Abstract
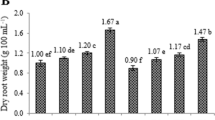
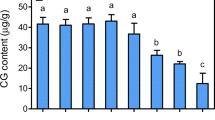
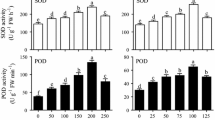
The major objective of the current study was to investigate the potential effects of differential pH levels on culture development and production of steviol glycosides and other polyphenolics content in submerge root cultures of Stevia rebaudiana. In vitro grown cultures require an optimum pH level for rapid growth and uniform production of secondary metabolites. Herein, varying media pH levels (5.0, 5.1, 5.2, 5.3, 5.4, 5.5, 5.6, 5.7, 5.8, 5.9 and 6.0) significantly influenced fresh and dry biomasses of adventitious root cultures. Among tested levels, adventitious root cultures favored high media pH (6.0) for accumulation of maximum fresh biomass (112.86 g l−1) and dry biomass (8.29 g l−1). In this study, we observed that lower pH level (5.1) strongly supported the production of steviosides (79.48 mg/g-DW) and rebaudioside-A (13.10 mg/g-DW) contents but reduced the polyphenolics content in adventitious cultures. However, dulcoside contents (2.57 mg/g-DW) in adventitious root cultures were found in higher quantities at pH level 5.8. Similarly, maximum total phenolics (70.06 mg/g-DW) and flavonoids (50.19 mg/g-DW) were observed on media having 5.8-pH level. The same pH level also improved DPPH-radical scavenging activities (92.67%). This study will offer an approach to enhance medicinal products in the in vitro tissues, rather than to over-exploit the wild plants and ultimately putting them on the brink of being endangered. Furthermore, the consistent production of secondary metabolites in these cultures could be scaled-up on bioreactor level, which will ultimately affect the society by potential introduction of cost-effective and biologically stable medicinal drugs.





Similar content being viewed by others
References
Ahmad, N., B.H. Haider, H. Fazal, M.A. Khan, and M.S. Afridi. 2014. Effect of reverse photoperiod on in vitro regeneration of piperine production in Piper nigrum L. Comptes Rendus Biologies 337: 19–28.
Ahmad, N., H. Fazal, B.H. Abbasi, M. Rashid, T. Mahmood, and N. Fatma. 2010. Efficient regeneration and antioxidant potential in regenerated tissues of Piper nigrum L. Plant Cell, Tissue and Organ Culture 102: 129–134.
Ahmadian, E., A. Lolaei, A. Mobasheri, and R. Bemana. 2013. Investigation of importance parameters of plant tissue (review). International Journal of Agriculture and Crop Sciences 5(8): 900–905.
Aman, N., F. Hadi, S.A. Khalil, R. Zamir, and N. Ahmad. 2013. Efficient regeneration for enhanced steviol glycosides production in Stevia rebaudiana (Bertoni). Comptes Rendus Biologies 336: 486–492.
Apelt, J., M. Bigl, P. Wunderlich, and R. Schliebs. 2004. Aging related increase in oxidative stress correlates with developmental pattern of beta-secretase activity and beta-amyloid plaque formation in transgenic Tg2576 mice with Alzheimer-like pathology. International Journal of Developmental Neuroscience 22(7): 475–484.
Arts, I.C.W., and P.C.H. Hollman. 2005. Polyphenols and disease risk in epidemiologic studies. The American Journal of Clinical Nutrition 81: 317–325.
Aziz, E.E., H. Al-Amier, and L.E. Craker. 2008. Influence of salt stress on growth and essential oil production in peppermint, pennyroyal, and apple mint. Journal of Herbs, Spices & Medicinal Plants 14: 77–87.
Bano, M.J., J. Lorente, J. Castillo, O. Benavente-Garcia, J.A. Del-Rio, and A. Ortuno. 2003. Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis, Antioxidant activity. Journal of Agricultural and Food Chemistry 51: 4247–4253.
Barz, W., S. Daniel, W. Hinderer, U. Jaques, H. Kessmann, J. Koster, and K. Tiemann. 1988. Elicitation and metabolism of phytoalexins in plant cell cultures. In Plant cell biotechnol. NATO ASI Series, ed. M. Pais, F. Mavituna, and J. Novais, 211–230. Berlin: Springer.
