Abstract
Introduction
For patients with epidermal growth factor receptor-mutated (EGFRm) locally advanced/metastatic non-small cell lung cancer (mNSCLC) whose disease has progressed on or after osimertinib and platinum-based chemotherapy (PBC), no uniformly accepted standard of care exists. Moreover, limited efficacy of standard treatments indicates an unmet medical need, which is being addressed by ongoing clinical investigations, including the HERTHENA-Lung01 (NCT04619004) study of patritumab deruxtecan (HER3‑DXd). However, because limited information is available on real-world clinical outcomes in such patients, early-phase trials of investigational therapies lack sufficient context for comparison. This study describes the real-world clinical characteristics, treatments, and outcomes for patients with EGFRm mNSCLC who initiated a new line of therapy following previous osimertinib and PBC, including a subset matched to the HERTHENA-Lung01 population.
Methods
This retrospective analysis used a US database derived from deidentified electronic health records. The reference cohort included patients with EGFRm mNSCLC who had initiated a new line of therapy between November 13, 2015 and June 30, 2021, following prior osimertinib and PBC. A subset of patients resembling the HERTHENA-Lung01 population was then extracted from the reference cohort; this matched subset was optimized using propensity score (PS) weighting. Endpoints were real-world overall survival (rwOS) and real-world progression-free survival (rwPFS). Confirmed real-world objective response rate (rwORR; partial/complete response confirmed ≥ 28 days later) was calculated for the response-evaluable subgroups of patients (with ≥ 2 response assessments spaced ≥ 28 days apart).
Results
In the reference cohort (N = 273), multiple treatment regimens were used, and none was predominant. Median rwPFS and rwOS were 3.3 and 8.6 months, respectively; confirmed rwORR (response evaluable, n = 123) was 13.0%. In the matched subset (n = 126), after PS weighting, median rwPFS and rwOS were 4.2 and 9.1 months, respectively; confirmed rwORR (response evaluable, n = 57) was 14.1%.
Conclusion
The treatment landscape for this heavily pretreated population of patients with EGFRm mNSCLC is fragmented, with no uniformly accepted standard of care. A high unmet need exists for therapeutic options that provide meaningful improvements in clinical benefit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Limited information is available on real-world treatment patterns and clinical outcomes in patients with epidermal growth factor receptor-mutated (EGFRm) locally advanced/metastatic non-small cell lung cancer (mNSCLC) previously treated with osimertinib and platinum-based chemotherapy (PBC). |
This study aimed to describe patient demographic and clinical characteristics, treatment patterns, and real-world clinical outcomes for patients in this setting, including a matched subset that met the inclusion criteria for the recent HERTHENA-Lung01 clinical trial of patritumab deruxtecan (HER3‑DXd). |
What was learned from the study? |
Within the fragmented treatment landscape for patients in this setting, no single regimen was used in > 10% of patients following previous treatment with osimertinib and PBC. |
Patients in the matched subset had median real-world progression-free survival of 4.2 (95% confidence interval [CI] 2.8–5.2) months and real-world overall survival of 9.1 (95% CI 7.4–11.4) months; confirmed real-world objective response rate was 14.1% (95% CI 3.7–33.1%). |
Overall, this study highlights the unmet medical need for more efficacious treatment options for EGFRm mNSCLC following failure of PBC and osimertinib and provides real-world context for evaluating the results of ongoing clinical trials such as HERTHENA-Lung01. |
Introduction
With an annual rate of 1.8 million deaths worldwide, approximately 125,000 of which occur in the USA, lung cancer remains the leading cause of cancer death [1, 2]. Because lung cancer symptoms do not typically occur until the disease has reached an advanced stage, delayed diagnosis is common [3]. In the USA (between 2013 and 2019), 53% of lung cancers had metastasized to distant locations at the time of diagnosis, 21% had spread regionally, and 21% were localized [4]; the majority of cases (80–85%) were non-small cell lung cancer (NSCLC) [5]. The 5-year relative survival rate in patients diagnosed with NSCLC between 2012 and 2018 was 28%; this rate fell to 9% in patients diagnosed with distant metastases [6]. A proportion of patients with NSCLC had tumors with an activating oncogenic mutation in epidermal growth factor receptor (EGFR). Such mutations were found in the tumors of approximately 15% of white and 50% of Asian patients diagnosed with NSCLC, and they occurred more frequently in women (43.7%) than in men (24.0%) [7,8,9]. Activating EGFR mutations, most commonly a deletion in exon 19 or an L858R point mutation in exon 21, are associated with tumor sensitivity to EGFR tyrosine kinase inhibitors (TKIs) [10,11,12].
