Abstract
Background
Imaging-based measures of atherosclerosis such as coronary artery calcium score (CACS) and coronary flow reserve (CFR) as well as carotid atherosclerotic plaque burden (cPB) are predictors of cardiovascular events in the general population. The objective of this study was to correlate CACS, cPB, myocardial blood flow (MBF), and CFR in patients with end-stage renal disease (ESRD).
Methods and results
39 patients (mean age 53 ± 12 years) with ESRD prior to kidney transplantation were enrolled. MBF and CFR were quantified at baseline and under hyperemia by 13N-NH3-PET/CT. CACS was calculated from low-dose CT scans acquired for PET attenuation correction. cPB was assessed by 3D ultrasound. Uni- and multivariate regression analyses between these and clinical parameters were performed. Median follow-up time for clinical events was 4.4 years. Kaplan–Meier survival estimates with log-rank test were performed with regards to cardiovascular (CV) events and death of any cause. CACS and cPB were associated in ESRD patients (r = 0.48; p ≤ 0.01). While cPB correlated with age (r = 0.43; p < 0.01), CACS did not. MBFstress was negatively associated with age (r = 0.44; p < 0.01) and time on dialysis (r = 0.42; p < 0.01). There were negative correlations between MBFstress and CACS (r = − 0.62; p < 0.001) and between MBFstress and cPB (r = − 0.43; p < 0.01). Age and CACS were the strongest predictors for MBFstress. CFR was impaired (< 2.0) in eight patients who also presented with higher cPB and higher CACS compared to those with a CFR > 2.0 (p = 0.06 and p = 0.4). In contrast to MBFstress, there was neither a significant correlation between CFR and CACS (r = − 0.2; p = 0.91) nor between CFR and cPB (r = − 0.1; p = 0.55). CV event-free survival was associated with reduced CFR and MBFstress (p = 0.001 and p < 0.001) but not with cPB or CACS.
Conclusions
CACS, cPB, and MBFstress are associated in patients with ESRD. Atherosclerosis is earlier detected by MBFstress than by CFR. CV event-free survival is associated with impaired CFR and MBFstress.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a major risk factor for the development of atherosclerosis and coronary artery disease (CAD) and patients with CKD are at increased risk for myocardial infarction, heart failure, and cardiac death.1,2 The pathophysiological interaction between the kidneys and the heart is termed the cardiorenal syndrome (CRS), with especially high risk for cardiovascular events in end-stage renal disease (ESRD) patients.3 Besides CAD of the large coronary arteries which often remains asymptomatic, microvascular disease, i.e., coronary microvascular dysfunction, is a frequent finding in this high-risk group.4
However, questions remain of how to assess cardiovascular risk in individual ESRD patients and how to identify patients who may benefit from further testing or a coronary intervention prior to transplantation.5,6 In suspected or known CAD the role of non-invasive myocardial perfusion imaging (MPI) in preselecting patients for coronary angiography is well established. Single photon emission computed tomography (SPECT) is a widely used imaging method with good diagnostic accuracy for the detection of relevant epicardial coronary artery disease in CKD patients.7 However, microvascular alterations may be missed by SPECT. Thus, quantitative myocardial perfusion positron emission computed tomography (PET) is the method of choice for quantitative MPI, as it additionally quantifies absolute myocardial blood flow (MBF) and coronary flow reserve (CFR) – increasing diagnostic accuracy and adding prognostic information.4,8,9 Recently, global CFR was proven to be an independent factor for risk stratification for all-cause and cardiovascular mortality in ESRD.4 Another imaging-derived parameter of atherosclerosis is the coronary artery calcium score (CACS), which also showed independent prognostic value in suspected CAD.11,12,–13
Atherosclerosis – as a systemic disease – also causes carotid artery stenosis and peripheral artery disease.10 Apart from the calculation of intima to media thickness,10,14 carotid ultrasound enables the quantitation of carotid artery atherosclerotic plaque burden (cPB).15 cPB was the strongest predictor of coronary artery calcium score (CACS) in asymptomatic, otherwise healthy individuals.16 In the general population, cPB and CACS can serve as imaging biomarkers of future cardiovascular events in asymptomatic adults.16 However, the relation between myocardial perfusion, CACS, and cPB has not yet been studied in ESRD patients. Therefore, the aim of this study was to correlate the results from myocardial perfusion PET with CACS and cPB in a cohort of ESRD patients prior to kidney transplantation and to evaluate if CFR or absolute MBF are better indicators for coronary artery pathology.