Berr, C., M.J. Richard, V. Gourlet, C. Garrel, and A. Favier. 2004. Enzymatic antioxidant balance and cognitive decline in aging—The EVA study. European Journal of Epidemiology 19(2): 133–138.
Bhatia, P., and N. Ashwath. 2005. Effect of medium pH on shoot regeneration from the cotyledonary explants of tomato. Biotechnol 4(1): 7–10.
Bidchol, A.M., A. Wilfred, P. Abhijna, and R. Harish. 2011. Free radical scavenging activity of aqueous and ethanolic extract of Brassica oleracea L. var. italica. Food and Bioprocess Technology 4(7): 1137–1143.
Bourdel-Marchasson, I., M.C. Delmas-Beauvieux, E. Peuchant, S. Richard-Harston, A. Decamps, B. Reignier, J.P. Emeriau, and M. Rainfray. 2001. Antioxidant defences and oxidative stress markers in erythrocytes and plasma from normally nourished elderly Alzheimer patients. Age Aging 30(3): 235–241.
Brandle, J., Sys, E.A., and Marsolais, A.A. 1998. Stevia plant named ‘RSIT 95-166-13’. USpatent. USPP10563.
Chen, Y., M. Yu, Z. Zhu, L. Zhang, and Q. Guo. 2013. Optimization of potassium chloride nutrition for proper growth, physiological development and bioactive component production in Prunella vulgaris L. PLoS ONE 8: 1–7.
Debnath, M., C. Malik, and B.P.S. Pand. 2006. Micropropagation a tool for production of high quality plants based medicines. Current Pharmaceutical Biotechnology 7: 33–49.
Dicosmo, F., and M. Misawa. 1985. Eliciting secondary metabolism in plant cell cultures. Trends in Biotechnology 3: 318–322.
Diwan, R., A. Shinde, and N. Malpathak. 2012. Phytochemical composition and antioxidant potential of Ruta graveolens L. in vitro culture lines. Journal of Botany 2012: 6. https://doi.org/10.1155/2012/685427.
Dixon, R.A., and N. Paiva. 1995. Stress induced phenylpropanoid metabolism. Plant Cell 7(7): 1085–1097.
El-Zefzafy, M.M., W.W. Mohamed, and M.S. Boghdady. 2015. Effect of pH on growth, protein profils and anatomy of Plectranthus amboinicus explants. International Journal of Life science and Pharma Research 5: 11–21.
Finn, C.E., J.J. Luby, C.J. Rosen, and P.D. Ascher. 1991. Evaluation in vitro of blueberry germplasm for higher pH tolerance. Journal of the American Society for Horticultural Science 116: 312–316.
Fujita, Y. 1988. Industrial production of shikonin and berberine. Applications of plant cell and tissue culture. Ciba Foundation Symposium 137, 228–38. Chichester: Wiley.
George, E.F., M.A. Hall, and G.J. De-Klerk. 2008. Plant propagation by tissue culture. Vol 1: The background, 3rd ed. Dordrecht: Springer.
Giri, L., P. Dhyani, S. Rawat, I.D. Bhatt, S.K. Nandi, R.S. Rawal, and V. Pande. 2012. In vitro production of phenolic compounds and antioxidant activity in callus suspension cultures of Habenaria edgeworthii: A rare Himalayan medicinal orchid. Industrial Crops and Products 39: 1–6.
Gorret, N., S.K. Rosli, S.F. Oppenheim, L.B. Willis, P.A. Lessard, C. Rha, and A.J. Sinskey. 2004. Bioreactor culture of oil palm (Elaeis guineensis) and effects of nitrogen source, inoculum size, and conditioned medium on biomass production. Journal of Biotechnology 108: 253–263.
Gurel, S., and Y. Gulsen. 1998. The effects of different sucrose, agar and pH levels on in vitro shoot production of Almond (Amygdalus communis L.). Turkish Journal of Botany 22: 363–373.
Hahlbrock, K., and D. Scheel. 1989. Physiology and molecular biology of phenylpropanoid metabolism. Annual Review of Plant Biology 40: 347–369.
Hahlbrock, K., J. Ebel, A. Oaks, J. Auden, and M. Liersch. 1974. Determination of specific growth stages of plant cell suspension cultures by monitoring conductivity changes in the medium. Planta 118: 75–84.