The current first-line standard-of-care therapy for patients in the USA with EGFR-mutated (EGFRm) locally advanced or metastatic NSCLC (mNSCLC) is osimertinib, a third-generation EGFR TKI [13]. Although rates of response to osimertinib in patients with EGFRm mNSCLC are high (80%) [14], the subsequent development of EGFR TKI resistance and disease progression are typical (median [95% confidence interval (CI)], 17.2 months [13.8–22.0 months]) [14,15,16]. Following disease progression on EGFR TKI therapy, patients with EGFRm mNSCLC are commonly treated with platinum-based chemotherapy (PBC), sometimes in combination with other drugs (immunotherapies, antiangiogenic therapies, other TKIs, or antibodies) [17,18,19].
For patients with EGFRm mNSCLC that has progressed on or after osimertinib and PBC, treatment options are diverse, but their use in the USA has not been adequately characterized. Data from the limited number of clinical trials of available salvage therapies for this patient population have shown limited efficacy (median progression-free survival [PFS], 2.7–3.2 months; median overall survival [OS], 7.5–10.6 months) [20, 21], indicating an unmet need for an efficacious and tolerable third-line therapy. This need for novel therapies in patients with EGFRm mNSCLC with progression following treatment with both osimertinib and PBC is being addressed by ongoing clinical trials, including the MARIPOSA-2 (NCT04988295) study of amivantamab [19], and the single-arm phase 2 HERTHENA-Lung01 study (NCT04619004) of patritumab deruxtecan (HER3‑DXd) [22]. However, because limited information is available on existing clinical outcomes in these patients, early-phase trials of new therapies lack context.
This study describes the real-world demographic and clinical characteristics, treatment patterns, and outcomes for patients with EGFRm mNSCLC who initiated a new line of therapy (LOT) following previous treatment with osimertinib and PBC. A subset of real-world patients matched to the population of HERTHENA-Lung01 was also described.
Methods
Study Design
This was a noninterventional, retrospective cohort study in real-world patients with EGFRm mNSCLC who received a new LOT after prior treatment with osimertinib and PBC in the setting of advanced NSCLC. The follow-up duration for study outcomes was from initiation (index date) of the new LOT (index LOT) to the last observed encounter in the source data (e.g., death, last confirmed activity date, or end of the study period, whichever came first). Analyses were conducted using the study reference cohort and a matched subset of that cohort, which was based on the selection criteria of and patient characteristics in the HERTHENA-Lung01 study.
Data Source
Data were provided by Flatiron Health (https://www.flatiron.com). The Flatiron Health database consists of deidentified electronic health records, with longitudinal patient-level structured and unstructured data, curated using technology-enabled abstraction [23, 24]. The data were sourced from more than three million patients across 280 cancer clinics (approximately 800 sites of care) in the USA. Approximately 80% of patients came from community practices and 20% from academic research centers. Patient-level data included demographics (e.g., age, sex, and race), diagnostic information (e.g., stage, pathology, molecular information, and radiology), extent of disease, laboratory values, treatments (e.g., LOT, dosing, and regimens), practice information (e.g., type and region), and patient outcomes (e.g., mortality, progression, and response events). LOTs were rule based and defined by expert oncology clinicians. Other enhanced data elements were curated for malignancies, treatment discontinuation, sites of metastases, radiation/surgical treatment, comorbidities, and selected adverse events. The Flatiron Health database includes distributions of patients with NSCLC according to age, sex, and geographic location similar to those of the Surveillance, Epidemiology, and End Results Program and the National Program of Cancer Registries [25]. The deidentified data were subject to obligations to prevent reidentification and protect patient confidentiality.
