Abstract
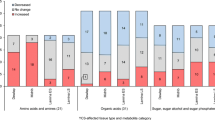
Understanding the metabolic and gene expression changes that accompany the expression of Yellow Canopy Syndrome (YCS) in sugarcane is important and could greatly assist in developing management strategies as well as in the identification of potential causal factors. Leaves representing two stages of development (leaves 4 and 6) from YCS symptomatic and YCS asymptomatic plants, from two seasons, were analysed using gas chromatography linked to mass spectrometry. More than 200 metabolites were detected in the leaf samples, and 84 of these could be identified. The results revealed intrinsic differences (p = 0.05) between the metabolomes of the YCS symptomatic and asymptomatic plants. It was evident that significant metabolic changes occurred well before the development of leaf yellowing. The major metabolic changes were associated with sugar metabolism, the pentose phosphate cycle, and phenylpropanoid and α-ketoglutarate metabolism. The diurnal changes of sucrose concentrations (low in the morning and high at the end of the day) are absent in the YCS symptomatic plants even before symptom expression. Comparing the leaf transcriptomes of the symptomatic and asymptomatic plants shows that a complex network of changes in gene expression underpins the observed changes in the metabolome.





Similar content being viewed by others
References
Afgan, E., C. Sloggett, N. Goonasekera, et al. 2015. Genomics virtual laboratory: A practical bioinformatics workbench for the cloud. PLoS ONE 10: e0140829.
Bonnett, G.D. 2013. Developmental stages (phenology). In Sugarcane: physiology, biochemistry, and functional biology, ed. P.H. Moore, and F.C. Botha, 35–53. New York: Wiley.
Braun, D.M., Y. Ma, N. Inada, M.G. Muszynski, and R.F. Baker. 2006. Tie-dyed1 regulates carbohydrate accumulation in maize leaves. Plant Physiology 142: 1511–1522.
Bundy, J.G., M.P. Davey, and M.R. Viant. 2009. Environmental metabolomics: A critical review and future perspectives. Metabolomics 5: 3–21.
Chinnaraja, C., and R. Viswanathan. 2015. Variability in yellow leaf symptom expression caused by the sugarcane yellow leaf virus and its seasonal influence in sugarcane. Phytoparasitica 43: 339–353.
Du, Y.-C., A. Nose, A. Kondo, and K. Wasano. 2000. Diurnal changes in photosynthesis in sugarcane leaves. II. Enzyme activities and metabolite levels relating to sucrose and starch metabolism. Plant Production Science 3: 9–16.
Gray, J., D. Caparrós-Ruiz, and E. Grotewold. 2012. Grass phenylpropanoids: regulate before using! Plant Science 184: 112–120.
Hill, C.B., J.D. Taylor, J. Edwards, et al. 2013. Whole-genome mapping of agronomic and metabolic traits to identify novel quantitative trait loci in bread wheat grown in a water-limited environment. Plant Physiology 162: 1266–1281.
Hu, C., J. Shi, S. Quan, et al. 2014. Metabolic variation between japonica and indica rice cultivars as revealed by non-targeted metabolomics. Scientific Reports 4: 5067.
Koch, K. 2004. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Current Opinion in Plant Biology 7: 235–246.
Lastdrager, J., J. Hanson, and S. Smeekens. 2014. Sugar signals and the control of plant growth and development. Journal of Experimental Botany 65: 799–807.
Ma, J., M. Hanssen, K. Lundgren, et al. 2011. The sucrose-regulated Arabidopsis transcription factor bZIP11 reprograms metabolism and regulates trehalose metabolism. New Phytologist 191: 733–745.
Macoy, D., W.-Y. Kim, S. Lee, and M. Kim. 2015. Biotic stress related functions of hydroxycinnamic acid amide in plants. Journal of Plant Biology 58: 156–163.
Marquardt, A., G. Scalia, P. Joyce, J. Basnayake, and F. C. Botha. 2016. Changes in photosynthesis and carbohydrate metabolism in sugarcane during the development of Yellow Canopy Syndrome (YCS). Functional Plant Biology 43: 523–533.