Hussain, A., I.A. Qarshi, H. Nazir, and I. Ullah. 2012a. Recent advances in plant in vitro culture, plant tissue culture: Current status and opportunities, 1–28. Rijeka: In Tech.
Hussain, M.S., S. Fareed, S. Ansari, M.A. Rahman, I.Z. Ahmad, and M. Saeed. 2012b. Current approaches toward production of secondary plant metabolites. Journal of Pharmacy and Bioallied Sciences 4(1): 10.
Jalil, M., M.S.M. Annuar, B.C. Tan, and N. Khalid. 2015. Effects of selected physicochemical parameters on Zerumbone production of Zingiber zerumbet Smith cell suspension culture. Evidence-based complementary and alternative medicine. https://doi.org/10.1155/2015/757514.
Juhasz, G.A., L.S. Simon, I. Velich, and P. Varro. 1997. Studies of non-ionic osmotic stress on bean callus and seedling cultures. Acta Horticulturae 44: 455–456.
Karuppusamy, S. 2009. A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. Journal of Medicinal Plants Research 3: 1222–1239.
Kim, S.H., and S.K. Kim. 2002. Effect of sucrose level and nitrogen source on fresh weight and anthocyanin production in cell suspention culture of ‘Sheridan’ Grape (Vitis spp.). Plant Biotechnology Journal 4: 2327–2330.
Kishore, P.B.K., and V. Dange. 1990. Sucrose metabolism in callus cultures of cotton during growth. Indian Journal of Experimental Biology 28: 352–355.
Kochian, L.V., O.A. Hoekenga, and A.P. Miguel. 2004. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annual Review of Plant Biology 55: 459–493.
Koyama, H., T. Toda, and T. Hara. 2001. Brief exposure to low pH stress causes irreversible damage to the growing root in Arabidopsis thaliana: Pectin Ca++ interaction may play an important role in proton rhizotoxicity. Journal of Experimental Botany 52: 361–368.
Lager, I.D.A., O. Andreasson, T.L. Dunbar, E. Andreasson, M.A. Escobar, and A.G. Rasmusson. 2010. Changes in external pH rapidly alter plant gene expression and modulate auxin and elicitor responses. Plant, Cell and Environment 33(9): 1513–1528.
Lakshmi, M.V., and V. Sridevi. 2009. Effect of pH and inoculum size on phenol degradation by Pseudomonas aeruginosa. International Journal of Chemical Sciences 7(4): 2246–2252.
Laukkanen, H., R.T. Julkunen, and A. Hohtola. 1997. Effect of different nitrogen nutrients on the viability, protein synthesis and tannin production of Scots pine callus. Physiologia Plantarum 100: 982–988.
Laukkanen, H., H. Soini, S. Kontunen-Soppela, A. Hohtola, and M. Viljanen. 2000. A mycobacterium isolated from tissue cultures of mature Pinus sylvestris interferes with growth of Scots pine seedlings. Tree Physiology 20: 915–920.
Lee, S.E., H.J. Hwang, J.S. Ha, H.S. Jeong, and J.H. Kim. 2003. Screening of medicinal plant extracts for antioxidant activity. Life Sciences 73: 167–179.
Lemus, M.R., G.A. Vega, B.L. Zura, and K. Ah-Hen. 2012. Stevia rebaudiana (Bertoni), source of a high-potency natural sweetener: A comprehensive review on the bio-chemical, nutritional and functional aspects. Food Chemistry 132: 1121–1132.
Li, Y., C. Guo, J. Yang, J. Wei, J. Xu, and S. Cheng. 2006. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chemistry 96: 254–260.
Madan, S., S. Ahmad, G.N. Singh, K. Kohli, Y. Kumar, R. Singh, and H. Garg. 2010. Stevia rebaudiana (Bert.) Bertoni—A review. Indian Journal of Natural Products 1: 267–286.
Maeda, H., T.L. Sage, G. Isaac, R. Welti, and D. DellaPenna. 2008. Tocopherols modulate extraplastidic poly unsaturated fatty acid metabolism in Arabidopsis at low temperature. The Plant Cell 20: 452–470.
Maisuthisakul, P., M. Suttajit, and R. Pongsawatmanit. 2007. Assessment of phenolic content and free radical scavenging capacity of some thai indigenous plants. Food Chemistry 100(4): 1409–1418.
Maisuthisakul, P., S. Pasuk, and P. Ritthiruangdej. 2008. Relationship of antioxidant properties and chemical composition of some Thai plants. Journal of Food Composition and Analysis 21: 229–240.
