Abstract
Background
Additional strategies are needed to refine the referral for diagnostic testing of symptomatic patients with suspected coronary artery disease (CAD). We aimed to compare various models to predict hemodynamically obstructive CAD.
Methods and results
Symptomatic patients with suspected CAD who underwent coronary artery calcium scoring (CACS) and sequential coronary computed tomography angiography (CCTA) and [15O]H2O positron emission tomography (PET) myocardial perfusion imaging were analyzed. Obstructive CAD was defined as a suspected coronary artery stenosis on CCTA with myocardial ischemia on PET (absolute stress myocardial perfusion ≤ 2.4 mL/g/min in ≥ 1 segment). Three models were developed to predict obstructive CAD-induced myocardial ischemia using logistic regression analysis: (1) basic model: including age, sex and cardiac symptoms, (2) risk factor model: adding number of risk factors to the basic model, and (3) CACS model: adding CACS to the risk factor model. Model performance was evaluated using discriminatory ability with area under the receiver-operating characteristic curves (AUC). A total of 647 patients (mean age 62 ± 9 years, 45% men) underwent CACS and sequential CCTA and PET myocardial perfusion imaging. Obstructive CAD with myocardial ischemia on PET was present in 151 (23%) patients. CACS was independently associated with myocardial ischemia (P < .001). AUC for the discrimination of ischemia for the CACS model was superior over the basic model and risk factor model (P < .001).
Conclusions
Adding CACS to the model including age, sex, cardiac symptoms and number of risk factors increases the accuracy to predict obstructive CAD with myocardial ischemia on PET in symptomatic patients with suspected CAD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traditionally, myocardial ischemia has been the gatekeeper for invasive coronary angiography and subsequent revascularization.1 However, many symptomatic patients with suspected coronary artery disease (CAD) do not have myocardial ischemia.2,3,4,5 Hence, alternative strategies are warranted in order to improve the referral for ischemia testing of this specific group of patients. Currently, European guidelines recommend physicians to estimate the pre-test probability of obstructive CAD—as a surrogate of myocardial ischemia– using the Diamond-Forrester approach by integrating age, sex and cardiac symptoms.6,7 Additional information on the clinical profile of patients, such as the presence and extent of risk factors for cardiovascular disease and coronary artery calcium (CAC), holds potential to further refine these often overestimating pre-test probabilities of myocardial ischemia.7,8 Coronary artery calcium scoring (CACS) seems particularly desirable since it is easily performed using non-contrast computed tomography (CT), requiring no intravenous contrast, low radiation exposure and lower costs (as compared to contrast-enhanced CT).9 Also, the extent of CACS has been described to correlate well with ischemia.10,11 Nevertheless, the optimal use of CACS in improving the pre-test probability assessment of ischemia has yet to be established in a large contemporary patient cohort.7 Therefore, the present study aimed to compare three models to predict obstructive CAD with myocardial ischemia on positron emission tomography (PET) in symptomatic patients with suspected CAD: (1) a basic model: including age, sex and cardiac symptoms, (2) a risk factor model: adding number of risk factors to the basic model, and (3) a CACS model: adding CACS to the risk factor model.
Methods
Study design and patients
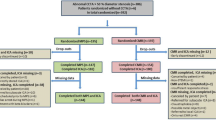
The study population included consecutive symptomatic patients with suspected CAD, who were referred for a PET/CT evaluation at the Turku University Hospital, Turku, Finland between 2007 and 2011. A detailed study design has been previously published.12 Of those enrolled, 717 patients underwent (1) CACS and (2) sequential coronary computed tomography angiography (CCTA) and [15O]H2O PET myocardial perfusion imaging to detect potential myocardial ischemia. The ethics committee of the Hospital District of South-West Finland approved the study protocol and waived the need for patients’ written informed consent. The study complied with the principles of the Declaration of Helsinki. Patients with unavailable data on cardiac symptoms (n = 25) or who failed to follow the sequential protocol (n = 45) were excluded. Hence, the present study consisted of 647 patients (Figure 1).