Analytic Cohorts
Flatiron Health provided a dataset consisting of patients with mNSCLC (NSCLC stages IIIB to IVB; International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] 162.x; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] C34.x or C39.9; along with consistent pathology) who had initiated a new LOT after prior treatment with osimertinib and PBC on or before December 31, 2021. These patients had ≥ 2 unique visit dates on or after January 1, 2011, and nonmissing relevant unstructured documents for abstraction. Further selection criteria, outlined below, were applied to establish the study reference cohort and the matched subset.
Study patients were eligible for the reference cohort if they (1) had evidence of structured activity within 90 days after diagnosis of mNSCLC, (2) were ≥ 18 years of age at their index date, (3) had a documented activating EGFR mutation (exon 19 deletion, L858R mutation) or could be inferred to have an activating EGFR mutation based on treatment with osimertinib and no evidence of other actionable genomic alterations (e.g., BRAF, KRAS, MET, RET, NTRK, ROS1 rearrangement, ALK rearrangement, or EGFR exon 20 mutation), and (4) initiated their index LOT between November 13, 2015 (US Food and Drug Administration approval of osimertinib) and June 30, 2021 (allowing ≥ 6 months of potential follow-up).
Selection criteria for the matched subset were adapted from those of the HERTHENA-Lung01 trial. Patients were eligible if they met the selection criteria for the reference cohort and did not have evidence of the following exclusions: (1) Eastern Cooperative Oncology Group (ECOG) performance status missing or > 1 based on the most proximal assessment within 90 days before or up to 7 days after the index date, (2) index date after March 31, 2021 (allowing ≥ 9 months of potential follow-up), (3) evidence of interstitial lung disease (including pulmonary fibrosis and radiation pneumonitis) on or within 12 months prior to the index date, (4) evidence of severe respiratory compromise on or within 6 months prior to the index date, (5) treatment with a clinical study drug within the qualifying index regimen, (6) treatment with anti-human epidermal growth factor receptor 3 (HER3) therapy or a topoisomerase I inhibitor on or before the index date, (7) any non-NSCLC primary malignancy on or within 12 months prior to the index date, (8) acute myocardial infarction on or within 6 months prior to the index date, (9) evidence of HIV or AIDS, (10) evidence of leptomeningeal disease on or within 12 months prior to the index date, or (11) evidence of clinically active spinal cord compression, evidence of brain metastasis, or treatment for brain metastasis within 3 months prior to the index date.
Within the reference cohort and the matched subset, response-evaluable patients were those with ≥ 2 response assessments ≥ 28 days apart after initiation of the index LOT (which was the minimum requirement to determine a confirmed response, defined as a complete/partial response confirmed ≥ 28 days later).
Study Measures
The primary outcomes evaluated were real-world progression-free survival (rwPFS), real-world overall survival (rwOS), and confirmed real-world objective response rate (rwORR) in the USA [25,26,27]. rwPFS was defined as the time from the index date to the earliest occurrence of real-world progression (rwP; documented as growth or worsening of mNSCLC by the treating clinician via radiological and/or pathological evidence) or death from any cause. Patients without a rwP event were censored at the last documented clinic notation. rwOS was defined as the time from the index date to death from any cause. In the source data, only the year and month of death were provided; thus, the date of death was imputed as the 15th of the month or the last documented encounter, whichever was later. Patients without a documented death were censored at the last structured activity in the electronic health record. Confirmed rwORR was defined as the proportion of patients who achieved a real-world partial or complete response (assessed by a treating physician or radiologist) confirmed ≥ 28 days from the initial response assessment among patients with ≥ 2 response assessments ≥ 28 days apart (response-evaluable patients) to align with the corresponding response measure in the HERTHENA-Lung01 trial. Patient demographic and clinical characteristics were assigned as the most recent measure at the index date.
Statistical Analysis and Propensity Score Weighting
Descriptive statistics were used to summarize all study variables at the time of initiation of the index LOT. Kaplan–Meier methods were used to evaluate time-to-event outcomes (rwPFS, rwOS). For the reference cohort, rwPFS and rwOS were also analyzed for subgroups based on ECOG performance status (unknown/0 or 1/≥ 2), prior immunotherapy (yes/no), EGFR mutation (exon 19 deletion/L858R mutation), history of liver metastases (yes/no [or unknown]), and history of brain metastases (yes/no [or unknown]). Confirmed rwORR was estimated as the proportion of patients with a Clopper–Pearson exact 95% CI.
