma A, Fu L, Wang Y (2020) A “on-off-on” fluorescence aptasensor using carbon quantum dots and graphene oxide for ultrasensitive detection of the major shellfish allergen arginine kinase. Microchem J 158:105171
Huang L, Sun D-W, Wu Z, Pu H, Wei Q (2021) Reproducible, shelf-stable, and bioaffinity SERS nanotags inspired by multivariate polyphenolic chemistry for bacterial identification. Anal Chim Acta 1167:338570
Gu HJ, Hao LL, Ye H, Ma PF, Wang ZP (2021) Nuclease-assisted target recycling signal amplification strategy for graphene quantum dot-based fluorescent detection of marine biotoxins. Microchim Acta 188:118
Zhang D, Pu H, Huang L, Sun D-W (2021) Advances in flexible surface-enhanced Raman scattering (SERS) substrates for nondestructive food detection: fundamentals and recent applications. Trends Food Sci Technol 109:690–701
Lee SY, Kim C, Kim HT (2015) Difference in chemical reactions in bulk plasma and sheath regions during surface modification of graphene oxide film using capacitively coupled NH3 plasma. J Appl Phys 118:103303
Wu Z, Pu H, Sun D-W (2021) Fingerprinting and tagging detection of mycotoxins in agri-food products by surface-enhanced Raman spectroscopy: principles and recent applications. Trends in Food Science & Technology 110:393–404
Sun D-W, Huang L, Pu H, Ma J (2021) Introducing reticular chemistry into agrochemistry. Chemical Society Reviews 50:1070–1110
Acknowledgements
The authors are grateful to the National Natural Science Foundation of China (32272466) for its support. This research was also supported by Guangdong Basic and Applied Basic Research Foundation (2021A1515010644, 2021A1515110396), Guangzhou Basic and Applied Basic Research Foundation (202201010008), the Contemporary International Collaborative Research Centre of Guangdong Province on Food Innovative Processing and Intelligent Control (2019A050519001), and the Common Technical Innovation Team of Guangdong Province on Preservation and Logistics of Agricultural Products (2023KJ145).
Funding
Open Access funding provided by the IReL Consortium See acknowledgements.
Author information
Authors and Affiliations
Contributions
First authorWriting - original draft, Formal analysis, Investigation. Second authorValidation, Funding acquisition, Resources. Third authorValidation, Funding acquisition, Resources. Last authorSupervision, Funding acquisition, Resources, Writing - review & editing.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, MX., Cheng, JH., Ma, J. et al. Advancing Aquatic Food Safety Detection Using Highly Sensitive Graphene Oxide and Reduced Graphene Oxide (GO/r-GO) Fluorescent Sensors. Food Eng Rev (2024). https://doi.org/10.1007/s12393-024-09375-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12393-024-09375-5