Image acquisition and analysis
Patients were scanned using a hybrid 64-detector row PET/CT scanner (GE Discovery VCT or GE D690, General Electric Medical Systems, Waukesha, Wisconsin). Protocols regarding image acquisition and analysis have been reported in detail.12,13
CACS
CACS was calculated from non-contrast CT scans according to the Agatston algorithm.14 Scores were categorized into 0, 1–99, 100–399 and ≥ 400.
Sequential CCTA and PET myocardial perfusion imaging
CCTA was performed using intravenous low-osmolar iodine (48–155 mL; 320–400 mg/mL) as a contrast agent.12,13 Prior to acquisition, intravenous metoprolol (0–30 mg) was administered to achieve heart rates < 60/min. Sublingual nitroglycerin (800 μg) or isosorbide dinitrate (1.25 mg) was administered to achieve maximal coronary vasodilatation. Subsequently, according to study design, all patients with a suspected obstructive stenosis ≥ 50% on CCTA by visual inspection of the attending physician underwent PET myocardial perfusion imaging to detect potential myocardial ischemia. PET myocardial perfusion imaging was performed using dynamic acquisition with [15O]H2O as a radiotracer (mean radioactivity: 1042 ± 117 MBq).12,13 At rest, [15O]H2O (Radiowater Generator, Hidex Oy, Finland) was intravenously injected over 15 s.13 For stress, adenosine (rate: 140 µg/kg/min) was infused 2 min before the stress scan to induce maximal vasodilation. Patients received instructions to avoid caffeine 24 h prior to the scan, considering its interaction with adenosine. Stress scans were quantitatively analyzed according to the 17-segment American Heart Association model using dedicated software (Carimas version 1.1.0, Turku, Finland) by an experienced physician, blinded to clinical or other data.15,16 Absolute stress myocardial perfusion was generated in mL/g/min for the segments and left ventricle as a whole (not for all).
Obstructive CAD-induced myocardial ischemia
The reference standard for myocardial ischemia was defined as an absolute stress myocardial perfusion ≤ 2.4 mL/g/min in ≥ 1 segment on PET.12 PET myocardial perfusion imaging was not performed in patients without a suspected obstructive stenosis on CCTA by study design. This specific group was considered to not have obstructive CAD-induced myocardial ischemia.
Statistical analysis
Normally and non-normally distributed continuous data are presented as means ± standard deviations (SD) and medians with interquartile ranges (IQR), respectively. Categorical data are presented as frequencies with percentages. First, comparisons of continuous data were performed with the Independent-Samples T test, Mann–Whitney U test, one-way analysis of variance or Kruskal–Wallis test, as appropriate. Comparisons of categorical data were performed using the χ2 test. Also, the Diamond-Forrest approach was applied to visualize the distribution of obstructive CAD with myocardial ischemia among patients according to age, sex and cardiac symptoms.6,7 Additionally, negative predictive values (NPV) and positive predictive values (PPV) were calculated with different cut-points of CACS. Second, models were developed for the prediction of obstructive CAD-induced myocardial ischemia using logistic regression analysis. Uni- and multivariate logistic regression analysis was performed to assess the association between selected variables versus myocardial ischemia. In a stepwise manner, three prediction models were defined: (1) basic model: including age, sex and cardiac symptoms, (2) risk factor model: adding number of risk factors to the basic model, and (3) CACS model: adding CACS to the risk factor model. Measures of association were expressed as odds ratios (OR) with 95% confidence intervals (CI). Goodness of model fit was compared with the likelihood ratio test. Third, performance of the models was evaluated using discriminatory ability. Discriminatory ability was assessed using area under the receiver-operating characteristic curves (AUC), integrated discrimination improvement (IDI) and net reclassification improvement (NRI). AUCs were compared with the DeLong's test.17,18 A two-sided P-value of < .05 was considered statistically significant, and all statistical analyses were performed with R (version 3.0.3, R Development Core Team, Vienna, Austria), SPSS software (version 26, SPSS IBM Corp., Armonk, New York) and MedCalc software (version 19.2.0, Ostend, Belgium).
