Abstract
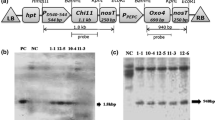
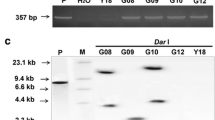
The E. coli mtlD gene encoding mannitol-1-phosphate dehydrogenase has been shown to be involved in the plant response to abiotic stresses such as salinity, drought and chilling. Nevertheless, in this study transgenic sugar beet lines containing the mtlD gene under control of a stress-inducible rd29A promoter were evaluated for their potential of resistance against three fungi including Alternaria alternata, Botrytis cinerea and Cercospora beticola. Southern blot analysis revealed the low copy number (1-3 copies) integration of the transgene in the transgenic lines. Expression profiling by semi-quantitative-RT-PCR analysis showed different levels of cold-inducible expression of mtlD in independent T1 transformants. Mannitol content quantified by HPLC analysis ranged from 1.8 to 3.7 µmol g−1 dry weight in different transgenic lines. Detached leaf bioassay showed that lower transgene expression levels in the transgenic line mt-LS4-32 under non-stressed conditions reduced the disease severity (DS) of A. alternata and B. cinerea by 49 to 87 %, respectively. At the whole-plant level, under non-stressed conditions, the transgenic line showed better performance to A. alternata with a delay of fungal symptom appearance. Following exposure to low temperature (4 °C), the transgenic line showed no fungal infection after 14 days of inoculation. Transgenic lines inoculated with C. beticola significantly (P < 0.001) showed higher resistance than the non-transformed control plants. DS in transgenic lines expressing mtlD gene was zero and 0.05 % compared to 25 % for the control. These results indicate that the mtlD gene can be used as a target to improve plant tolerance to both abiotic and biotic stresses.







Similar content being viewed by others
References
Abebe, T., A.C. Guenzi, B. Martin, and J.C. Cushman. 2003. Tolerance of mannitol-accumulating transgenic wheat to water stress and salinity. Plant Physiology 131: 1748–1755.
Alvarez, M.E., R.I. Pennell, P.J. Meijer, A. Ishikawa, R.A. Dixon, and C. Lamb. 1998. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92: 773–784.
Bauer, M., J. Libantova, J. Moravcikova, and I. Bekesiova. 2003. Transgenic tobacco plants constitutively expressing acidic chitinase from cucumber. Biologia 53: 749–758.
Behnam, B., A. Kikuchi, F. Celebi-Toprak, M. Kasuga, K. Yamaguchi-Shinozaki, and K.N. Watanabe. 2007. Arabidopsis rd29A:DREB1A enhances freezing tolerance in transgenic potato. Plant Cell Reports 26: 1275–1282.
Cai, D.G., M. Kleine, S. Kifle, H.J. Harloff, N.N. Sandal, K.A. Marcker, R.M. KleinLankhorst, E.M.J. Salentijn, W. Lange, W.J. Stiekema, U. Wyss, F.M.W. Grundler, and C. Jung. 1997. Positional cloning of a gene for nematode resistance in sugar beet. Science 275: 832–834.
Celebi-Toprak, F., B. Behnam, G. Serrano, M. Kasuga, K. Yamaguchi-Shinozaki, H. Naka, J.A. Watanabe, S. Yamanaka, and K.N. Watanabe. 2005. Tolerance to salt stress in transgenic tetrasomic tetraploid potato, Solanum tuberosum cv. Desiree appears to be induced by DREB1A gene and rd29A promoter of Arabidopsis thaliana. Breeding Science 55: 311–320.
Chen, T.H.H., and N. Murata. 2002. Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. Current Opinion of Plant Biology 5: 250–257.
Chen, X., and Z. Guo. 2008. Tobacco OPBP1 enhances salt tolerance and disease resistance of transgenic rice. International Journal of Molecular Science 9: 2601–2613.
Chiang, Y.J., C. Stushnoff, A.E. Mesay, M.L. Jones, and H.J. Bohnert. 2005. Overexpression of mannitol-1-phosphate dehydrogenase increases mannitol accumulation and adds protection against chilling injury in petunia. Journal of the American Society for Horticultural Science 130: 605–610.
D’Halluin, K., M. Bossout, M. Bonne, B. Mazur, J. Leemans, and J. Botterman. 1992. Transformation of sugar beet (Beta vulgaris L.) and evaluation of herbicide resistance in transgenic plants. Biotechnology 10: 309–314.