For the analyses of the matched subset and its subgroup of response-evaluable patients, real-world patients were propensity score (PS) weighted so that the distribution of baseline characteristics would resemble that in the HERTHENA-Lung01 trial population. Propensity scores were estimated using logistic regression to model the log odds of HERTHENA-Lung01 trial enrollment as a linear function of the set of nine prespecified prognostic factors (age, sex, race, history of smoking, ECOG performance status, prior LOTs, prior immunotherapy, history of liver metastases, and history of brain metastases) [28, 29]. Covariate balance between the real-world and trial cohorts was assessed before and after PS weighting using standardized mean differences, with a prespecified threshold of ± 0.2. Additionally, the effective sample size (ESS) was calculated to reflect the impact of PS weighting on the amount of information retained from the real-world cohorts. ESS can be interpreted in this context as the number of independent unweighted observations that achieve the same precision (or variance) as the weighted observations.
Ethics Statement
This study was exempt from review by an institutional review board because the database was compliant with the Health Insurance Portability and Accountability Act, the data did not include any identifiable patient information, and the study did not involve human subjects, as defined by 45 CFR 46.102(f)(2). Permission was granted from the database owner (Flatiron Health) to access the database.
Results
Patient Populations and Propensity Score Weighting
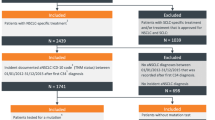
The initial database sample consisted of 409 patients. After all inclusion and exclusion criteria were applied (Fig. 1), the reference cohort included 273 patients, with a median follow-up of 7.3 months (range 0.0–60.8 months). Within the reference cohort, 123 were response evaluable, with a median follow-up of 11.4 months (range 3.1–60.8 months). After the inclusion and exclusion criteria modeled on the HERTHENA-Lung01 study were applied (Fig. 1), the matched subset included 126 patients, with a median follow-up of 9.2 months (range 0.03–59.9 months). Within the matched subset, 57 were response evaluable, with a median follow-up of 15.2 months (range 3.9–59.9 months).
Patient selection diagram and analytic cohorts. AIDS acquired immunodeficiency syndrome, ECOG Eastern Cooperative Oncology Group, EGFR epidermal growth factor receptor, EHR electronic health record, HER3 human epidermal growth factor receptor 3, HIV human immunodeficiency virus, ILD interstitial lung disease, mNSCLC metastatic non-small cell lung cancer, PBC platinum-based chemotherapy, PS propensity score. aIndex date was defined as the date of initiation of a new line of therapy after prior treatment with osimertinib and platinum-based chemotherapy. bDocumented evidence of an activating EGFR mutation (exon 19 deletion, L858R mutation) or no evidence of other mutations (e.g., BRAF, KRAS, MET, RET, NTRK, ROS1 rearrangement, ALK rearrangement, or EGFR exon 20 mutation). cThe exclusion criteria for the matched cohort were based on the entry criteria for the HERTHENA-Lung01 study
For the matched subset, PS weighting based on the nine prespecified prognostic factors achieved covariate balance (standardized mean difference within ± 0.2 for each variable) between the matched subset and the HERTHENA-Lung01 target population (Supplementary Materials Table S1), with ESS = 78. However, covariate balance was not achieved for the response-evaluable patients within the matched subset. On the basis of the clinical judgment that sex was the least impactful covariate, the sex variable was excluded, and PS weighting of the response-evaluable patients was repeated based on the eight remaining prespecified prognostic factors. With sex excluded, covariate balance was achieved (Supplementary Materials Table S1), with ESS = 26.
Demographic and Clinical Characteristics
The median age of the reference cohort at the index LOT was 67 years (interquartile range, 60–74 years), and most patients were female (67.4%), white (61.2%), and treated in a community practice setting (79.1%) (Table 1). In the reference cohort, 73% of patients had an ECOG performance status of ≤ 1 at the index date, 39.2% had a history of brain metastases, and 27.8% had a history of liver metastases. The median number of prior LOTs was 3. The response-evaluable patients within the reference cohort had demographic and clinical characteristics similar to those of the full cohort.