Results
Patients
Baseline characteristics of the patients are shown in Table 1. In total, 647 patients (mean age 62 ± 9 years, 45% men) underwent CACS and sequential CCTA and [15O]H2O PET myocardial perfusion imaging for ischemia assessment. CCTA ruled out an obstructive stenosis in 338 patients; they were considered to not have obstructive CAD-induced myocardial ischemia (and did not undergo PET myocardial perfusion imaging by the sequential study design) (Figure 1). CCTA revealed a suspected obstructive stenosis in 309 patients. Obstructive CAD with myocardial ischemia on PET was present in 151 (23% out of 647) patients. Patients with myocardial ischemia were older (63 ± 8 years vs. 61 ± 10 years, P = .002), more often male (72% vs. 37%, P < .001) and presented more frequently with typical angina (37% vs. 22%, P < .001) as compared to patients without ischemia. In addition, patients with myocardial ischemia had more risk factors for cardiovascular disease (P < .001) and used more medications (P ≤ .007). The distribution of ischemia among patients based on the Diamond-Forrester approach according to age, sex and cardiac symptoms was demonstrated in Supplemental Table 1.
Imaging findings
CACS
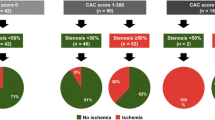
Median CACS of the patients was 32 (IQR 0–281) (Table 2). In total, 225 (35%) and 422 (65%) patients had CACS = 0 and CACS ≥ 1, respectively. Patients with obstructive CAD-induced myocardial ischemia had a higher CACS as compared to patients without ischemia (422 (IQR 117–1047) vs. 5 (IQR 0–136), P < .001). The majority of patients with ischemia had CACS ≥ 400 (53%). Moreover, the frequency of ischemia increased with higher CACS categories: 2% for CACS = 0, 17% for CACS = 1–99, 30% for CACS = 100–399 and 64% for CACS ≥ 400 (P < .001) (Figure 2). Consequently, the NPV of CACS = 0 was 97.8% (95% CI 94.9–99.1%) and this value slightly varied according to the cardiac symptoms at presentation: 98.5% for patients with non-anginal pain or atypical angina, 97.6% for patients with typical angina and 96.0% for patients with dyspnea at exertion (Fig. 3). Conversely, the PPV of CACS ≥ 1 was only 34.6% (95% CI 32.7–36.5%) and also differed according to symptomatic status: 29.7% for patients with non-anginal or atypical angina, 45.5% for patients with typical angina and 31.1% for patients with dyspnea at exertion. When the cut-point was set at CACS < 100 versus CACS ≥ 100, NPV and PPV were 91.6% (95% CI 88.9–93.7%) and 46.5% (95% CI 42.4–50.6%), respectively.
Bar graphs of obstructive CAD-induced myocardial ischemia by CACS. CACS, coronary artery calcium scoring; CAD, coronary artery disease; PET, positron emission tomography. Definitions: *In patients with obstructive CAD-induced myocardial ischemia, a median of 5 segments (IQR 1–13 segments) for CACS = 0, 6 segments (IQR 3–12 segments) for CACS = 1–99, 7 segments (IQR 4–12 segments) for CACS = 100–399 and 12 segments (IQR 6–16 segments) for CACS ≥ 400 was affected
Sequential CCTA and PET myocardial perfusion imaging
Details regarding sequential CCTA and PET myocardial perfusion imaging are shown in Figure 1. In patients with obstructive CAD-induced myocardial ischemia, a median of 10 segments (IQR 5–15 segments) was affected. Patients with myocardial ischemia had a reduced global stress myocardial perfusion as compared to patients without ischemia on PET (2.3 ± .7 mL/g/min vs. 3.9 ± .9 mL/g/min, P < .001) (Table 2).