Methods
Study Population
39 patients (14 female; 36%), mean age 53 ± 12 years (range 26-69) with ESRD on dialysis [12 peritoneal dialysis (PD); 27 hemodialysis (HD)], were prospectively enrolled in the study prior to kidney transplantation. Exclusion criteria were renal failure in a pre-dialysis state, prior kidney transplantation, or known CAD in patients individual history. All patients were asymptomatic at the time of imaging. Detailed patient characteristics are provided in Table 1.
PET Acquisition
A 128-slice PET/CT scanner (Biograph mCT, Siemens Healthcare) was used for PET imaging. Patients fasted for > 4 hour and refrained from caffeine or theophylline for 24 hour before imaging. A low-dose CT scan of the heart (100 kV, 60 mA with CARE Dose, 0.5 second per rotation, Pitch 1.2; 3 mm reconstructed slice thickness) for attenuation correction of PET data was acquired during shallow breathing. For the rest study 13N-NH3 (352 ± 38 MBq) was given in an intravenous bolus. List-mode 3-dimensional PET images were acquired for 14 minutes. Infusion of adenosine was started through a separate intravenous line (0.14 µg/kg, 6 minutes) and a second dose of 13N-NH3 (354 ± 39 MBq) was infused at the half time of adenosine infusion, followed by a 14 minutes. list-mode acquisition. Quality control of PET and CT for attenuation correction was performed. List-mode data were resampled to attenuation-corrected static, electrocardiographically gated, and dynamic images (17 frames; 12 × 10 seconds; 3 × 20 seconds and 2 × 30 seconds). Emission data were corrected for randoms, dead time, scatter, and attenuation. All images were reconstructed iteratively by a 3D attenuation-weighted ordered-subsets expectation maximization iterative reconstruction algorithm and time-of-flight (OSEM 3D; 2i21s, Gaussian smoothing at 8 mm full-width at half-maximum; zoom of 1).
PET data were analyzed using commercially available software (syngo.PET Myocardial Blood Flow and MI Cardiology; syngo.via; Siemens Healthcare, Germany).
Analysis of Regional Perfusion
Static perfusion images were reangulated to short, horizontal, and vertical long axis slices for visual analysis. Each of the 17 myocardial segments was scored on a scale of 0-4 (normal, mildly, moderately, severely decreased, or absent perfusion) by two independent and experienced readers blinded to other data. Summed stress score (SSS), summed rest score (SRS), and summed difference score (SDS) were calculated. Abnormal perfusion was defined as a SSS ≥ 4. Significant ischemia was defined as a SDS ≥ 3. Coronary angiography was performed in these selected cases.
Quantification of MBF, CFR, and Left Ventricular Function
Absolute MBF (ml·g·min) was calculated from the dynamic stress and rest scans corrected for motion. Automated factor analysis was used to generate arterial input function and myocardial tissue time-activity curves. Hyperemic MBF (MBFstress) was corrected for myocardial 13N-NH3 activity (from the rest study). MBFstress and resting MBF (MBFrest) were calculated by fitting the time-activity curves to a 2-compartment kinetic model as described elsewhere.17 MBFrest was normalized for cardiac work by dividing by the rate–pressure product and multiplying the result by 10.000.18 CFR was determined as the ratio of MBFstress to corrected MBFrest. Stress and resting left ventricular ejection fraction (LVEFstress and LVEFrest) were calculated from the ECG-triggered PET data using the Corridor 4DM software (Invia, Ann Arbor, USA).
Coronary Artery Calcium Scoring
CACS assessment was performed using the low-dose, non-contrast multidetector CT scans for attenuation correction without prospective electrocardiography-gated acquisition. CACS was calculated by the Agatston method accounting for the area of coronary artery calcification in CT (> 130 Hounsfield units, preset value by the calcium scoring software, Siemens Healthcare, Germany) and expressed as Agatston score19 into the following categories: ≤ 100; 101-400, 401-1000 and > 1000.20
3D Carotid Ultrasound
The Philips iU22 ultrasound system (Philips Healthcare, Bothell, Washington) with a dedicated 3D transducer was used. All scans were performed by physicians with advanced experience in head and neck ultrasound imaging. The protocol included 3D imaging of the left and right common carotid artery and its bifurcation as far as assessable. All 3D-ultrasound recordings were analyzed using dedicated software for semiautomatic plaque quantification (Philips QLAB-VPQ). Carotid plaque burden (cPB) was expressed in mm3.16 cPB patients were stratified in the following tertiles: ≤ 154 mm3, 155-344 mm3, and ≥ 345 mm3 (13 patients each).
