Abstract
A 66-year-old woman with rheumatoid arthritis (RA) who had been receiving methotrexate (MTX) for 2 years presented with tarry stools. Contrast-enhanced computed tomography (CT) of the abdomen revealed irregular wall thickening in the ileocecal region and multiple low-contrast masses in both lobes of the liver. Lower gastrointestinal endoscopy revealed a type 2 tumor in the ileocecal region with a semi-peripheral ulcer. Histological examination of liver and colon biopsies showed other iatrogenic immunodeficiency-associated lymphoproliferative disorder (Oi-LPD), diffuse large B-cell lymphoma type, with positivity for Epstein-Barr virus DNA. After withdrawal of MTX, the LPD lesions disappeared and the patient achieved remission. We considered this to be a sporadic case of Oi-LPD, diffuse large B-cell lymphoma type, in the liver and colon due to treatment with MTX. There has been no previous report of this condition with simultaneous hepatic and colonic lesions, and the present case is thought to be highly informative in relation to the pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lymphoproliferative disorders (LPDs) arise in patients who have been treated with immunosuppressants, and diffuse large B-cell lymphoma (DLBCL) is the most common type. One such immunosuppressant, methotrexate (MTX), is the first choice for patients with rheumatoid arthritis (RA) [1, 2]. EBV reactivation associated with reduced immunocompetence is known to be responsible for the development of MTX-LPD. As MTX administration for RA tends to be prolonged, development of MTX-LPD is known to be a severe complication [3]. Here we report our experience with a RA patient who developed MTX-LPD, DLBCL type, in the liver and colon.
Case presentation
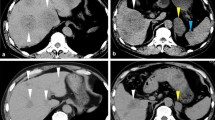
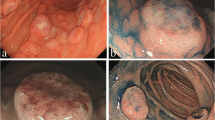
A 66 year-old woman was admitted to our hospital with a 3 day history of tarry stools. She had RA and had been treated with salazosulfapyridine, iguratimod and MTX for 2 years. Her RA symptoms were well controlled. Her abdomen was flat, soft, and not tender. Severe anemia (hemoglobin 7.6 g/dL) was evident, and the serum levels of C-reactive protein and soluble interleukin-2 receptor were 9.19 mg/dL and 2,630 U/mL, respectively. Epstein–Barr virus (EBV) DNA was detected in the peripheral blood at 3.60 log IU/ml (Table 1). Contrast-enhanced CT revealed a mass with enhancement in the ileocecal region (Fig. 1A), and multiple nodular masses without enhancement in the liver and para-aortic region (Fig. 1B and C). Contrast-enhanced magnetic resonance imaging (MRI) with gadolinium-ethoxybenzyl diethylenetriamine-pentaacetic acid (Gd-EOB-DTPA) showed multiple tumors in the liver with central necrosis (Fig. 1D-G). Lower gastrointestinal endoscopy revealed a mass in the ileocecal region which was suspected malignant tumor (Fig. 2). Liver metastasis of ileocecal cancer or malignant lymphoma was suspected, and ultrasound-guided biopsy of the liver tumors was performed. Histological examination of the liver and colon specimens revealed proliferation of CD20-positive large lymphoid cells with necrosis and in situ hybridization (ISH) demonstrated Epstein-Barr encoding region (EBER)-1 positivity. Histological examination showed diffuse proliferation of mostly large lymphoid cells with necrosis in the liver (Fig. 3A and B) and colon, although there was no atypia in the epithelium of the surface mucosa of colon. Immunohistochemically, these large lymphoid cells were CD20 ( +) (Fig. 3C), CD30 ( +) (Fig. 3D), CD10 ( +) (Fig. 3E), CD 23 (−) (Fig. 3F), BCL2 (−) (Fig. 3G), BCL6 (−) (Fig. 3H), and MUM1 (−) (Fig. 3I). The Ki-67 labeling index was 80% (Fig. 3J). EBER positivity was detected by ISH (Fig. 3K). Immunostaining of biopsies from the colon yielded similar findings (Fig. 3L and M). The final diagnosis was Oi-DLBCL based on the WHO classification revised fourth edition, as the multiple tumors began to shrink after MTX withdrawal.
Abdominal imaging modalities. Contrast-enhanced computed tomography (CT) showed a mass with enhancement in the ileocecal region (A, arrow), and multiple nodular masses without enhancement in the liver (B) and para-aortic region (C, arrow). Penetrating vessels running through the tumor were detected in the liver (B, arrow). Contrast-enhanced magnetic resonance imaging (MRI) with gadolinium-ethoxybenzyl diethylenetriamine-penta-acetic acid (Gd-EOB-DTPA) showed that multiple tumors in the liver were lower contrast than the liver parenchyma in portal phase (D). T1-weighted images showed low signal in all lesions (E). The coarse lesion was centrally high signal on T2-weighted images (F) and low signal on DWI images (G)
Histological findings in the liver and colon specimens. Histological examination showed diffuse proliferation of mostly large lymphoid cells with necrosis (A, B). Immunohistochemically, the liver specimens revealed CD20 ( +) (C), CD30 ( +) (D), CD10 ( +) (E), CD 23 (−) (F), BCL2 (−) (G), BCL6 (−) (H), MUM1 (−) (I), and Ki-67 labeling index 80% (J). EBV encoding RNA (EBER) positivity was detected by in situ hybridization (ISH) (K). Similarly, histology of the colon specimens also revealed CD20 ( +) (L) and EBER-ISH positivity (M)
Observation was continued, and CT of the abdomen four months later revealed spontaneous shrinkage of most of the tumors (Fig. 4A). The serum level of sIL-2R improved spontaneously (312 IU/ml), and EBV DNA in the peripheral blood decreased to less than the standard value. After the withdrawal of MTX, the patient experienced no deterioration in her joints. There has been no sign of recurrence for 8 months (Fig. 4B and 5).