Prediction of obstructive CAD with myocardial ischemia
Model development using logistic regression analysis
In the univariable analysis, age, male sex, typical angina, all individual cardiac risk factors (except for family history of CAD) and the number of risk factors per-patient were each associated with obstructive CAD-induced myocardial ischemia (P ≤ .005). Furthermore, CACS was a significant univariable predictor of myocardial ischemia, both as a continuous (P < .001) and categorized score (P < .001) (Table 3). In the multivariable analysis, prediction models of ischemia were defined using a stepwise approach: (1) basic model: including age, sex and cardiac symptoms, (2) risk factor model: adding number of risk factors to the basic model, and (3) CACS model: adding CACS to the risk factor model (Table 4). In the CACS model, male sex (OR 4.686 (95% CI 2.921–7.518), P < .001), typical angina (OR 4.555 (1.636–12.682), P = .004), dyspnea at exertion (OR 3.026 (95% CI 1.078–8.495), number of risk factors (OR 1.461 (95% CI 1.191–1.793), P < .001) and CACS (OR 1.002 (95% CI 1.001–1.002), P < .001) remained independently associated with myocardial ischemia. Importantly, adding CACS to the risk factor model resulted in a significantly better fit of the model (χ2 = 200 vs. χ2 = 126, P < .001).
Model performance using discriminatory ability
AUC for the discrimination of obstructive CAD with myocardial ischemia was .746 (95% CI .701–.791) for the basic model, .790 (95% CI .751–.830) for the risk factor model and .849 (95% CI .813–.884) for the CACS model (Figure 4). The CACS model had a significantly better discriminatory ability than the basic model (P < .001) and risk factor model (P < .001). Also, the CACS model provided incremental predictive information over the basic model (IDI = .176, P < .001 and NRI = .633, P < .001) and risk factor model (IDI = .125, P < .001 and NRI = .440, P < .001) (Supplemental Table 2).
Discussion
The present study evaluated 647 symptomatic patients with suspected CAD from a large contemporary patient cohort, who underwent CACS and sequential CCTA and [15O]H2O PET myocardial perfusion imaging for ischemia assessment. We compared three models to predict obstructive CAD with myocardial ischemia on PET: (1) a basic model, (2) a risk factor model and (3) a CACS model. CACS was strongly and independently associated with myocardial ischemia. Moreover, by incorporating CACS into the pre-test probability assessment, the discrimination of ischemia significantly improved compared to the basic model and risk factor model. These findings suggest a possible role for routine CACS detection in symptomatic patients in order to refine referral for ischemia testing, by either triaging them away from (in case of low CACS) or towards (in case of high CACS) this test. Particularly, the NPV of CACS = 0 was excellent (97.8%) irrespective of the cardiac symptoms at presentation (96.0–98.5%). Our approach is an example of the stepwise application of non-invasive imaging tests, which in turn could lead to more cost-effective care.
