Abstract
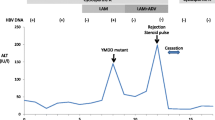
Re-infection by the hepatitis C virus (HCV) occurs rapidly after liver transplantation (LT), and spontaneous clearance of HCV is rare under immunosuppressive conditions. Here, we report on two patients who underwent LT to treat liver cirrhosis and hepatocellular carcinoma. The immunosuppressants prescribed were short-term corticosteroids, tacrolimus, and mycophenolate mofetil. A 50-year-old woman underwent LT, with her brother as the donor. She acquired HCV of serological type 1 after LT; the HCV RNA level was 6.0 logIU/mL. Corticosteroids were discontinued within 24 days, with a total dose of 669 mg (adjusted) prednisolone (PSL). The serum alanine aminotransferase (ALT) level increased to 700 U/L by day 55 post-LT. Surprisingly, HCV RNA was not detected on day 87. A 52-year-old man underwent LT, with his sister as the donor. He became rapidly re-infected with HCV of serological type 2; the HCV RNA level was 6.9 logIU/mL. Corticosteroids were given for 24 days, with a total dose of 827 mg (adjusted) PSL. The serum ALT level increased continuously and his HCV cleared 115 days after LT. Both donor and recipient had the major IL28B genotype. HCV was eliminated spontaneously, even under immunosuppressive conditions, after PSL discontinuation without interferon treatment. Minimal use of immunosuppressants and the presence of hepatitis may have contributed to HCV clearance. However, it is important to evaluate additional relevant cases.


Similar content being viewed by others
References
Gane E. The natural history and outcome of liver transplantation in hepatitis C virus-infected recipients. Liver Transpl. 2003;9:S28–34.
Berenguer M, Prieto M, Rayón JM, et al. Natural history of clinically compensated hepatitis C virus-related graft cirrhosis after liver transplantation. Hepatology. 2000;32:852–8.
Gallegos-Orozco JF, Yosephy A, Noble B, et al. Natural history of post-liver transplantation hepatitis C: a review of factors that may influence its course. Liver Transpl. 2009;15:1872–81.
Gane EJ, Portmann BC, Naoumov NV, et al. Long-term outcome of hepatitis C infection after liver transplantation. N Engl J Med. 1996;334:815–20.
Wali M, Harrison RF, Gow PJ, et al. Advancing donor liver age and rapid fibrosis progression following transplantation for hepatitis C. Gut. 2002;51:248–52.
Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J Viral Hepat. 2006;13:34–41.
Doughty AL, Zekry A, Spencer JD, et al. Spontaneous clearance of hepatitis C virus infection post-liver transplantation is associated with rapidly changing quasispecies: a single case report. Liver Transpl. 2000;6:648–53.
Casanovas-Taltavull T, Ercilla MG, Gonzalez CP, et al. Long-term immune response after liver transplantation in patients with spontaneous or post-treatment HCV-RNA clearance. Liver Transpl. 2004;10:584–94.
Neumann UP, Berg T, Bahra M, et al. Long-term outcome of liver transplants for chronic hepatitis C: a 10-year follow-up. Transplantation. 2004;77:226–31.
Samonakis DN, Cholongitas E, Triantos CK, et al. Sustained, spontaneous disappearance of serum HCV-RNA under immunosuppression after liver transplantation for HCV cirrhosis. J Hepatol. 2005;43:1091–3.
Suneetha PV, Mederacke I, Heim A, et al. Spontaneous clearance of chronic hepatitis C after liver transplantation: are hepatitis C virus-specific T cell responses the clue? Liver Transpl. 2008;14:1225–7.
Weber NK, Trotter JF. Spontaneous clearance of hepatitis C virus after liver transplantation. Transplantation. 2009;87:1102–3.
Dale CH, Burns P, McCutcheon M, et al. Spontaneous clearance of hepatitis C after liver and renal transplantation. Can J Gastroenterol. 2009;23:265–7.
Haque M, Hashim A, Greanya ED, et al. Spontaneous clearance of hepatitis C infection post-liver transplant: a rare but real phenomenon? A case report and review of the literature. Ann Hepatol. 2010;9:202–6.