Discussion
RA patients are known to have a 2- to 4-fold higher incidence of malignant lymphoma as a complication than the general population [4]. Although MTX is used as a first-line treatment for patients with RA, it carries a risk of LPD [2]. When LPD occurs, biologics are often used in the RA patients as an alternative to MTX [5]. LPD, including malignant lymphoma, has been found to occur in RA patients receiving MTX, and this is termed MTX-LPD. The WHO classifies it as immunodeficiency-associated LPD. EBV infection is said to be a complication in about half of MTX-LPD cases, and EBV reactivation has been implicated [6,7,8]. It is thought that the EBV gene releases factors similar to growth factors, transcription factors, and apoptosis inhibitors, causing B lymphocytes to transform into lymphoblasts, exhibiting tumor-like growth [9]. The most common sites of MTX-LPD are lymph nodes [10] and tonsils [11], but thyroid [12], lung [13], liver [14], and colon lesions [15] have also been reported.
Imaging modalities, such as US and CT, can aid the diagnosis of hepatic lymphoma/MTX-LPD. A previous report has indicated that penetrating vessels running through the tumor visualized by US was helpful for diagnosis of hepatic lymphoma [16]. In the present case, penetrating vessels running through the tumor were detected by CT scan. The evidence suggests that MTX-LPD should be considered in RA patients who are receiving MTX medications when CT or US shows a hepatic mass with signs of penetrating vessels.
A search of PubMed using the terms “MTX-LPD” and “hepatic”, or “MTX-LPD” and “liver”, yielded 12 available studies published in English between 2000 and 2022 [10, 14, 17,18,19,20,21,22,23,24,25,26]. In all cases the patients had been treated with immunosuppressants such as MTX, steroids, infliximab, and tacrolimus. All had taken MTX for more than 24 months, and the median total dose of MTX was 1932 mg (960–6000 mg). Ten patients showed reactivation of EBV. The present patient had taken MTX for 24 months (total dose, 960 mg) and also showed reactivation of EBV. MTX-LPD involving the liver along with other parenchymal organs must be differentiated from cancer metastasis. As shown in Table 2, five patients with hepatic MTX-LPD accompanied by other parenchymal organ lesions involvement of the adrenal gland (n = 2), multiple lymph nodes (n = 1), spleen (n = 1), and colon (n = 1, this case).
In the listed MTX-LPD, DLBCL type cases (n = 8), MTX was discontinued and three of the patients achieved complete remission. Three additional patients achieved complete remission with chemotherapy. Two patients were treated by partial hepatectomy and had no recurrence. Two patients with MTX-LPD, Hodgkin lymphoma type, also stopped using MTX. One of them suffered relapse even after withdrawal of MTX, and the other underwent partial hepatectomy and had no recurrence. Our present patient achieved complete remission after MTX had been withdrawn. These findings suggest that immediate withdrawal of MTX is recommended for patients with hepatic MTX-LPD, DLBCL type. Our patient had two lesions; these may have been concurrent, or one may have been due to metastasis from the colon to the liver. Because of the multiple lymph node metastases and the histological similarities of the lesions, the possibility that the colon lesion had metastasized to the liver could not be ruled out. To our knowledge, this is the first case of MTX-LPD involving both the liver and colon to have been reported in English.
Conclusion
We have experienced a case of MTX-LPD, DLBCL type, in which multiple liver masses and colon lesions were concurrent. Multiple systemic masses in RA patients receiving MTX are suggestive of MTX-LPD, and early discontinuation of MTX should be considered.
References
Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26.
Ohe R, Yang S, Yamashita D, et al. Pathogenesis of follicular thymic hyperplasia associated with rheumatoid arthritis. Pathol Int. 2022;72:252–60.
Saito S, Takeuchi T. Immune response in LPD during methotrexate administration (MTX-LPD) in rheumatoid arthritis patients. J Clin Exp Hematop. 2019;59:145–55.
Anderson LA, Gadalla S, Morton LM, et al. Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int J Cancer. 2009;125:398–405.
Katsuyama T, Sada KE, Yan M, et al. Prognostic factors of methotrexate-associated lymphoproliferative disorders associated with rheumatoid arthritis and plausible application of biological agents. Mod Rheumatol. 2017;27:773–7.