Dalmay, T., A. Hamilton, S. Rudd, S. Angell, and D.C. Baulcombe. 2000. An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 101: 543–553.
Dana, M.M., J.A. Pintor-Toro, and B. Cubero. 2006. Transgenic tobacco plants overexpressing chitinases of fungal origin show enhanced resistance to biotic and abiotic stress agents. Plant Physiology 142: 722–730.
Das, M., H. Chauhan, A. Chhibbar, Q.M.R. Haq, and P. Khurana. 2011. High-efficiency transformation and selective tolerance against biotic and abiotic stress in mulberry, Morus indica cv. K2, by constitutive and inducible expression of tobacco osmotin. Transgenic Research 20: 231–246.
Daub, M.E., and M. Ehrenshaft. 2000. The photoactivated cercospora toxin cercosporin: Contributions to plant disease and fundamental biology. Annual review of Phytopathology 38: 461–490.
Dellaporta, S.L., J. Wood, and J.B. Hicks. 1983. A plant DNA minipreparation version II. Plant Molecular Biology Reporter 1: 19–21.
FAO. 2009. Agribusiness handbook: Sugar beet white sugar.
Finn, T.E., L. Wang, D. Smolilo, N.A. Smith, R. White, et al. 2011. Transgene expression and transgene-induced silencing in diploid and autotetraploid Arabidopsis. Genetics 187: 409–423.
Fondy, B.R., and D.R. Geiger. 1977. Sugar selectivity and other characteristics of phloem loading in Beta vulgaris L. Plant Physiology 59: 953–960.
Fujita, M., Y. Fujita, Y. Noutoshi, F. Takahashi, Y. Narusaka, K. Yamaguchi-Shinozaki, and K. Shinozaki. 2006. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Current Opinion Plant Biology 9: 436–442.
Hema, R., R.S. Vemanna, S. Sreeramulu, C.P. Reddy, M. Senthil-Kumar, and M. Udayakumar. 2014. Stable expression of mtlD gene imparts multiple stress tolerance in Finger Millet. PLoS ONE 9: e99110.
Hobbs, S.L.A., P. Kpodar, and C.M.O. Delong. 1990. The effect of T-DNA copy number, position and methylation on reporter gene expression in tobacco transformants. Plant Molecular Biology 15: 851–864.
Hobbs, S.L.A., T.D. Warkentin, and C.M.O. DeLong. 1993. Transgene copy number can be positively or negatively associated with transgene expression. Plant Molecular Biology 21: 17–26.
Hu, L., H. Lu, Q. Liu, X. Chen, and X. Jiang. 2005. Overexpression of mtlD gene in transgenic Populus tomentosa improves salt tolerance through accumulation of mannitol. Tree Physiology 25: 1273–1281.
Ivic, S.D., R.C. Sicher, and A.C. Smigocki. 2001. Growth habit and sugar accumulation in sugar beet (Beta vulgaris L.) transformed with a cytokinin biosynthesis gene. Plant Cell Reports 20: 770–773.
Jafari, M., P. Norouzi, M.A. Malboobi, B. Ghareyazie, M. Valizadeh, S.A. Mohammadi, and M. Mousavi. 2009. Enhanced resistance to a lepidopteran pest in transgenic sugar beet plants expressing synthetic cry1Ab gene. Euphytica 165: 333–344.
Jayaraj, J., and Z.K. Punja. 2007. Combined expression of chitinase and lipid transfer protein genes in transgenic carrot plants enhances resistance to foliar fungal pathogens. Plant Cell Reports 26: 1539–1546.
Jennings, D.B., M.E. Daub, D.M. Pharr, and J.D. Williamson. 2002. Constitutive expression of a celery mannitol dehydrogenase in tobacco enhances resistance to the mannitol-secreting fungal pathogen Alternaria alternata. Plant Journal 32: 41–49.
Karakas, B., P. Ozias-Akins, C. Stushnoff, M. Suefferheld, and M. Rieger. 1997. Salinity and drought tolerance of mannitol accumulation transgenic tobacco. Plant Cell Environment 20: 609–616.
Kasuga, M., S. Miura, K. Shinozaki, and K. Yamaguchi-Shinozaki. 2004. A combination of the Arabidopsis DREB1A gene and stress inducible rd29A promoter improved drought- and low-temperature stress tolerance in tobacco by gene transfer. Plant Cell Physiology 45: 346–350.
