Abstract
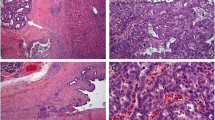
Intrahepatic mucinous cholangiocarcinoma (IHMC) is rare and behaves notoriously; however, the details of the clinicopathological characteristics of IHMC remain unknown. A 70-year-old man was admitted for examination of the hepatic mass in the S1 segment. He underwent extended left hepatic lobectomy. Histopathological evaluation demonstrated mixed papillary carcinoma that comprised well to moderately differentiated tubular adenocarcinoma and signet-ring cell carcinoma with large amounts of mucus lakes. Tumor was relapsed 9 months after surgery. Although he received chemotherapy with the combination of gemcitabine and cisplatin, he had renal failure and discontinued the chemotherapy. He received palliative radiotherapy for metastasis in the cervical spine. Then, the patient treated with S-1, however, he died 16 months after the initial diagnosis. The autopsy findings showed multiple nodules in the lungs, pleura, kidneys, adrenal glands, stomach, pancreas, and lymph nodes. Histological examination revealed that all nodules were IHMC. Next-generation sequencing revealed that somatic mutations in ADGRB3, TAF1L and EPHA3 may affect carcinogenesis, and those in TAF1, EPHA3, PIK3C2B, FN1, ERBB3, BRIP1, SYNE1 and TGFBR2 may affect metastasis. Molecular carcinogenesis of IHMC may be distinct from that of ordinary cholangiocarcinoma. Further studies are needed to elucidate the genetic mutations and their functions in IHMC.





Similar content being viewed by others
Abbreviations
- IHMC:
-
Intrahepatic mucinous cholangiocarcinoma
- MC:
-
Mucinous carcinoma
- CECT:
-
Contrast-enhanced computed tomography
- MRI:
-
Magnetic resonance imaging
- T2WI:
-
T2-weighted image
- DWI:
-
Diffusion-weighted image
- ADC:
-
Apparent diffusion coefficient
- UICC:
-
The Union for International Cancer Control
- GEM:
-
Gemcitabine
- CDDP:
-
Cisplatin
- NGS:
-
Next-generation sequencing
- FFPE:
-
Formalin-fixed, paraffin-embedded
- CNVs:
-
Copy number variations
- WHO:
-
World Health Organization
- HCC:
-
Hepatocellular carcinoma
- IHCC:
-
Intrahepatic cholangiocarcinoma
References
Laohawetwanit T, Klaikaew N. Pathological aspects of mucinous cholangiocarcinoma: a single-center experience and systematic review. Pathol Int. 2020;70:661–70.
Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26–42.
Fujimoto A, Furuta M, Shiraishi Y, et al. Whole-genome mutational landscape of liver cancers displaying biliary phenotype reveals hepatitis impact and molecular diversity. Nat Commun. 2015;6:6120.
Zou S, Li J, Zhou H, et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat Commun. 2014;5:5696.
Fujimoto A, Furuta M, Totoki Y, et al. Whole-genome mutational landscape and characterization of noncoding and structural mutations in liver cancer. Nat Genet. 2016;48:500–9.
Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47:1003–10.
Nakagaki T, Tamura M, Kobashi K, et al. Profiling cancer-related gene mutations in oral squamous cell carcinoma from Japanese patients by targeted amplicon sequencing. Oncotarget. 2017;8:59113–22.
Wada K, Kondo F, Kubosawa H, et al. Combined hepatocellular and mucinous carcinoma. Acta Pathol Jpn. 1986;36:285–91.
Bu-Ghanim M, Suriawinata A, Killackey M, et al. Invasive colloid carcinoma arising from intraductal papillary neoplasm in a 50-year-old woman with primary sclerosing cholangitis. Semin Liver Dis. 2004;24:209–13.
Chow LT, Ahuja AT, Kwong KH, et al. Mucinous cholangiocarcinoma: an unusual complication of hepatolithiasis and recurrent pyogenic cholangitis. Histopathology. 1997;30:491–4.
Kai K, Ide Y, Miyoshi A, et al. A case of mucinous cholangiocarcinoma showing features of hepatocellular carcinoma. Pathol Int. 2013;63:419–21.
Kang GH, Moon HS, Lee ES, et al. A case of colloid carcinoma arising in association with intraductal papillary neoplasm of the liver. Korean J Gastroenterol = Taehan Sohwagi Hakhoe chi. 2012;60:386–90.