Follow-Up
Clinical follow-up was performed every 6 months. This included clinical patient interview, laboratory data, and recording of clinical events. End-points were major cardiovascular (CV) events and death from any cause.
Statistical Analysis
Analysis of discrete variables was performed using the Chi squared test. Continuous variables were compared either by a two-sided Student’s t test for unpaired samples or by one-way analysis of variance (ANOVA) with a Bonferroni/Tamhane correction. Univariate and multivariate logistic regression analyses were performed to test for associations between continuous variables. Pearson’s correlation or Spearman-Rho tests were used for the comparison of parametric normally or not normally distributed variables. Variables significantly correlated in the univariate analysis were included in a multivariate stepwise regression model. Two-sided p values < 0.05 were considered significant. Kaplan–Meier survival curves with log-rank test were performed for the analysis of patients survival. Analyses were performed with SPSS software package (IBM SPSS Statistics, Version 22.0. Armonk, NY).
All procedures performed were in accordance with the ethical standards of the institutional and research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments. The study was approved by the institutional review board of the Medical Association of Westfalen-Lippe and the Faculty of Medicine, University of Münster. Informed consent was given by all patients.
Results
Detailed characteristics of the patient cohort are provided in Table 1.
CACS and cPB
22 (56%) individuals had a CACS < 100, eight patients (21%) had a CACS between 101 and 400 and nine patients (23%) had a CACS > 401 (six of which > 1000). CACS was not correlated with age (r = 0.27; p = 0.97; Figure 1A) or with the time on dialysis (r = 0.28; p = 0.08; Figure 1B). CACS did not differ between the PD and HD subgroups (Table 1).
cPB correlated with age (r = 0.43; p = 0.007; Figure. 2A) but not with the time on dialysis (r = 0.18; p = 0.28; Figure. 2B). cPB was significantly higher in the HD as compared to the PD subgroup (Table 1). cPB showed a moderate association with CACS (r = 0.48; p = 0.002).
MBF, CFR, and Left Ventricular Function
MBFrest ranged from 0.7 to 2.2 ml·g·min and MBFstress ranged from 1.6 - 4.6 ml·g·min. Individuals with MBFstress < 2 ml·g·min (N = 3) presented with higher cPB (393 ± 219 vs 268 ± 197 mm3) and higher CACS (2136 ± 1583 vs. 299 ± 671) compared to those with MBFstress > 2 ml/g/min. Mean MBFstress did not differ between patients on PD or HD (Table 1). There was a negative correlation between MBFstress and CACS (r = − 0.62; p < 0.0001; Figure. 3A) and between MBFstress and cPB (r = − 0.43; p = 0.007; Figure. 3B). MBFstress impaired with increasing CACS and cPB, respectively. In contrast, CFR remained stable with increasing CACS and cPB (Table 2), since MBFrest decreased. Moreover, there was a moderate correlation between MBFstress and LVEFrest (r = 0.32; p = 0.05) but there was no correlation between CFR and LVEFrest (r = 0.14; p = 0.94).
MBFstress was negatively associated with age (r = 0.44; p < 0.01) and the time on dialysis (r = 0.42; p < 0.01). MBFstress decreased from 3.16 ± 0.58 ml·g·min. in patients < 1 year on dialysis to 2.46 ± 0.56 ml·g·min. in patients > 3 years on dialysis (p < 0.05; Figure 4A). In contrast, CFR did not change (p = 1.0; Figure. 4B).
CFR ranged from 1.4 to 4.7 in the total cohort. There were also no significant differences between patients on PD or HD (Table 1). CFR was impaired (< 2.0) in eight patients (20%; p = 0.47). These individuals also presented with higher cPB (394 ± 169 vs 247 ± 197 mm3) and a higher CACS (679 ± 1119 vs 379 ± 833) compared to those with a CFR > 2.0 (p = 0.06 and p = 0.4, respectively). There was neither a significant correlation between CFR and CACS (r = − 0.2; p = 0.91) nor between CFR and cPB (r = − 0.1; p = 0.55). There were inverse correlations between MBFrest and hemoglobin (r = − 0.39; p = 0.015) and between CFR and hemoglobin (r = 0.32; p = 0.048).