Chin JL, Nicholas RM, Russell J, et al. Spontaneous clearance of hepatitis C infection after liver transplantation from IL28B rs12979860 CC donors. Eur J Gastroenterol Hepatol. 2012;24:1110–2.
Raghuraman S, Park H, Osburn WO, et al. Spontaneous clearance of chronic hepatitis C virus infection is associated with appearance of neutralizing antibodies and reversal of T-cell exhaustion. J Infect Dis. 2012;205:763–71.
Ciesek S, Steinmann E, Iken M, et al. Glucocorticosteroids increase cell entry by hepatitis C virus. Gastroenterology. 2010;138:1875–84.
Somsouk M, Lauer GM, Casson D, et al. Spontaneous resolution of chronic hepatitis C virus disease after withdrawal of immunosuppression. Gastroenterology. 2003;124:1946–9.
Neumann UP, Neuhaus P. Discussion on spontaneous resolution of chronic hepatitis C virus after withdrawal of immunosuppression. Gastroenterology. 2004;126: 627 (author reply 628).
Nakagawa M, Sakamoto N, Tanabe Y, et al. Suppression of hepatitis C virus replication by cyclosporin a is mediated by blockade of cyclophilins. Gastroenterology. 2005;129:1031–41.
Samonakis DN, Germani G, Burroughs AK. Immunosuppression and HCV recurrence after liver transplantation. J Hepatol. 2012;56:973–83.
Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401.
Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105–9.
Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801.
Grebely J, Page K, Sacks-Davis R, et al. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology. 2014;59:109–20.
Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61:S58–68.
Hoffmann FS, Schmidt A, Dittmann Chevillotte M, et al. Polymorphisms in melanoma differentiation-associated gene 5 link protein function to clearance of hepatitis C virus. Hepatology. 2015;61:460–70.
Bhagat V, Foont JA, Schiff ER, et al. Spontaneous clearance of hepatitis C virus after liver transplantation in two patients coinfected with hepatitis C virus and human immunodeficiency virus. Liver Transpl. 2008;14:92–5.
Hanson RG, Peters MG, Hoofnagle JH. Effects of immunosuppressive therapy with prednisolone on B and T lymphocyte function in patients with chronic type B hepatitis. Hepatology. 1986;6(2):173–9.
Rauch A, Kutalik Z, Descombes P, et al. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338–45, 45.e1–7.
El Awady MK, Bader El Din NG, Abdel Aziz Riad M, et al. Predictors of disease recurrence post living donor liver transplantation in end stage chronic HCV patients. Dis Markers. 2014;2014:202548.
Lange CM, Moradpour D, Doehring A, et al. Impact of donor and recipient IL28B rs12979860 genotypes on hepatitis C virus liver graft reinfection. J Hepatol. 2011;55:322–7.
Fukuhara T, Taketomi A, Motomura T, et al. Variants in IL28B in liver recipients and donors correlate with response to peg-interferon and ribavirin therapy for recurrent hepatitis C. Gastroenterology. 2010;139:1577–85, 85.e1–3.
Grebely J, Dore GJ, Kim AY, et al. Genetics of spontaneous clearance of hepatitis C virus infection: a complex topic with much to learn. Hepatology. 2014;60:2127–8.
Acknowledgments
This work was supported by the Takako Satake Award administered by Tokyo Women’s Medical University and a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (#26461024-0001) to T.K.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest:
Tomomi Kogiso, Etsuko Hashimoto, Yuichi Ikarashi, Kazuhisa Kodama, Makiko Taniai, Nobuyuki Torii, Hiroto Egawa, Masakazu Yamamoto, and Katsutoshi Tokushige declare that they have no conflict of interest.
Human/Animal Rights:
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008(5).
Informed Consent:
Informed consent was obtained from all patients for being included in the study.
Rights and permissions
About this article
Cite this article
Kogiso, T., Hashimoto, E., Ikarashi, Y. et al. Spontaneous clearance of HCV accompanying hepatitis after liver transplantation. Clin J Gastroenterol 8, 323–329 (2015). https://doi.org/10.1007/s12328-015-0602-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12328-015-0602-y