After PS weighting, the distribution of baseline characteristics of the matched subset and its subgroup of response-evaluable patients resembled that of the HERTHENA-Lung01 trial population [22] (Table 2). The median age was 63 years in both the HERTHENA-Lung01 trial population and the matched subset and 62 years in the response-evaluable patients of the matched subset. Most patients had an ECOG performance status of 1 (matched subset, 67.9%; response-evaluable patients, 66.3%; HERTHENA-Lung01 population, 66.5%), and similar proportions of patients had a history of brain metastases (matched subset, 48.9%; response-evaluable patients, 42.7%; HERTHENA-Lung01 population, 32.1%) or a history of liver metastases (matched subset, 36.1%; response-evaluable patients, 33.9%; HERTHENA-Lung01 population, 34.0%).
Real-World Treatment Patterns
In the reference cohort, no single treatment regimen among the index LOTs was used in > 10% of patients (Table 3, Supplementary Materials Table S2). A majority of patients (51.6%) received combination therapies as the index LOT. The most represented treatment type was chemotherapy other than PBC (23.1%), followed by immunotherapy as monotherapy (17.2%), immunotherapy in combination with another agent (15.4%), EGFR TKI monotherapy (15.4%), EGFR TKI in combination with nonimmunotherapy (12.1%), PBC (11.7%), any regimen containing a clinical study drug (4.0%), and other regimen (1.1%) (Supplementary Materials Table S3).
Real-World PFS and OS
In the reference cohort, median rwPFS was 3.3 months (95% CI 2.8–4.4 months; Fig. 2a). The reference cohort subgroup analyses (Supplementary Materials Fig. S1) showed that rwPFS appeared to be shorter in patients with a history of liver metastases (median, 2.3 months [95% CI 1.7–2.8 months]) than in those without (median, 4.4 months [95% CI 3.4–5.2 months]). Median rwOS was 8.6 months (95% CI 7.4–9.8 months; Fig. 2b), and the subgroup analyses (Supplementary Materials Fig. S1) showed that patients with a history of liver metastases had shorter rwOS (median, 4.9 months [95% CI 3.9–8.5 months]) than those without (median, 9.5 months [95% CI 8.6–12.0 months]).
In the matched subset, median rwPFS was 4.2 months (95% CI 2.8–5.2 months) after PS weighting to the HERTHENA-Lung01 trial population (Fig. 2c). Median rwOS was 9.1 months (95% CI 7.4–11.4 months) after PS weighting to the HERTHENA-Lung01 trial population (Fig. 2d).
Confirmed Real-World ORR
Confirmed rwORR was only evaluable in patients with ≥ 2 response assessments ≥ 28 days apart. In the reference cohort (response-evaluable patients, n = 123 [45% of reference cohort]), confirmed rwORR was 13.0% (95% CI 7.6–20.3%; Table 4). In the matched subset (response-evaluable patients, n = 57 [45% of matched subset]), confirmed rwORR was 14.1% (95% CI 3.7–33.1%) after PS weighting to the HERTHENA-Lung01 trial population (Table 4).
Discussion
This study described real-world treatment patterns and outcomes, which have not been previously characterized in the literature, following prior therapy with osimertinib and PBC in US patients with EGFRm mNSCLC. This analysis showed that for real-world patients with EGFRm mNSCLC, treatment strategies following previous osimertinib and PBC were fragmented; in the reference cohort, no index treatment regimen was received by more than 10% of patients.
Previous studies using the Flatiron Health database have established that real-world data can provide informative comparisons to clinical trial data regarding outcome measures in NSCLC, including rwPFS, rwOS, and rwORR [26, 30]. To characterize the unmet need in clinical practice and provide context for results of the phase 2 HERTHENA-Lung01 trial, we estimated these measures in a real-world population that was matched (using selection criteria and PS weighting) to the HERTHENA-Lung01 patient population to resemble the distribution of baseline characteristics. We found median rwPFS of 4.2 months (95% CI 2.8–5.2 months) and median rwOS of 9.1 months (95% CI 7.4–11.4 months) in the matched subset after PS weighting to the HERTHENA-Lung01 trial. In contrast, HERTHENA-Lung01 reported median PFS of 5.5 months (95% CI 5.1–6.4 months) and median OS of 11.9 months (95% CI 10.9–13.1 months) in patients with EGFRm mNSCLC treated with HER3-DXd after previous treatment with osimertinib and PBC [22]. The analysis in response-evaluable patients found that the rwORR to the index LOTs was 13.0% (95% CI 7.6–20.3%) in the reference cohort and 14.1% (95% CI 3.7–33.1%) in the matched subset after PS weighting. In contrast, HERTHENA-Lung01 reported a confirmed ORR of 29.2% (95% CI 23.1–35.9%) [22].