Khare, N., D. Goyary, N.K. Singh, P. Shah, M. Rathore, S. Anandhan, D. Sharma, M. Arif, and Z. Ahmed. 2010. Transgenic tomato cv. Pusa Uphar expressing a bacterial mannitol-1-phosphate dehydrogenase gene confers abiotic stress tolerance. Plant Cell Tissue Organ Culture 103: 267–277.
Lamb, C., and R.A. Dixon. 1997. The oxidative burst in plant disease resistance. Annual review of plant physiology and plant molecular biology 48: 251–275.
Lechtenberg, B., D. Schuberty, A. Forsbachz, M. Gils, and R. Schmidt. 2003. Neither inverted repeat T-DNA configurations nor arrangements of tandemly repeated transgenes are sufficient to trigger transgene silencing. Plant Journal 34: 507–551.
Lennefors, B.L., E.I. Savenkov, J. Bensefelt, E. Wremerth-Weich, P. Roggen, and S. Tuvesson. 2006. dsRNA-mediated resistance to Beet Necrotic Yellow Vein Virus infections in sugar beet (Beta vulgaris L.). Molecular Breeding 18: 313–325.
Maheswari, M., Y. Varalaxmi, A. Vijayalakshmi, S.K. Yadav, P. Sharmila, B. Venkateswarlu, M. Vanaja, and P. Pardha Saradhi. 2010. Metabolic engineering using mtlD gene enhances tolerance to water deficit and salinity in sorghum. Biologia Plantarum 54: 647–652.
Mannerlof, M., B.L. Lennerfors, and P. Tenning. 1996. Reduced titer of BNYVV in transgenic sugar beets expressing the BNYVV coat protein. Euphytica 90: 293–299.
Mannerlof, M., S. Tuvesson, P. Steen, and P. Tenning. 1997. Transgenic sugar beet tolerant to glyphosate. Euphytica 94: 83–91.
Matzke, M.A., and A.J.M. Matzke. 1995. How and why do plants inactivate homologous (trans)genes? Plant Physiology 107: 679–685.
McCabe, M.S., U.B. Mohapatra, S.C. Debnath, J.B. Power, and M.R. Davey. 1999. Integration, expression and inheritance of two linked T-DNA marker genes in transgenic lettuce. Molecular Breeding 5: 329–344.
Owens, L.D., and T.M. Heutte. 1997. A single amino acid substitution in the antimicrobial defense protein cecropin B is associated with diminished degradation by leaf intercellular fluid. Molecular Plant Microbe Interaction 10: 525–528.
Prabhavathi, V., and M.V. Rajam. 2007. Mannitol-accumulating transgenic eggplants exhibitenhanced resistance to fungal wilts. Plant Science 173: 50–54.
Prabhavathi, V., J.S. Yadav, and P.A. Kumar. 2002. Abiotic stress tolerance in transgenic eggplant (Solanum melongena L.) by introduction of bacterial mannitol phospho dehydrogenase gene. Molecular Breeding 9: 137–147.
Rahnama, H., H. Vakilian, H. Fahimi, and B. Ghareyazie. 2011. Enhanced salt stress tolerance in transgenic potato plants (Solanum tuberosum L.) expressing a bacterial mtlD gene. Acta Physiologia Plantarum 33: 1521–1532.
Ruperez, P., and G. Toledano. 2003. Celery by-products as a source of mannitol. European Food Research and Technology 216: 224–226.
Salmeron, J.M., and B. Vernooij. 1998. Transgenic approaches to microbial disease resistance in crop plants. Current Opinion Plant Biology 1: 347–352.
Shane, W.W., and P.S. Teng. 1992. Impact of Cercospora leaf spot on root weight, sugar yield, and purity of Beta vulgaris. Plant Disease 76: 812–820.
Sharma, P., A.B. Jha, R.S. Dubey, and M. Pessarakli. 2012. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. Journal of Botany. doi:10.1155/2012/217037.
Shen, B., G. Jensen, and H.J. Bohnert. 1997. Increased resistance to oxidative stress in transgenic plants by targeting mannitol biosynthesis to chloroplast. Plant Physiology 113: 1177–1183.
Smirnoff, N. 1998. Plant resistance to environmental stress. Current Opinion in Biotechnology 9: 214–219.
Smirnoff, N., and Q.J. Cumbes. 1989. Hydroxyl radical-scavenging activity of compatible solutes. Phytochemistry 28: 1057–1060.