Martins, M.C.M., M. Hejazi, J. Fettke, et al. 2013. Feedback inhibition of starch degradation in arabidopsis leaves mediated by trehalose 6-phosphate. Plant Physiology 163: 1142–1163.
Morkunas, I., and L. Ratajczak. 2014. The role of sugar signaling in plant defense responses against fungal pathogens. Acta Physiologiae Plantarum 36: 1607–1619.
Payyavula, R.S., R.K. Singh, and D.A. Navarre. 2013. Transcription factors, sucrose, and sucrose metabolic genes interact to regulate potato phenylpropanoid metabolism. Journal of Experimental Botany 64: 5115–5131.
Rajcan, I., and M. Tollenaar. 1999. Source:sink ratio and leaf senescence in maize: II. Nitrogen metabolism during grain filling. Field Crops Research 60: 255–265.
Rodziewicz, P., B. Swarcewicz, K. Chmielewska, A. Wojakowska, and M. Stobiecki. 2014. Influence of abiotic stresses on plant proteome and metabolome changes. Acta Physiologiae Plantarum 36: 1–19.
Roessner, U., A. Luedemann, D. Brust, et al. 2001. Metabolic profiling allows comprehensive phenotyping of genetically or environmentally modified plant systems. Plant Cell 13: 11–29.
Rolland, F., B. Moore, and J. Sheen. 2002. Sugar sensing and signaling in plants. Plant Cell 14: S185–S205.
Schauer, N., and A.R. Fernie. 2006. Plant metabolomics: Towards biological function and mechanism. Trends in Plant Science 11: 508–516.
Shahri, W., S. Sabhi Ahmad, and I. Tahir. 2015. Sugar signaling in plant growth and development. In Plant signaling: Understanding the molecular crosstalk, ed. K.R. Hakeem, R.U. Rehman, and I. Tahir, 93–116. New Dehli: Springer.
Slewinski, T.L., R.F. Baker, A. Stubert, and D.M. Braun. 2012. Tie-dyed2 encodes a callose synthase that functions in vein development and affects symplastic trafficking within the phloem of maize leaves. Plant Physiology 160: 1540–1550.
Smeekens, S., J. Ma, J. Hanson, et al. 2010. Sugar signals and molecular networks controlling plant growth. Current Opinion in Plant Biology 13: 273–278.
Tauzin, A.S., and T. Giardina. 2014. Sucrose and invertases, a part of the plant defense response to the biotic stresses. Frontiers in Plant Science. doi:10.3389/fpls.2014.00293.
Thiagarajah, M.R., L.A. Hunt, and J.D. Mahon. 1981. Effects of position and age on leaf photosynthesis in corn (Zea mays). Canadian Journal of Botany 59: 28–33.
Thomas, H. 2013. Senescence, ageing and death of the whole plant. New Phytologist 197: 696–711.
Trethewey, R.N., and A.M. Smith. 2000. Starch metabolism in leaves. In Photosynthesis: Physiology and metabolism, ed. R.C. Leegood, T.D. Sharkey, and S. Caemmerer, 205–231. Dordrecht: Springer.
Weise, S.E., K.J. van Wijk, and T.D. Sharkey. 2011. The role of transitory starch in C3, CAM, and C4 metabolism and opportunities for engineering leaf starch accumulation. Journal of Experimental Botany 62: 3109–3118.
Wingler, A., T.L. Delatte, L.E. O’Hara, et al. 2012. Trehalose 6-phosphate is required for the onset of leaf senescence associated with high carbon availability. Plant Physiology 158: 1241–1251.
Xia, J., I.V. Sinelnikov, B. Han, and D.S. Wishart. 2015. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Research 43: 251–257.
Funding
This study was funded by Sugar Research Australia (Grant Number 2015016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Marquardt, A., Scalia, G., Wathen-Dunn, K. et al. Yellow Canopy Syndrome (YCS) in Sugarcane is Associated with Altered Carbon Partitioning in the Leaf. Sugar Tech 19, 647–655 (2017). https://doi.org/10.1007/s12355-017-0555-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-017-0555-1