Significant correlations are summarized in Table 3. In a stepwise multivariate regression analysis hemoglobin proved as strongest predictor for MBFrest. Age and CACS were the strongest predictors for MBFstress whereas only age was predictive for cPB.
Ischemia
Reversible stress perfusion defects were observed in four patients (10%). In these cases ischemia ranged from 4 to 9%. Global CFR was ≥ 2.0 in all cases and MBFstress was impaired (< 2.0 ml·g·min) in only one case. None of the patients had significant coronary artery stenoses > 50% in subsequent coronary angiography.
Predictive Value
10 patients (26%; two men, eight women) presented with CACS < 100 and cPB < 154 mm3 (= lower tertile limit), whereas 29 patients (74%; 23 men, six women) presented with either CACS > 100 or cPB > 154 mm3 or both. None of the patients with low CACS and cPB presented with impaired CFR or MBFstress. Among the patients with high CACS and/or cPB only eight patients (27.5%) showed impaired CFR and three (10%) impaired MBFstress. Patients with low CACS and cPB were older by trend (55.3 ± 8.8 vs 47.3 ± 17.7 years; p = 0.2) but there were no differences regarding the time on dialysis (2.4 ± 3.1 vs 2.5 ± 2.4 years; p = 0.91).
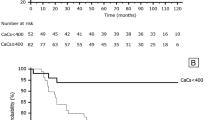
Median follow-up time was 4.4 years (mean 3.6 ± 1.1 years). In the follow-up, there were four major cardiovascular events (MACE): 2 myocardial infarctions, 1 stroke, and 1 pulmonary embolism. There were six deaths, all by non-cardiovascular causes. CV event-free survival was associated by reduced CFR and MBFstress (p = 0.001 and p < 0.001) but not with cPB or CACS (Figure. 5). On the other hand overall survival could not be predicted by any of these variables (all p > 0.25).
Discussion
We investigated the association between quantitative MBF, CACS and cPB in patients with ESRD assigned for kidney transplantation evaluation. Our main findings were: MBFstress but not CFR was significantly correlated with CACS and cPB. The strongest predictors for MBFstress were CACS and age, whereas cPB was best predicted by age. On the other hand the strongest predictor for CACS was MBFstress. Particularly the combination of low CACS and low cPB resulted in a high negative predictive value for preserved MBF and CFR.
The BioImage Study investigated the relationship of several (imaging) biomarkers and cardiovascular outcome in a large population of asymptomatic adults without known cardiovascular or renal disease.16 Here, a distinct correlation between CACS and cPB was described. In another study cPB was shown to be predictive of mortality and to occur more frequently in patients on HD than on PD,21 the latter also observed in our study. In line with the BioImage Study, we found a significant correlation between CACS and cPB also in patients with ESRD. In a multivariate regression analysis the strongest predictors for CACS and cPB were MBFstress and age. Thus, the results of our study are in line with the current understanding of atherosclerosis as a systemic and diffuse disease. Interestingly, cPB was only moderately associated with and not predictive of MBFstress in the multivariate analysis. Instead, CACS turned out to be predictive for MBFstress. This is not surprising since CACS reflects the extent of coronary artery atherosclerosis which directly influences myocardial perfusion and the degree of atherosclerosis in the carotid arteries does not necessarily preclude coronary atherosclerosis with a similar extent. Our findings indicate that none of the imaging parameters is able to predict the others with sufficient accuracy in patients with ESRD. Importantly, CACS is not limited to intimal calcification but also superposed by smooth muscle cell mediated, metabolic media calcification in patients with ESRD.22 This also could explain the only moderate correlation between CACS and cPB, since cPB is rather a marker of ‘classical’ atherosclerosis resulting from subintimal plaque development. Due to this different pathophysiology, the clinical value of CACS in CKD patients still remains controversial.13 Interestingly, CACS did not correlate with age. This finding may be explained by the different pathophysiology of media calcification but also (at least partly) by the distinctly younger age of patients studied here as compared with a classical CAD population.