Snyder, G.W., J.C. Ingersoll, and A.C. Smigocki. 1999. Introduction of pathogen defense genes and a cytokinin biosynthesis gene into sugar beet (Beta vulgaris L.) by Agrobacterium or particle bombardment. Plant Cell Reports 18: 829–834.
Staerkel, C., M.J. Boenisch, C. Kroger, J. Bormann, W. Schafer, and D. Stahl. 2013. CbCTB2, an O-methyltransferase is essential for biosynthesis of the phytotoxin cercosporin and infection of sugar beet by Cercospora beticola. BMC Plant Biology 13: 50.
Stoop, J.M.H., J.D. Williamson, and D.M. Pharr. 1996. Mannitol metabolism in plants: a method for coping with stress. Trends in Plant Science 1: 139–144.
Su, J., P.L. Chen, and R. Wu. 1999. Transgene expression of mannitol-1-phosphate dehydrogenase enhanced the salt stress tolerance of the transgenic rice seedlings. Scientific Agriculture Sinica 32: 101–103.
Tang, W. 2002. Regeneration of transgenic Ioblolly pine expressing genes for salt tolerance. Journal of Forestry Research 13: 1–6.
Tang, W., X. Peng, and R.J. Newton. 2005. Enhanced tolerance to salt stress in transgenic loblolly pine simultaneously expressing two genes, encoding mannitol-1-phosphate dehydrogenase and lucitol-6-phosphate dehydrogenase. Plant Physiology and Biochemistry 43: 139–146.
Tarczynski, M.C., R.G. Jensen, and H.J. Bohnert. 1992. Expression of a bacterial mtlD gene in transgenic tobacco leads to production and accumulation of mannitol. Proceedings of the National Academy of Sciences United States of America 89: 2600–2604.
Tarczynski, C., R.G. Jensen, and H.J. Bohnert. 1993. Stress protection of transgenic tobacco by production of the osmolite mannitol. Science 259: 508–510.
Thomas, J.C., M. Sepahi, B. Arendall, and H.J. Bohnert. 1995. Enhancement of seed germination in high salinity by engineering mannitol expression in Arabidopsis thaliana. Plant Cell Environment 18: 801–806.
Waditee, R., N.H. Bhuiyan, E. Hirata, T. Hibino, Y. Tanaka, M. Shikata, and T. Takabe. 2007. Metabolic engineering for betaine accumulation in microbes and plants. Journal of Biological Chemistry 282: 34185–34193.
Wang, H., D. Huang, R. Lu, J. Liu, Q. Qian, and X. Peng. 2000. Salt tolerance of transgenic rice (0ryza sativa L.) with mtlD gene and gutD gene. Chinese Science Bulletin 45: 1685–1690.
Wilhite, S.E., T.C. Elden, V. Puizdar, S. Armstrong, and A.C. Smigocki. 2000. Inhibition of aspartyl and serine proteinases in midgut of sugar beet root maggot with proteinase inhibitors. Entomologia Experimentalis et Applicata 97: 229–233.
Yamaguchi-Shinozaki, K., and K. Shinozaki. 1994. A novel cis acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6: 251–264.
Yao, Q., L. Cong, J.L. Chang, K.X. Li, G.X. Yang, and G.Y. He. 2006. Low copy number gene transfer and stable expression in a commercial wheat cultivar via particle bombardment. Journal of Experimental Botany 57: 3737–3746.
Zhu, B., T.H.H. Chen, and P.H. Li. 1995. Activation of two osmotin like genes by abiotic stimuli and fungal pathogen in transgenic potato plants. Plant Physiology 108: 929–937.
Zimmermann, M.H., and H. Ziegler. 1975. List of sugars and sugar alcohols in sieve-tube exudates. In Encyclopedia of plant physiology, ed. M.H. Zimmermann, and J.A. Milburn, 479–503. New York: Springer.
Acknowledgments
The authors thank the Bioscience and Biotechnology Institute of Urmia University for providing the necessary laboratory facilities. The authors would also like to thank Dr. Y. Ghosta, Dep. of Plant Protection, University of Urmia, Urmia, Iran, for providing the fungal isolates A. alternata and B. cinerea.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goudarzi, A., Jafari, M., Safaie, N. et al. Transgenic Sugar Beet Expressing a Bacterial Mannitol-1-Phosphate Dehydrogenase (mtlD) Gene Shows Enhanced Resistance to Fungal Pathogens. Sugar Tech 18, 192–203 (2016). https://doi.org/10.1007/s12355-015-0379-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-015-0379-9