Martin, S.M., and D. Rose. 1976. Growth of plant cell (Ipomea) suspension cultures at controlled pH levels. Canadian Journal of Botany 54: 1265–1270.
Mead, J.F. 1976. Free radical mechanisms of lipid damage and consequences for cellular membranes. In Free radicals in biology, vol. 1, ed. W.A. Pryor, 51–68. New York: Academic Press.
Meydani, M. 2001. Antioxidants and cognitive function. Nutrition Reviews 59(8): 75–80.
Middleton, E., C. Kandaswami, and T.C. Theoharides. 2000. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacological Reviews 52: 673–675.
Moog, P.R., and W. Bruggemann. 1994. Iron reductase systems on the plant plasma membrane. A review. Plant and Soil 165: 241–260.
Murashige, T., and F. Skoog. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum 15: 473–497.
Murthy, H.N., E.J. Hahn, and K.Y. Paek. 2008. Adventitious roots and secondary metabolism. Chinese Journal of Biotechnology 24: 711–716.
Naik, P.M., S.H. Manohar, N. Praveen, and H.N. Murthy. 2010. Effects of sucrose and pH levels on in vitro shoot regeneration from leaf explants of Bacopa monnieri and accumulation of bacoside A in regenerated shoots. Plant Cell, Tissue and Organ Culture 100: 235–239.
Ncube, N.S., A.J. Afolayan, and A.L. Okoh. 2008. Assessment techniques of anti microbial properties of natural compounds of plant origin current methods and future trends. African Journal of Biotechnology 7(12): 1797–1806.
Ostrolucka, M.G., A. Gajdosova, E. Ondruskova, A. Lateekova, and G. Libiakova. 2010. Effect of medium pH on axillary shoot proliferation of selected Vaccinium vitis-idaea L. Cultivars. Acta Biologica Cracoviensia Series Botanica 52(2): 92–96.
Ostrolucka, M.G., G. Libiakova, E. Ondruskova, and A. Gajdosova. 2004. In vitro propagation of Vaccinium species. Acta Universitatis Latviensis 670: 7–15.
Owens, P.R., L.P. Wilding, L.M. Lee, and B.E. Herbert. 2005. Evaluation of platinum electrodes and three electrode potential standards to determine electrode quality. Soil Science Society of America Journal 69(5): 1541–1550.
Ozcankaya, R., and N. Delibas. 2002. Malondialdehyde, superoxide dismutase, melatonin, iron, copper, and zinc blood concentrations in patients with Alzheimer disease cross-sectional study. Croatian Medical Journal 43(1): 28–32.
Pandhair, V., and B.S. Sekhon. 2006. Reactive oxygen species and antioxidants in plants. An overview. Journal of Plant Biochemistry and Biotechnology 15: 71–78.
Pieta, P., P. Sionetti, and P. Mauri. 1998. Anti-oxidant activity of selected medicinal plant. Journal of Agriculture and Food Chemistry 46: 4487–4490.
Poonnachit, U., and R. Darnell. 2004. Effect of ammonium and nitrate on ferric chelate reductase and nitrate reductase in Vaccinium species. Annals of Botany 93: 399–405.
Ramanand, Lal M. 2004. An efficient protocol for in vitro micropropagation of sugarcane. Sugar Tech 6: 85–87.
Rossi-Hassani, B.D., and J.P. Zryd. 1994. Instabilite genetique chez Portulaca grandiflora (Hook). Annales de Genetique 37: 53–59.
Saenz-Carbonell, L.A., I.E. Maldonado-Mendoza, O. Moreno-Valenzula, R. Ciau-Uitz, M. Lopez-Meyer, and V.M. Loyola-Vargas. 1990. Effect of the medium pH on the release of secondary metabolites from roots of Datura stramonium, Catharanthus roseus, and Tagetes patula cultured in vitro. Applied Biochemistry and Biotechnology 38: 257–267.
Sairkar, P., M.K. Chandravanshi, N.P. Shukla, and N.N. Mehrotra. 2009. Mass production of an economically important medicinal plant Stevia rebaudiana using in vitro propagation techniques. Journal of Medicinal Plants Research 3: 266–270.