Another factor associated with MBFstress was the time on dialysis. This finding supports the assumption that the duration of renal replacement therapy has a strong impact on myocardial perfusion, mainly due to rarefication of small intramyocardial vessels.23,24,25 We found impaired CFR in 20% of our patients, which is less than reported in other studies33 and most probably due to the relatively young study cohort. Prior investigations have suggested that CKD is associated with abnormal coronary vasodilator function,26 which results from multiple mechanisms including decreased capillary density,27 vascular remodeling,28 and endothelial dysfunction.29,30 Early stage microvascular disease may be the reason for impaired MBFstress in our patient cohort. This can be assumed since global CFR was still preserved and significant epicardial CAD could be excluded by angiography in selected cases with mild to moderate regional ischemia. Thus, we interpret the data in that way that regional ischemia is most probably related to regional microvascular dysfunction, since technical image artifacts could be excluded. This can be missed by the assessment of global CFR.
Although preliminary results based on a rather small patient cohort should always be interpreted with care, our results indicate that the combination of low cPB and low CACS may have a high negative predictive value with regards to the exclusion of CAD. On the other hand the positive predictive value of CACS and cPB for CFR and MBF was low. Thus, it would be worth to evaluate in a larger study if the assessment of cPB and CACS may replace MPI in the evaluation prior to kidney transplantation listing in cases of normal findings. But on the other hand in this context importantly, our results indicate that cardiovascular event-free survival may be not predicted by cPB or CACS but only by CFR and MBFstress. These findings are in line with previous study results in distinctly larger ESRD patient cohorts already showing the prognostic value of CFR in this particular patient group, although in these studies a lower cutoff value for CFR than in our study was used.4,31
Notably striking was the observation that CFR was independently associated with the current Hb value and was neither correlated with CACS or cPB nor with age or the time on dialysis. Increased MBFrest has been already observed in mild CKD and was correlated with the degree of GFR.32 Anemia induces a compensatory increase in MBFrest via increased nitric oxide release33 which naturally also affects CFR. Although in former investigations impaired CFR was highly prevalent in patients with CKD (4, 35; example Figure 6), our results suggest that MBFstress may be a better parameter for the early assessment of microvascular dysfunction. Variability of MBFrest in the individual person is widely known and is probably related to differences in myocardial work load.35 Although corrected for cardiac work increased MBFrest due to other factors as metabolic oxygen demand or an enhanced activation of the sympathetic nervous and renin–angiotensin–aldosterone system34 which is chronically activated in patients with ESRD cannot be ruled out. Therefore, when interpreting CFR, changes in MBFrest due to alterations in metabolic demand need to be considered.36,37,38 Altogether, the results of our study raise the question if absolute MBFstress may be an earlier and more robust marker for CAD in patients with ESRD than CFR. This hypothesis is additionally supported by the finding that only MBFstress was significantly correlated with LVEF(rest) but not CFR. In former studies absolute MBFstress already shown to be more accurate than CFR in detecting CAD in non-CKD patients.35,37 Interestingly, MBFrest was higher in patients without or minimal CACS and low cPB, a finding which is most probably caused by the higher frequency of females in these groups. Most studies report of higher MBFrest in females, although the mechanisms of this phenomenon remain uncertain.38
Study Limitations
The small size of the patient cohort is certainly a limitation of our study. Furthermore, the study population was young and time on dialysis was relatively short. These factors may explain the relatively low number of pathological findings. Another limitation is the use of a non-ECG-triggered, low-dose CT for CACS although CACS on that basis is feasible.39,40 Thus, a dedicated state-of-the-art CACS-CT was not performed additionally in order to avoid futile additional radiation exposure.
New Knowledge Gained
Our results indicate that MBFstress is a marker of subclinical atherosclerosis in patients with ESRD, whereas CFR may miss early stages of vascular dysfunction in this patient cohort. Nevertheless, impaired CFR and MBFstress are predictors for CV event-free survival whereas cPB and CACS may be not.
Conclusions
Although CACS and cPB as parameters of coronary and systemic atherosclerosis are associated in patients with ESRD, only absolute MBFstress but not CFR correlates with CACS and cPB. Furthermore, MBFstress impairs with increasing time on renal replacement therapy. MBFrest and CFR are regularly influenced by actual hemoglobin values. Thus, MBFstress may serve as a robust parameter for the detection of microvascular disease in patients with ESRD than CFR.
Overall, our results indicate that quantitative MPI cannot be replaced by the assessment of CACS or cPB only. Nevertheless, there may be a good negative predictive value. CV event-free survival is associated with impaired CFR and MBFstress only.