CACS in asymptomatic patients: preventative care
Anatomical imaging with CACS has been initially introduced as a screening tool for CAD in asymptomatic patients with the aim of improving cardiovascular risk assessment and guiding primary preventative care.19,20,21 Regarding cardiovascular risk assessment, various large long-term population-based studies have uniformly reported on the association between CACS and major adverse cardiac events in asymptomatic patients without known CAD.22,23,24,25 Especially, a CACS = 0 has been linked to a very low risk of adverse events (power of zero).23,26,27 Regarding preventative care strategies, it has been clearly demonstrated that a CACS = 0 can reclassify a large subset of asymptomatic patients (44%) in whom statins would have been otherwise considered or recommended (atherosclerotic cardiovascular disease risk score ≥ 5%) according to existing guidelines.28
CACS in symptomatic patients: ischemia
On the other hand, anatomical imaging in symptomatic patients with suspected CAD has the aim to identify hemodynamically obstructive CAD (coronary artery stenosis ≥ 50%) that causes ischemia.29 Few studies have reported on the association between CACS and myocardial ischemia on PET in symptomatic patients with suspected CAD.30,31,32 Schenker et al. analyzed 695 symptomatic patients with suspected CAD, who underwent CACS and PET myocardial perfusing imaging using a hybrid PET/CT scanner.30 In line with our results, a stepwise increase was demonstrated in the frequency of myocardial ischemia with increasing CACS (16% for CACS = 0 to 49% for CACS ≥ 1000). Furthermore, adding CACS to a model including age, sex, cardiac symptoms and risk factors improved the discrimination of ischemia significantly (AUC .72 vs. AUC .67, P < .001). Likewise, Esteves et al. evaluated 84 symptomatic patients with a low-intermediate likelihood of CAD, who were admitted to the chest pain unit and underwent CACS plus myocardial ischemia testing with PET.31 Applying this strategy, a strong association was shown between CACS = 0 and the absence of myocardial ischemia, yielding a negative predictive value of 100%. Again, these results were overall highly consistent with the findings in the current study, showing only a 2% prevalence of myocardial ischemia in patients with CACS = 0. Similar findings were derived from studies using single photon emission computed tomography as the reference standard for myocardial ischemia.11,29 However, it should be noted that PET has enhanced diagnostic performance over single photon emission computed tomography, in particular when myocardial perfusion is quantitatively analyzed.33 Additionally, all latest generation PET scanners are combined with a CT scanner into a hybrid system, of which the low-dose non-gated CT transmission scan can be used to not only perform attenuation correction of the PET images but also to perform visual assessment of CAC.34,35 With the rapid development of artificial intelligence with sophisticated algorithms, this approach holds potential for the automated assessment of CAC from non-gated CT scans.36,37
Limitations
Some limitations of the present study need to be addressed. First, our study had a retrospective observational design with limitations such as (unmeasured) confounding factors and selection bias. For instance, of those enrolled in the registry, CACS was performed per protocol in all patients for risk stratification purposes, but not analyzed in some patients due to logistical or technical reasons.12 Second, PET myocardial perfusion imaging was not performed in patients without suspected obstructive stenosis on CCTA according to study design. Absence of myocardial ischemia in this specific group of patients was therefore assumption-based, but in line with published literature.31 Nevertheless, we acknowledge that diffuse, heterogenous CAD or microvascular dysfunction could have contributed to downstream myocardial perfusion abnormalities.38,39,40,41 Unfortunately, we were not able to analyze this in detail due to the sequential design of the study. Third, PET myocardial perfusion findings were solely interpreted on a per-patient basis, since CACS was not available on a per-vessel basis. Lastly, utilizing CACS as a gatekeeper to ischemia testing still needs prospective and randomized data. However, our study adds to the wealth of data suggesting that patients with CACS = 0 are at low risk (but not risk free). To this end it should be emphasized that clinical decisions should always be individualized.
New knowledge gained
The stepwise application of non-invasive imaging tests, including an initial CACS, can potentially refine the referral for ischemia testing of symptomatic patients with CAD.
Conclusion
In symptomatic patients with suspected CAD, a CACS model including age, sex, cardiac symptoms, number of risk factors and CACS allows for accurate and superior prediction of obstructive CAD with myocardial ischemia on PET.
Abbreviations
- AUC:
-
Area under the receiver-operating characteristic curve
- CAC:
-
Coronary artery calcium
- CACS:
-
Coronary artery calcium scoring
- CAD:
-
Coronary artery disease
- CCTA:
-
Coronary computed tomography angiography
- CT:
-
Computed tomography
- IDI:
-
Integrated discrimination improvement
- NPV:
-
Negative predictive value
- NRI:
-
Net reclassification improvement
- PET:
-
Positron emission tomography
- PPV:
-
Positive predictive value
References
Neumann F-J, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J 2019;40:87‐165.
Tonino PA, Fearon WF, De Bruyne B, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol 2010;55:2816‐21.
Park SJ, Kang SJ, Ahn JM, et al. Visual-functional mismatch between coronary angiography and fractional flow reserve. JACC Cardiovasc Interv 2012;5:1029‐36.