Mizukami Y, Ohta H, Arisato S, et al. Case report: mucinous cholangiocarcinoma featuring a multicystic appearance and periportal collar in imaging. J Gastroenterol Hepatol. 1999;14:1223–6.
Morita D, Kagata Y, Ogata S, et al. Combined hepatocellular carcinoma and cholangiocarcinoma with components of mucinous carcinoma arising in a cirrhotic liver. Pathol Int. 2006;56:222–6.
Motoo Y, Sawabu N, Minamoto T, et al. Rapidly growing mucinous cholangiocarcinoma. Intern Med (Tokyo, Japan). 1993;32:116–21.
Oshiro T, Esaki M. A case of intrahepatic cholangiocarcinoma with marked mucus production. Jpn J Clin Oncol. 2011;41:1388.
Sasaki M, Nakanuma Y, Shimizu K, et al. Pathological and immunohistochemical findings in a case of mucinous cholangiocarcinoma. Pathol Int. 1995;45:781–6.
Sonobe H, Enzan H, Ido E, et al. Mucinous cholangiocarcinoma featuring a unique microcystic appearance. Pathol Int. 1995;45:292–6.
Chi Z, Bhalla A, Saeed O, et al. Mucinous intrahepatic cholangiocarcinoma: a distinct variant. Hum Pathol. 2018;78:131–7.
Hagiwara K, Araki K, Yamanaka T, et al. Resected primary mucinous cholangiocarcinoma of the liver. Surg Case Rep. 2018;4:41.
Sumiyoshi T, Shima Y, Okabayashi T, et al. Mucinous cholangiocarcinoma: clinicopathological features of the rarest type of cholangiocarcinoma. Ann Gastroenterol Surg. 2017;1:114–21.
Lee DH, Lee JM. Primary malignant tumours in the non-cirrhotic liver. Eur J Radiol. 2017;95:349–61.
Kee HJ, Ahn KY, Choi KC, et al. Expression of brain-specific angiogenesis inhibitor 3 (BAI3) in normal brain and implications for BAI3 in ischemia-induced brain angiogenesis and malignant glioma. FEBS Lett. 2004;569:307–16.
Zhang Y, Luo J, Liu Z, et al. Identification of hub genes in colorectal cancer based on weighted gene co-expression network analysis. 2021. Biosci Rep. https://doi.org/10.1042/bsr20211280.
Wassarman DA, Sauer F. TAF(II)250: a transcription toolbox. J Cell Sci. 2001;114:2895–902.
Lee DH, Gershenzon N, Gupta M, et al. Functional characterization of core promoter elements: the downstream core element is recognized by TAF1. Mol Cell Biol. 2005;25:9674–86.
Juven-Gershon T, Kadonaga JT. Regulation of gene expression via the core promoter and the basal transcriptional machinery. Dev Biol. 2010;339:225–9.
Tavassoli P, Wafa LA, Cheng H, et al. TAF1 differentially enhances androgen receptor transcriptional activity via its N-terminal kinase and ubiquitin-activating and -conjugating domains. Mol Endocrinol. 2010;24:696–708.
Filippakopoulos P, Picaud S, Mangos M, et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell. 2012;149:214–31.
Zhong S, Yan H, Chen Z, et al. Overexpression of TAF1L promotes cell proliferation, migration and invasion in esophageal squamous cell carcinoma. J Cancer. 2019;10:979–89.
Lawrenson ID, Wimmer-Kleikamp SH, Lock P, et al. Ephrin-A5 induces rounding, blebbing and de-adhesion of EphA3-expressing 293T and melanoma cells by CrkII and Rho-mediated signalling. J Cell Sci. 2002;115:1059–72.
Padthaisong S, Thanee M, Namwat N, et al. A panel of protein kinase high expression is associated with postoperative recurrence in cholangiocarcinoma. BMC Cancer. 2020;20:154.
Hynes NE, MacDonald G. ErbB receptors and signaling pathways in cancer. Curr Opin Cell Biol. 2009;21:177–84.
Shi F, Telesco SE, Liu Y, et al. ErbB3/HER3 intracellular domain is competent to bind ATP and catalyze autophosphorylation. Proc Natl Acad Sci U S A. 2010;107:7692–7.
Berger MB, Mendrola JM, Lemmon MA. ErbB3/HER3 does not homodimerize upon neuregulin binding at the cell surface. FEBS Lett. 2004;569:332–6.