Abbreviations
- CKD:
-
Chronic kidney disease
- CAD:
-
Coronary artery disease
- ESRD:
-
End-stage renal disease
- MPI:
-
Myocardial perfusion imaging
- MBF:
-
Myocardial blood flow
- CFR:
-
Coronary flow reserve
- cPB:
-
Carotid artery atherosclerotic plaque burden
- CACS:
-
Coronary artery calcium score
- PD:
-
Peritoneal dialysis
- HD:
-
Hemodialysis
References
Bock JS, Gottlieb SS. Cardiorenal syndrome: New perspectives. Circulation 2010;121:2592-600.
Shlipak MG, Massie BM. The clinical challenge of cardiorenal syndrome. Circulation 2004;110:1514-7.
Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G. Characteristics of sudden death in hemodialysis patients. Kidney Int 2006;69:2268-73.
Shah N, Charytan D, Murthy V, Skali Lami H, Veeranna V, Cheezum MK, et al. Prognostic value of coronary flow reserve in patients with dialysis-dependent ESRD. J Am Soc Nephrol 2016;27:1823-9.
Lentine KL, Hurst FP, Jindal RM, Villines TC, Kunz JS, Yuan CM, et al. Cardiovascular risk assessment among potential kidney transplant candidates: Approaches and controversies. Am J Kidney Dis 2010;55:152-67.
Friedman SE, Palac RT, Zlotnick DM, Chobanian MC, Costa SP. A call to action: Variability in guidelines for cardiac evaluation before renal transplantation. Clin J Am Soc Nephrol 2011;6:1185-91.
Kim JK, Kim SG, Kim HJ, Song YR. Cardiac risk assessment by gated single-photon emission computed tomography in asymptomatic end-stage renal disease patients at the start of dialysis. J Nucl Cardiol 2012;19:438-47.
Bengel FM. Leaving relativity behind: Quantitative clinical perfusion imaging. J Am Coll Cardiol 2011;58:749-51.
Fukushima K, Javadi MS, Higuchi T, Lautamäki R, Merrill J, Nekolla SG, et al. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med 2011;52:726-32.
Salonen JT, Salonen R. Ultrasonographically assessed carotid morphology and the risk of coronary heart disease. Arterioscler Thromb 1991;11:1245-9.
Hecht HS. Coronary artery calcium scanning: Past, present, and future. JACC Cardiovasc Imaging 2015;8:579-96.
Moody WE, Lin EL, Stoodley M, McNulty D, Thomson LE, Berman DS, et al. Prognostic utility of calcium scoring as an adjunct to stress myocardial perfusion scintigraphy in end stage renal disease. Am J Cardiol 2016;117:1387-96.
Bashir A, Moody WE, Edwards NC, Ferro CJ, Townend JN, Steeds RP. Coronary artery calcium assessment in CKD: Utility in cardiovascular disease risk assessment and treatment? Am J Kidney Dis 2015;65:937-48.
Kato A, Takita T, Maruyama Y, Kumagai H, Hishida A. Impact of carotid atherosclerosis on long-term mortality in chronic hemodialysis patients. Kidney Int 2003;64:1472-9.
Sillesen H, Muntendam P, Adourian A, Entrekin R, Garcia M, Falk E, et al. Carotid plaque burden as a measure of subclinical atherosclerosis: Comparison with other tests for subclinical arterial disease in the High Risk Plaque BioImage study. JACC Cardiovasc Imaging 2012;5:681-9.
Baber U, Mehran R, Sartori S, Schoos MM, Sillesen H, Muntendam P, et al. Prevalence, impact, and predictive value of detecting subclinical coronary and carotid atherosclerosis in asymptomatic adults: the BioImage study. J Am Coll Cardiol 2015;65:1065-74.
El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: Comparison with (13)N-ammonia PET. J Nucl Med 2009;50:1062-71.
Czernin J, Muller P, Chan S, Brunken RC, Porenta G, Krivokapich J, et al. Influence of age and hemodynamics on myocardial blood flow and flow reserve. Circulation 1993;88:62-9.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 1990;15:827-32.
Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ. Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology 2003;228:826-33.
Mutluay R, Degertekin CK, Poyraz F, Yılmaz MI, Yücel C, Turfan M, et al. Dialysis type may predict carotid intima media thickness and plaque presence in end-stage renal disease patients. Adv Ther 2012;29:370-82.
Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol 2004;15:2857-67.