Layland J, Oldroyd KG, Curzen N, et al. Fractional flow reserve vs. angiography in guiding management to optimize outcomes in non-ST-segment elevation myocardial infarction: the British Heart Foundation FAMOUS-NSTEMI randomized trial. Eur Heart J 2015;36:100‐11.
Ahmadi A, Stone GW, Leipsic J, et al. Association of coronary stenosis and plaque morphology with fractional flow reserve and outcomes. JAMA Cardiol 2016;1:350‐7.
Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical-diagnosis of coronary-artery disease. New Engl J Med 1979;300:1350‐8.
Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J 2020;41:407‐77.
Winther S, Bottcher M, Jorgensen HS, et al. Coronary calcium score may replace cardiovascular risk factors as primary risk stratification tool before kidney transplantation. Transplantation 2016;100:2177‐87.
Gerber TC, Carr JJ, Arai AE, et al. Ionizing radiation in cardiac imaging: a science advisory from the American Heart Association Committee on Cardiac Imaging of the Council on Clinical Cardiology and Committee on Cardiovascular Imaging and Intervention of the Council on Cardiovascular Radiology and Intervention. Circulation 2009;119:1056‐65.
Berman DS, Wong ND, Gransar H, et al. Relationship between stress-induced myocardial ischemia and atherosclerosis measured by coronary calcium tomography. J Am Coll Cardiol 2004;44:923‐30.
He ZX, Hedrick TD, Pratt CM, et al. Severity of coronary artery calcification by electron beam computed tomography predicts silent myocardial ischemia. Circulation 2000;101:244‐51.
Maaniitty T, Stenstrom I, Bax JJ, et al. Prognostic value of coronary CT angiography with selective PET perfusion imaging in coronary artery disease. JACC Cardiovasc Imaging 2017;10:1361‐70.
Kajander S, Joutsiniemi E, Saraste M, et al. Cardiac positron emission tomography/computed tomography imaging accurately detects anatomically and functionally significant coronary artery disease. Circulation 2010;122:603‐13.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827‐32.
Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 2002;105:539‐42.
Nesterov SV, Han C, Maki M, et al. Myocardial perfusion quantitation with 15O-labelled water PET: high reproducibility of the new cardiac analysis software (Carimas). Eur J Nucl Med Mol Imaging 2009;36:1594‐602.
Pencina MJ, D’Agostino RB Sr, D’Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157‐72.
Harrell FE, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA-J Am Med Assoc 1982;247:2543‐6.
Sangiorgi G, Rumberger JA, Severson A, et al. Arterial calcification and not lumen stenosis is highly correlated with atherosclerotic plaque burden in humans: a histologic study of 723 coronary artery segments using nondecalcifying methodology. J Am Coll Cardiol 1998;31:126‐33.
Detrano R, Guerci AD, Carr JJ, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 2008;358:1336‐45.
Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol 2018;72:434‐47.
Bild DE, Detrano R, Peterson D, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2005;111:1313‐20.
Erbel R, Mohlenkamp S, Moebus S, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol 2010;56:1397‐406.
Vliegenthart R, Oudkerk M, Hofman A, et al. Coronary calcification improves cardiovascular risk prediction in the elderly. Circulation 2005;112:572‐7.
Ferencik M, Pencina KM, Liu T, et al. Coronary artery calcium distribution is an independent predictor of incident major coronary heart disease events results from the Framingham Heart Study. Circ-Cardiovasc Imag 2017. https://doi.org/10.1161/CIRCIMAGING.117.006592.
Budoff MJ, Young R, Burke G, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J 2018;39:2401‐8.
Budoff MJ, Mayrhofer T, Ferencik M, et al. Prognostic value of coronary artery calcium in the PROMISE study (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017;136:1993‐2005.
Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association Cholesterol Management Guidelines MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015;66:1657‐68.
