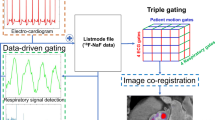
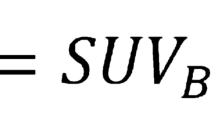Abstract
Background
Challenges to cardiac PET-CT include patient motion, prolonged image acquisition and a reduction of counts due to gating. We compared two analytical tools, FusionQuant and OsiriX, for quantification of gated cardiac 18F-sodium fluoride (18F-fluoride) PET-CT imaging.
Methods
Twenty-seven patients with aortic stenosis were included, 15 of whom underwent repeated imaging 4 weeks apart. Agreement between analytical tools and scan-rescan reproducibility was determined using the Bland–Altman method and Lin’s concordance correlation coefficients (CCC).
Results
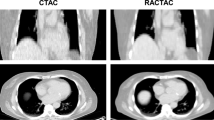
Image analysis was faster with FusionQuant [median time (IQR) 7:10 (6:40-8:20) minutes] compared with OsiriX [8:30 (8:00-10:10) minutes, p = .002]. Agreement of uptake measurements between programs was excellent, CCC = 0.972 (95% CI 0.949-0.995) for mean tissue-to-background ratio (TBRmean) and 0.981 (95% CI 0.965-0.997) for maximum tissue-to-background ratio (TBRmax). Mean noise decreased from 11.7% in the diastolic gate to 6.7% in motion-corrected images (p = .002); SNR increased from 25.41 to 41.13 (p = .0001). Aortic valve scan-rescan reproducibility for TBRmax was improved with FusionQuant using motion correction compared to OsiriX (error ± 36% vs ± 13%, p < .001) while reproducibility for TBRmean was similar (± 10% vs ± 8% p = .252).
Conclusion
18F-fluoride PET quantification with FusionQuant and OsiriX is comparable. FusionQuant with motion correction offers advantages with respect to analysis time and reproducibility of TBRmax values.



Similar content being viewed by others
Abbreviations
- PET:
-
Positron emission tomography
- CT:
-
Computed tomography
- ECG:
-
Electrocardiogram
- ROI:
-
Region of interest
- SNR:
-
Signal-to-noise ratio
- TBR:
-
Tissue-to-background ratio
- SUV:
-
Standardized uptake value
References
Rubeaux M, Doris MK, Alessio A, Slomka PJ. Enhancing cardiac PET by motion correction techniques. Curr Cardiol Rep 2017;19:14.
Doris MK, Rubeaux M, Pawade T, Otaki Y, Xie Y, Li D, et al. Motion-corrected imaging of the aortic valve with (18)F-NaF PET/CT and PET/MRI: A feasibility study. J Nucl Med 2017;58:1811-4.
Rubeaux M, Joshi NV, Dweck MR, Fletcher A, Motwani M, Thomson LE, et al. Motion correction of 18F-NaF PET for imaging coronary atherosclerotic plaques. J Nucl Med 2016;57:54-9.
Doris MK, Otaki Y, Krishnan SK, Kwiecinski J, Rubeaux M, Alessio A, et al. Optimization of reconstruction and quantification of motion-corrected coronary PET-CT. J Nucl Cardiol 2018. https://doi.org/10.1007/s12350-018-1317-5.
Pawade TA, Cartlidge TR, Jenkins WS, Adamson PD, Robson P, Lucatelli C, et al. Optimization and reproducibility of aortic valve 18F-fluoride positron emission tomography in patients with aortic stenosis. Circ Cardiovasc Imaging 2016;9(10):e005131.
Le Meunier L, Slomka PJ, Dey D, Ramesh A, Thomson LE, Hayes SW, et al. Enhanced definition PET for cardiac imaging. J Nucl Cardiol 2010;17:414-26.
Dweck MR, Jones C, Joshi NV, Fletcher AM, Richardson H, White A, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012;125:76-86.
Vercauteren T, Pennec X, Perchant A, Ayache N. Symmetric log-domain diffeomorphic registration: A demons-based approach. Med Image Comput Comput Assist Interv 2008;11:754-61.
Rubeaux M, Joshi N, Dweck, Fletcher A, Motwani M, LE Thomson, et al. Demons versus level-Set motion registration for coronary (18)F-sodium fluoride PET. Proc SPIE Int Soc Opt Eng 2016. https://doi.org/10.1117/12.2217179.
Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307-10.
Ludbrook J. Confidence in Altman-Bland plots: A critical review of the method of differences. Clin Exp Pharmacol Physiol 2010;37:143-9.
Blau M, Ganatra R, Bender MA. 18 F-fluoride for bone imaging. Semin Nucl Med 1972;2:31-7.
Derlin T, Richter U, Bannas P, Begemann P, Buchert R, Mester J, et al. Feasibility of 18F-sodium fluoride PET/CT for imaging of atherosclerotic plaque. J Nucl Med 2010;51:862-5.
Derlin T, Wisotzki C, Richter U, Apostolova I, Bannas P, Weber C, et al. In vivo imaging of mineral deposition in carotid plaque using 18F-sodium fluoride PET/CT: Correlation with atherogenic risk factors. J Nucl Med 2011;52:362-8.
Irkle A, Vesey AT, Lewis DY, Skepper JN, Bird JL, Dweck MR, et al. Identifying active vascular microcalcification by (18)F-sodium fluoride positron emission tomography. Nat Commun 2015;6:7495.
McKenney-Drake ML, Territo PR, Salavati A, Houshmand S, Persohn S, Liang Y, et al. (18)F-NaF PET imaging of early coronary artery calcification. JACC Cardiovasc Imaging 2016;9:627-8.