Tornig J, Amann K, Ritz E, Nichols C, Zeier M, Mall G. Arteriolar wall thickening, capillary rarefaction and interstitial fibrosis in the heart of rats with renal failure: The effects of ramipril, nifedipine and moxonidine. J Am Soc Nephrol 1996;7:667-75.
Amann K, Tyralla K. Cardiovascular changes in chronic renal failure–pathogenesis and therapy. Clin Nephrol 2002;58:S62-72.
Parnham S, Gleadle JM, Leong D, Grover S, Bradbrook C, Woodman RJ, et al. Myocardial perfusion is impaired in asymptomatic renal and liver transplant recipients: A cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 2015;17:56.
Chade AR, Brosh D, Higano ST, Lennon RJ, Lerman LO, Lerman A. Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease. Kidney Int 2006;69:266-71.
Amann K, Breitbach M, Ritz E, Mall G. Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol 1998;9:1018-22.
Schwarz U, Buzello M, Ritz E, Stein G, Raabe G, Wiest G, et al. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant 2000;15:218-23.
Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Dorbala S, et al. Coronary vascular dysfunction and prognosis in patients with chronic kidney disease. JACC Cardiovasc Imaging 2012;5:1025-34.
Naya M, Murthy VL, Taqueti VR, Foster CR, Klein J, Garber M, et al. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med 2014;55:248-55.
Charytan DM, Skali H, Shah NR, Veeranna V, Cheezum MK, Taqueti VR, Kato T, Bibbo CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Coronary flow reserve is predictive of the risk of cardiovascular death regardless of chronic kidney disease stage. Kidney Int 2018;93:501-9.
Koivuviita N, Tertti R, Jarvisalo M, Pietilä M, Hannukainen J, Sundell J, et al. Increased basal myocardial perfusion in patients with chronic kidney disease without symptomatic coronary artery disease. Nephrol Dial Transplant 2009;24:2773-9.
Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, et al. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood 2006;107:566-74.
Paz Y, Morgenstern R, Weinberg R, Chiles M, Bhatti N, Ali Z, et al. Relation of coronary flow reserve to other findings on positron emission tomography myocardial perfusion imaging and left heart catheterization in patients with end-stage renal disease being evaluated for kidney transplant. Am J Cardiol 2017;120:1909-12.
Schindler TH. Myocardial blood flow: Putting it into clinical perspective. J Nucl Cardiol 2016;23:1056-71.
Schindler TH, Dilsizian V. PET-determined hyperemic myocardial blood flow: Further progress to clinical application. J Am Coll Cardiol 2014;64:1476-8.
Srivastava AV, Ananthasubramaniam K. Guiding coronary revascularization using PET stress myocardial perfusion imaging: The proof is in the pudding. J Nucl Cardiol 2016;24:975-9.
Chareonthaitawee P, Kaufmann PA, Rimoldi O, Camici PG. Heterogeneity of resting and hyperemic myocardial blood flow in healthy humans. Cardiovasc Res 2001;50:151-61.
Einstein AJ, Johnson LL, Bokhari S, Son J, Thompson RC, Bateman TM, et al. Agreement of visual estimation of coronary artery calcium from low-dose CT attenuation correction scans in hybrid PET/CT and SPECT/CT with standard Agatston score. J Am Coll Cardiol 2010;56:1914-21.
Xie X, Zhao Y, de Bock GH, de Jong PA, Mali WP, Oudkerk M, et al. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: Systematic review and meta-analysis. Circ Cardiovasc Imaging 2013;6:514-21.
Acknowledgements
Open Access funding provided by Projekt DEAL. We thank Nemanja Avramovic for excellent technical assistance, patient preparation and performing PET acquisitions.
Disclosure
The authors have indicated that they have no financial conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Funding
This work was supported by grants of the German Research Foundation, Bonn, Germany (DFG SFB 656 C06, C07 and C11, and clinician scientist position to A.V.) No relationships with industry.
Christian Wenning, Alexis Vrachimis, Stefan Reuter and Michael Schäfers are equal first and senior authorships.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wenning, C., Vrachimis, A., Pavenstädt, HJ. et al. Coronary artery calcium burden, carotid atherosclerotic plaque burden, and myocardial blood flow in patients with end-stage renal disease: A non-invasive imaging study combining PET/CT and 3D ultrasound. J. Nucl. Cardiol. 28, 2660–2670 (2021). https://doi.org/10.1007/s12350-020-02080-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-020-02080-w