Bavishi C, Argulian E, Chatterjee S, Rozanski A. CACS and the frequency of stress-induced myocardial ischemia during MPI: a meta-analysis. JACC Cardiovasc Imaging 2016;9:580‐9.
Schenker MP, Dorbala S, Hong EC, et al. Interrelation of coronary calcification, myocardial ischemia, and outcomes in patients with intermediate likelihood of coronary artery disease: a combined positron emission tomography/computed tomography study. Circulation 2008;117:1693‐700.
Esteves FP, Sanyal R, Santana CA, Shaw L, Raggi P. Potential impact of noncontrast computed tomography as gatekeeper for myocardial perfusion positron emission tomography in patients admitted to the chest pain unit. Am J Cardiol 2008;101:149‐52.
Fathala A, AlJefri A, Alsugair A, Abouzied M. Coronary artery calcification detected by PET/CT scan as a marker of myocardial ischemia/coronary artery disease. Nucl Med Commun 2011;32:273‐8.
Gulati M, Levy PD, Mukherjee D, et al. AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021. https://doi.org/10.1161/CIR.0000000000001029.
Di Carli MF. Integrating coronary artery calcium and functional imaging: redundant or complementary? JACC Cardiovasc Imaging 2021;14:2453.
Einstein AJ, Johnson LL, Bokhari S, et al. Agreement of visual estimation of coronary artery calcium from low-dose CT attenuation correction scans in hybrid PET/CT and SPECT/CT with standard Agatston score. J Am Coll Cardiol 2010;56:1914‐21.
Isgum I, de Vos BD, Wolterink JM, et al. Automatic determination of cardiovascular risk by CT attenuation correction maps in Rb-82 PET/CT. J Nucl Cardiol 2018;25:2133‐42.
Lessmann N, van Ginneken B, Zreik M, et al. Automatic calcium scoring in low-dose chest CT using deep neural networks with dilated convolutions. IEEE Trans Med Imaging 2018;37:615‐25.
Gould KL, Nakagawa Y, Nakagawa K, et al. Frequency and clinical implications of fluid dynamically significant diffuse coronary artery disease manifest as graded, longitudinal, base-to-apex myocardial perfusion abnormalities by noninvasive positron emission tomography. Circulation 2000;101:1931‐9.
De Bruyne B, Hersbach F, Pijls NH, et al. Abnormal epicardial coronary resistance in patients with diffuse atherosclerosis but “Normal” coronary angiography. Circulation 2001;104:2401‐6.
Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol 2013;62:1639‐53.
Gould KL, Johnson NP. Coronary physiology beyond coronary flow reserve in microvascular angina: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:2642‐62.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Wang is supported by a research grant from the University of Turku. Dr. Saraste received speaker or consultancy fees from Amgen, Abbott, Astra Zeneca, Bayer, Boehringer Ingelheim and Pfizer. Dr. Knuuti received consultancy fees from GE Healthcare and AstraZeneca and speaker fees from GE Healthcare, Bayer, Lundbeck, Boehringer Ingelheim, Pfizer and Merck, outside of the submitted work. Dr. Bax received speaker fees from Abbot Vascular and Edwards Lifesciences. The Department of Cardiology, Leiden University Medical Center, Leiden, the Netherlands has received unrestricted research grants from Bayer, Abbott Vascular, Medtronic, Biotronik, Boston Scientific, GE Healthcare and Edwards Lifesciences. The remaining authors have no relevant disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com
The authors have also provided an audio summary of the article, which is available to download as ESM, or to listen to via the JNC/ASNC Podcast.
Funding: Dr. Butcher received funding from European Society of Cardiology (ESC Research Grant App000080404).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van den Hoogen, I.J., Wang, X., Butcher, S.C. et al. Incorporating coronary artery calcium scoring in the prediction of obstructive coronary artery disease with myocardial ischemia: a study with sequential use of coronary computed tomography angiography and positron emission tomography imaging. J. Nucl. Cardiol. 30, 178–188 (2023). https://doi.org/10.1007/s12350-022-03132-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-022-03132-z