Hop H, de Boer SA, Reijrink M, Kamphuisen PW, de Borst MH, Pol RA, et al. (18)F-sodium fluoride positron emission tomography assessed microcalcifications in culprit and non-culprit human carotid plaques. J Nucl Cardiol 2018. https://doi.org/10.1007/s12350-018-1325-5.
Bellinge JW, Francis RJ, Majeed K, Watts GF, Schultz CJ. In search of the vulnerable patient or the vulnerable plaque: (18)F-sodium fluoride positron emission tomography for cardiovascular risk stratification. J Nucl Cardiol 2018. https://doi.org/10.1007/s12350-018-1360-2.
Janssen T, Bannas P, Herrmann J, Veldhoen S, Busch JD, Treszl A, et al. Association of linear (1)(8)F-sodium fluoride accumulation in femoral arteries as a measure of diffuse calcification with cardiovascular risk factors: A PET/CT study. J Nucl Cardiol 2013;20:569-77.
Dweck MR, Chow MW, Joshi NV, Williams MC, Jones C, Fletcher AM, et al. Coronary arterial 18F-sodium fluoride uptake: A novel marker of plaque biology. J Am Coll Cardiol 2012;59:1539-48.
Dweck MR, Khaw HJ, Sng GK, Luo EL, Baird A, Williams MC, et al. Aortic stenosis, atherosclerosis, and skeletal bone: Is there a common link with calcification and inflammation? Eur Heart J 2013;34:1567-74.
Kitagawa T, Yamamoto H, Toshimitsu S, Sasaki K, Senoo A, Kubo Y, et al. (18)F-sodium fluoride positron emission tomography for molecular imaging of coronary atherosclerosis based on computed tomography analysis. Atherosclerosis 2017;263:385-92.
Oliveira-Santos M, Castelo-Branco M, Silva R, Gomes A, Chichorro N, Abrunhosa A, et al. Atherosclerotic plaque metabolism in high cardiovascular risk subjects - A subclinical atherosclerosis imaging study with (18)F-NaF PET-CT. Atherosclerosis 2017;260:41-6.
Forsythe RO, Dweck MR, McBride OMB, Vesey AT, Semple SI, Shah ASV, et al. (18)F-sodium fluoride uptake in abdominal aortic aneurysms: The SoFIA(3) study. J Am Coll Cardiol 2018;71:513-23.
Marchesseau S, Seneviratna A, Sjoholm AT, Qin DL, Ho JXM, Hausenloy DJ, et al. Hybrid PET/CT and PET/MRI imaging of vulnerable coronary plaque and myocardial scar tissue in acute myocardial infarction. J Nucl Cardiol 2017. https://doi.org/10.1007/s12350-017-0918-8.
ClinicalTrials.gov. National Library of Medicine (U.S.). 18F-Fluoride Assessment of Aortic Bioprosthesis Durability and Outcome. https://ClinicalTrials.gov/show/NCT02304276. Accessed 18 Aug 2018.
ClinicalTrials.gov. National Library of Medicine (U.S.). Study Investigating the Effect of Drugs Used to Treat Osteoporosis on the Progression of Calcific Aortic Stenosis. https://ClinicalTrials.gov/show/NCT02132026. Accessed 18 Aug 2018.
ClinicalTrials.gov. National Library of Medicine (U.S.). Bicuspid Aortic Valve Stenosis and the Effect of vItamin K2 on Calcium metabolism on 18F-NaF PET/MRI. https://ClinicalTrials.gov/show/NCT02917525. Accessed 18 Aug 2018.
Hong I, Jones J, Casey M (2014) Ultrafast Elastic Motion Correction via Motion Deblurring. 2014 IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC). Seattle, WA; 2014. p. 2–3.
Cal-Gonzalez J, Li X, Heber D, Rausch I, Moore SC, Schafers K, et al. Partial volume correction for improved PET quantification in (18)F-NaF imaging of atherosclerotic plaques. J Nucl Cardiol 2017;25:1742-6.
Disclosure
The authors have indicated that they have no financial conflict of interest.
Author information
Authors and Affiliations
Contributions
DM, MKD, JK, DD, DEN, MRD, and PJS participated in conception and design of the study; DM, MKD, SC, JK, TAP, and FECMP were actively involved in collecting data and data analysis; DM, MRK, and PJS drafted the manuscript; all authors gave final approval of the submitted manuscript.
Corresponding author
Additional information
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Funding
This research was supported in part by Grant R01HL135557 from the National Heart, Lung, and Blood Institute/National Institute of Health (NHLBI/NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. D. M. was supported by The Glorney-Raisbeck Fellowship Program, Corlette Glorney Foundation, and The New York Academy of Medicine. The study was also supported by a Grant (“Cardiac Imaging Research Initiative”) from the Miriam & Sheldon G. Adelson Medical Research Foundation. David Newby (CH/09/002, RE/13/3/30183), Marc Dweck (FS/14/78), and Mhairi Doris (FS/17/79/33226) are supported by the British Heart Foundation. David Newby is also the recipient of a Wellcome Trust Senior Investigator Award (WT103782AIA). Marc Dweck is the recipient of the Sir Jules Thorn Award for biomedical research (15/JTA).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Massera, D., Doris, M.K., Cadet, S. et al. Analytical quantification of aortic valve 18F-sodium fluoride PET uptake. J. Nucl. Cardiol. 27, 962–972 (2020). https://doi.org/10.1007/s12350-018-01542-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12350-018-01542-6