Kikuchi K, Miyazaki Y, Tanaka A, et al. Methotrexate-related epstein-barr virus (EBV)-associated lymphoproliferative disorder–so-called “Hodgkin-like lesion”–of the oral cavity in a patient with rheumatoid arthritis. Head Neck Pathol. 2010;4:305–11.
Kitamura N, Sugiyama K, Nagasawa Y, et al. Involvement of Epstein-Barr virus in the development and spontaneous regression of methotrexate-associated lymphoproliferative disorder in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2022;40:1330–5.
Afonso C, Roque A, Almeida C, et al. Methotrexate-associated lymphoproliferative disorder in a patient with psoriasis: a case report and review of the literature. Case Rep Hematol. 2022;2022:7178065.
Grywalska E, Markowicz J, Grabarczyk P, et al. Epstein-Barr virus-associated lymphoproliferative disorders. Postepy Hig Med Dosw (Online). 2013;67:481–90.
Tsukazaki Y, Shinohara T, Tanaka K, et al. Hepatosplenic Hodgkin lymphoma without lymphadenopathy following reversible methotrexate-associated lymphoproliferative disorder. Mod Rheumatol. 2017;27:372–5.
Watanabe T, Teratani Y. Unusual manifestation of methotrexate-associated lymphoproliferative disorder as a palatal mass. BMJ Case Rep. 2022. https://doi.org/10.1136/bcr-2022-250616.
Hiruma M, Tsuboi K, Hirose T. Methotrexate-associated lymphoproliferative disorder in the thyroid gland of a patient with chronic thyroiditis. J Med Ultrason. 2001;2023(50):575–6.
Suemori K, Hasegawa H, Ishizaki J, et al. Methotrexate-associated lymphoproliferative disease with multiple pulmonary nodules in a patient with rheumatoid arthritis. Intern Med. 2015;54:1421–5.
Omameuda T, Miyato H, Sata N, et al. Primary hepatic methotrexate-associated lymphoproliferative disorder associated with Epstein-Barr virus reactivation and accompanied by spontaneous necrosis: a case report. Medicine (Baltimore). 2022;101: e31993.
Shirakabe K, Mizokami K. Methotrexate-associated lymphoproliferative disease detected as a colorectal mass lesions: a case report. J Surg Case Rep. 2023. https://doi.org/10.1093/jscr/rjad098.
Takasumi M, Okai K, Asano T, et al. A case of methotrexate-associated lymphoproliferative disorder diagnosed by liver biopsy. Nihon Shokakibyo Gakkai Zasshi. 2015;112(1):115–22.
Soubrier M, Arrestier S, Bouloudian S, et al. Epstein-Barr virus infection associated hepatic lymphoma in a patient treated with methotrexate for rheumatoid arthritis. Joint Bone Spine. 2006;73:218–9.
Fujita T, Tanabe M, Iida E, et al. Multi-modality imaging findings of methotrexate-related Epstein-Barr virus-associated hepatic tumor. Clin Imaging. 2013;37:962–4.
Tatsumi G, Ukyo N, Hirata H, et al. Primary hepatic lymphoma in a patient with rheumatoid arthritis treated with methotrexate. Case Rep Hematol. 2014;2014: 460574.
Kawahara A, Tsukada J, Yamaguchi T, et al. Reversible methotrexate-associated lymphoma of the liver in rheumatoid arthritis: a unique case of primary hepatic lymphoma. Biomark Res. 2015;3:10.
Miyagawa K, Shibata M, Noguchi H, et al. Methotrexate-related primary hepatic lymphoma in a patient with rheumatoid arthritis. Intern Med. 2015;54:401–5.
Ohkura Y, Shindoh J, Haruta S, et al. Primary adrenal lymphoma possibly associated with Epstein-Barr virus reactivation due to immunosuppression under methotrexate therapy. Medicine (Baltimore). 2015;94: e1270.
Matsumoto R, Numata K, Doba N, et al. A case of multiple hepatic lesions associated with methotrexate-associated lymphoproliferative disorder. J Med Ultrason. 2001;2016(43):545–51.
Takei D, Abe T, Amano H, et al. Methotrexate-associated primary hepatic malignant lymphoma following hepatectomy: a case report. Int J Surg Case Rep. 2017;31:5–9.
Ono R, Kumagae T, Uojima H, et al. Hepatic methotrexate-associated lymphoproliferative disorders identified by multiple liver tumors: a case report and review of the literature. J Med Case Rep. 2019;13:196.
Ito N, Masuda T, Yamaguchi K, et al. Pneumonia and meningoencephalitis due to varicella-zoster virus reinfection and Epstein-Barr virus reactivation in a patient with rheumatoid arthritis. Intern Med. 2022;61:2961–5.
Acknowledgements
This work was supported by JSPS KAKENHI Grant number JP21K06901.
Funding
This work was supported by JSPS KAKENHI under JP21K06901 for Rintaro Ohe
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical approval and consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nagata, Y., Akiba, S., Horiuchi, H. et al. A case of methotrexate-related lymphoproliferative disease showing multiple liver lesions in a patient with rheumatoid arthritis. Clin J Gastroenterol (2024). https://doi.org/10.1007/s12328-024-01963-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12328-024-01963-6