Saito, A., C.M. Maier, P. Narasimhan, T. Nishi, Y.S. Song, F. Yu, J. Liu, Y.S. Lee, C. Nito, H. Kamada, R.L. Dodd, L.B. Hsieh, B. Hassid, E.E. Kim, M. Gonzalez, and P.H. Chan. 2005. Oxidative stress and neuronal death survival signaling in cerebral ischemia. Molecular Neurobiology 31(1–3): 105–116.
Seigler, D. 1998. Flavonoid and secondary metabolism, 151–192. Norwell: Kluwer.
Sheeja, R.R., and B. Lawrence. 2015. Phytochemical screening of the leaves of Stevia rebaudiana, Bertoni. International Journal of Current Microbiology and Applied Sciences 4: 344–347.
Shi, Q.H., Z.J. Zhu, J. Li, and Q.Q. Qian. 2006. Combined effects of excess Mn and low pH on oxidative stress and antioxidant enzymes in cucumber roots. Agricultural Sciences in China 5: 767–772.
Singh, A.K. 2005. Physiological and biochemical basis of in vitro morphogenesis in sugarcane hybrids. Dissertation Ph.D. Thesis, MJP Rohilkhand University Bareilly.
Sivanandhan, G., M. Arun, S. Mayavan, M. Rajesh, T.S. Mariashibu, M. Manickavasagam, N. Selvaraj, and A. Ganapathi. 2012. Chitosan enhances withanolides production in adventitious root cultures of Withania somnifera (L.) Dunal. Industrial Crops and Products 37: 124–129.
Skerget, M., P. Kotnik, M. Hadolin, A.R. Hras, M. Simonic, and Z. Knez. 2005. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chemistry 89: 191–198.
Sudha, G., and G.A. Ravishankar. 2003. Elicitation of anthocyanin production in callus cultures of Daucus carota and the involvement of methyl jasmonate and salicylic acid. Acta. Physiologiae Plantarum 25: 249–256.
Tabachnik, L., and D.E. Kester. 1977. Shoot culture for almond and almond peach hybrid clones in vitro. Horticultural Science 12: 545–547.
Taware, A.S., D.S. Mukadam, A.M. Chavan, and S.D. Tawar. 2010. Comparative studies of in vitro and in vivo grown plants and callus of Stevia rebaudiana (Bertoni). International Journal of Integrative Biology 9(1): 10–15.
Thorpe, T., C. Stasolla, E.C. Yeung, G.-J. De-Klerk, A. Roberts, and E.F. George. 2008. The components of plant tissue culture media II: Organic additions, osmotic and pH effects and support systems. In Plant propagation by tissue culture. Vol. 1, The background, 115–175, 3rd ed, ed. E.F. George, et al., 115–173. Dordrecht: Springer.
Tuteja, N., and S. Mahajan. 2007. Further characterization of calcineurin B-like protein and its interacting partner CBL-interacting protein kinase from Pisum sativum. Plant Signaling & Behaviour 2: 358–361.
Wang, H., G.J. Provan, and K. Helliwell. 2004. Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chemistry 87: 307–311.
Westcott, R.J., and G.G. Henshaw. 1976. Phenolic synthesis and phenylalanine ammonia-lyase activity in suspension cultures of Acer pseudoplatanus L. Planta 131: 67–73.
Williams, J.G.K., A.R. Kubelk, K.J. Livak, J.A. Rafalski, and S.V. Tingey. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Research 18: 6531–6535.
Wong, C., H. Li, K. Cheng, and F. Chen. 2006. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chemistry 97: 705–711.
Yu, H.M., M. Lu, and X.G. He. 2005. The techniques of plant tissue culture and its frequent problems and resolutions. Journal of Shandong Education Institute 20: 101–103.
Author information
Authors and Affiliations
Contributions
NA performed the major experiment and developed adventitious root cultures of Stevia and finally investigated the phenolic and flavonoid content. AR designed the experiment and thoroughly checked the whole manuscript. NA wrote the manuscript and performed antioxidant activity and HPLC analysis for Steviol glycosides. HF provided plant materials.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest regarding this manuscript.
Rights and permissions
About this article
Cite this article
Ahmad, N., Rab, A., Ahmad, N. et al. Differential pH-Induced Biosynthesis of Steviol Glycosides and Biochemical Parameters in Submerge Root Cultures of Stevia rebaudiana (Bert.). Sugar Tech 20, 734–744 (2018). https://doi.org/10.1007/s12355-018-0589-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-018-0589-z