Abstract
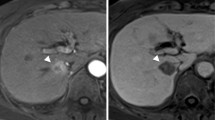
We report on a 62-year-old man with chronic hepatitis C who developed rapidly growing hepatocellular carcinoma (HCC) after achieving sustained virological response at post-treatment week 24 (SVR 24) by direct-acting antiviral (DAA) treatment. In 2008, he failed interferon therapy at 56 years of age. He received daclatasvir plus asunaprevir for 24 weeks after confirmation of no liver tumor by abdominal ultrasonography. He had no advanced liver fibrosis. Three months after initiation of DAA treatment, a liver tumor measuring 6 mm in diameter was detected by ultrasonography and confirmed with magnetic resonance imaging. After achieving SVR 24, the tumor increased in size to 16 mm. Two months later, a tumor biopsy was performed, and histology revealed moderately to poorly differentiated HCC. The patient’s alpha-fetoprotein (AFP) level was within the normal range, but the Lens culinaris agglutinin-reactive fraction of AFP level was elevated. The diameter of the tumor increased to 32 mm at 2 months after diagnosis. Lymph node metastasis in porta hepatis was found by positron emission tomography at 4 months after diagnosis. The patient received hepatic arterial infusion chemotherapy and radiation therapy, but died later. Careful monitoring is required during and after DAA treatment because HCC can grow fast even in patients with normal AFP and no advanced liver fibrosis.



Similar content being viewed by others
References
Hoofnagle JH, Mullen KD, Jones DB, et al. Treatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. A preliminary report. N Engl J Med. 1986;315:1575–8.
Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82.
Russo MW, Fried MW. Side effects of therapy for chronic hepatitis C. Gastroenterology. 2003;124:1711–9.
Pawlotsky JM. New hepatitis C therapy: the toolbox, strategies, and challenges. Gastroenterology. 2014;146:1176–92.
Majumdar A, Kitson MT, Roberts SK, et al. Systematic review; current concepts and challenges for the direct-acting era in hepatitis C cirrhosis. Aliment Pharmacol Ther. 2016;43:1276–92.
Kobayashi M, Suzuki F, Fujiyama S, et al. Sustained virologic response by direct antiviral agents reduces the incidence of hepatocellular carcinoma in patients with HCV infection. J Med Virol. 2017;89:476–83.
Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727–33.
Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–6.
Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26.
Carr BI, Kanke F, Wise M, et al. Clinical evaluation of lens culinaris agglutinin-reactive alpha-fetoprotein and des-gamma-carboxy prothrombin in histologically proven hepatocellular carcinoma in the United States. Dig Dis Sci. 2007;52:776–82.
Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, des-gamma carboxy prothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110–8.
Nguyen K, Jimenez M, Moghadam N, et al. Decrease of alpha-fetoprotein in patients with cirrhosis treated with direct-acting antivirals. J Clin Transl Hepatol. 2017;5:43–9.
Yamashita F, Tanaka M, Satomura S, et al. Prognostic significance of lens culinaris agglutinin A-reactive alpha-fetoprotein in small hepatocellular carcinoma. Gastroenterology. 1996;114:996–1001.
Li DK, Chung RT. Impact of hepatitis C virus eradication on hepatocellular carcinogenesis. Cancer. 2015;121:2874–82.
Makiyama A, Itoh Y, Kasahara A, et al. Characteristics of patients with chronic hepatitis C who develop hepatocellular carcinoma after a sustained response to inferferon therapy. Cancer. 2014;101:1616–22.
Hiramatsu N, Oze T. Takehara T. Suppression of hepatocellular carcinoma development in hepatitis C patients given interferon-based antiviral therapy. Hepatol Res. 2015;45:152–61.
Hung C-H, Lee C-M, Wang J-H, et al. Impact of diabetes mellitus on incidence of hepatocellular carcinoma in chronic hepatitis C patients treated with interferon-based antiviral therapy. Int J Cancer. 2011;128:2344–52.
Yamashita N, Ohho A, Yamasaki A, et al. Hepatocarcinogenesis in chronic hepatitis C patients achieving a sustained virological response to interferon; significance of lifelong periodic cancer screening for improving outcomes. J Gastroenterol. 2014;49:1504–13.
Asahina Y, Tsuchiya K, Nishimura T, et al. Alpha-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013;58:1253–62.
Oze T, Hiramatsu N, Yakushijin T, et al. Post-treatment levels of alpha-fetoprotein predict incidence of hepatocellular carcinoma after interferon therapy. Clin Gastroenterol Hepatol. 2014;12:1186–95.
Ikeda M, Fujiyama S, Tanaka M, et al. Risk factors for development of hepatocellular carcinoma in patients with chronic hepatitis C after sustained response to interferon. J Gastroenterol. 2005;40:148–56.
Villani R, Facciorusso A, Bellanti F, et al. DAAs rapidly reduce inflammation but increase serum VEGF level: a rationale for tumor risk during anti-HCV treatment. PLoS One. 2016;. doi:10.1371/journal.pone.0167934.
Yamaguchi R, Yano H, Iemura A, et al. Expression of vascular endothelial growth factor in human hepatocellular carcinoma. Hepatology. 1998;28:68–77.
Serti E, Chepa-Lotrea X, Kim YJ, et al. Successful interferon-free therapy of chronic hepatitis C virus infection normalizes natural killer cell function. Gastroenterology. 2015;149:190–200.
Spaan M, van Oord G, Kreefft K, et al. Immunological analysis during interferon-free therapy for chronic hepatitis C virus infection reveals modulation of the natural killer cell compartment. J Infect Dis. 2016;213:216–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Human rights
All procedures followed have been performed in accordance with the ethical standards laid down in the Declaration of Helsinki and its amendments.
Informed consent
This study does not require informed consent.
Rights and permissions
About this article
Cite this article
Kawaguchi, T., Ide, T., Koga, H. et al. Rapidly growing hepatocellular carcinoma after direct-acting antiviral treatment of chronic hepatitis C. Clin J Gastroenterol 11, 69–74 (2018). https://doi.org/10.1007/s12328-017-0789-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-017-0789-1