Zhang K, Sun J, Liu N, et al. Transformation of NIH 3T3 cells by HER3 or HER4 receptors requires the presence of HER1 or HER2. J Biol Chem. 1996;271:3884–90.
Fedi P, Pierce JH, di Fiore PP, et al. Efficient coupling with phosphatidylinositol 3-kinase, but not phospholipase C gamma or GTPase-activating protein, distinguishes ErbB-3 signaling from that of other ErbB/EGFR family members. Mol Cell Biol. 1994;14:492–500.
Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–30.
Massagué J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103:295–309.
Massagué J. TGFbeta in cancer. Cell. 2008;134:215–30.
Noë M, Niknafs N, Fischer CG, et al. Genomic characterization of malignant progression in neoplastic pancreatic cysts. Nat Commun. 2020;11:4085.
Puckelwartz MJ, Kessler E, Zhang Y, et al. Disruption of nesprin-1 produces an Emery Dreifuss muscular dystrophy-like phenotype in mice. Hum Mol Genet. 2009;18:607–20.
Ito Y, Takeda T, Sasaki Y, et al. Expression and clinical significance of the erbB family in intrahepatic cholangiocellular carcinoma. Pathol Res Pract. 2001;197:95–100.
Ruzzenente A, Fassan M, Conci S, et al. Cholangiocarcinoma heterogeneity revealed by multigene mutational profiling: clinical and prognostic relevance in surgically resected patients. Ann Surg Oncol. 2016;23:1699–707.
Kim GA, Lee HC, Kim MJ, et al. Incidence of hepatocellular carcinoma after HBsAg seroclearance in chronic hepatitis B patients: a need for surveillance. J Hepatol. 2015;62:1092–9.
Castoria G, Migliaccio A, Bilancio A, et al. PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. Embo J. 2001;20:6050–9.
Sadhu C, Masinovsky B, Dick K, et al. Essential role of phosphoinositide 3-kinase delta in neutrophil directional movement. J Immunol. 2003;170:2647–54.
Graupera M, Guillermet-Guibert J, Foukas LC, et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453:662–6.
Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–41.
Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90.
Chikh A, Ferro R, Abbott JJ, et al. Class II phosphoinositide 3-kinase C2β regulates a novel signaling pathway involved in breast cancer progression. Oncotarget. 2016;7:18325–45.
Liu Z, Li X, Ma J, et al. Integrative analysis of the IQ motif-containing GTPase-activating protein family indicates that the IQGAP3-PIK3C2B axis promotes invasion in colon cancer. Onco Targets Ther. 2020;13:8299–311.
Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci. 2002;115:3861–3.
Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol. 2005;24:389–99.
Gao W, Liu Y, Qin R, et al. Silence of fibronectin 1 increases cisplatin sensitivity of non-small cell lung cancer cell line. Biochem Biophys Res Commun. 2016;476:35–41.
Moroz A, Delella FK, Lacorte LM, et al. Fibronectin induces MMP2 expression in human prostate cancer cells. Biochem Biophys Res Commun. 2013;430:1319–21.
He Y, Andersen GR, Nielsen KH. Structural basis for the function of DEAH helicases. EMBO Rep. 2010;11:180–6.
Seal S, Thompson D, Renwick A, et al. Truncating mutations in the Fanconi anemia J gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nat Genet. 2006;38:1239–41.
Cantor SB, Bell DW, Ganesan S, et al. BACH1, a novel helicase-like protein, interacts directly with BRCA1 and contributes to its DNA repair function. Cell. 2001;105:149–60.
Alix-Panabières C, Cayrefourcq L, Mazard T, et al. Molecular portrait of metastasis-competent circulating tumor cells in colon cancer reveals the crucial role of genes regulating energy metabolism and DNA repair. Clin Chem. 2017;63:700–13.
Rizeq B, Sif S, Nasrallah GK, et al. Novel role of BRCA1 interacting C-terminal helicase 1 (BRIP1) in breast tumour cell invasion. J Cell Mol Med. 2020;24:11477–88.
Chakravarty D, Gao J, Phillips SM, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017;2017:1–16.
Funding
This study was supported in part by JSPS KAKENHI Grant Number 16H06279 (PAGS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from the spouse of the patient for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Masaki, Y., Akutsu, N., Adachi, Y. et al. Genomic analysis of an aggressive case with metastatic intrahepatic mucinous cholangiocarcinoma. Clin J Gastroenterol 15, 809–817 (2022). https://doi.org/10.1007/s12328-022-01649-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-022-01649-x