This study provides real-world evidence reflective of recent US clinical practice in the context of EGFRm mNSCLC and highlights the limitations of available treatment strategies following prior treatment with osimertinib and PBC. Although optimal post-osimertinib approaches are evolving [19, 31], there is an ongoing need for clinical trials with these patients to identify optimal treatment strategies, as prior real-world studies suggest outcomes may be improved with therapy tailored to address acquired resistance mechanisms [32]. While the results of our study are reflective of current clinical practice in the USA, the methodology is associated with the following limitations, and results should be interpreted with caution. In clinical trials, tumor size changes are evaluated with scheduled radiographic images using standardized criteria, often by blinded independent central review, to provide robust, consistent assessments of tumor changes. In contrast, real-world responses are based on clinical assessments that are unblinded and may differ in timing and methodology; therefore, differences in ascertainment of outcomes could exist. The source database consisted of aggregated electronic health records and physicians’ notes; therefore, information on key study variables may not have been consistently documented. Furthermore, undocumented data may not have been missing at random; to the degree that missingness was associated with study outcomes, estimates may be biased. Additionally, the sample sizes of the analytical cohorts were relatively small, particularly for the response-evaluable subgroups. For the rwORR analyses, more than half of the study population did not have the requisite assessments to be included in the calculation. However, it is likely that our estimates of confirmed rwORR were overestimates, as patients may have been excluded from the response-evaluable subgroups because they had disease progression, died, or initiated a new LOT prior to meeting the assessment criteria. Median rwPFS and rwOS were longer in the response-evaluable patients than in the full reference cohort or the matched subset (Supplementary Materials Table S4). This study was not powered for comparative analyses; no hypotheses or statistical tests were prespecified or conducted, and the precision of estimates is generally limited. Finally, cross-study comparisons should always be treated with caution.
Conclusion
This study provides real-world context for the interpretation of results from ongoing randomized trials evaluating novel therapeutic strategies for these patients with EGFRm mNSCLC that have progressed on the current EGFR TKI-based standard of care, including those from the HERTHENA-Lung01 trial. Several limitations inherent to the study design and data source should be kept in mind including differences in assessment measures compared with the clinical trial setting, and limitations in data availability within the medical records used for this analysis. Taken together, the results of this analysis still reinforce the unmet medical need for more efficacious treatments in this population of heavily pretreated patients with EGFRm mNSCLC and support the need for ongoing clinical trials in these patients.
Data Availability
The data that support the findings of this study have been originated by Flatiron Health, Inc. Requests for data sharing by license or by permission for the specific purpose of replicating results in this manuscript can be submitted to publicationsdataaccess@flatiron.com.
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49.
Mayo Clinic. Lung cancer: symptoms and causes. https://www.mayoclinic.org/diseases-conditions/lung-cancer/symptoms-causes/syc-20374620. Accessed 1 Feb 2024
National Cancer Institute/Surveillance Epidemiology and End Results Program. Cancer stat facts: lung and bronchus cancer. https://seer.cancer.gov/statfacts/html/lungb.html. Accessed 1 Feb 2024
American Cancer Society. Key statistics for lung cancer: American Cancer Scociety. https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html. Accessed 1 Feb 2024
American Cancer Society. Lung cancer survival rates: American Cancer Society. https://www.cancer.org/cancer/lung-cancer/detection-diagnosis-staging/survival-rates.html. Accessed 1 Feb 2024
Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–67.
Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9(2):154–62.
Zhang YL, Yuan JQ, Wang KF, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7(48):78985–93.
He J, Huang Z, Han L, Gong Y, Xie C. Mechanisms and management of 3rd‑generation EGFR‑TKI resistance in advanced non‑small cell lung cancer (Review). Int J Oncol. 2021;59(5):90.
Hsu WH, Yang JC, Mok TS, Loong HH. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol. 2018;29(suppl_1):i3–9.
Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97(5):339–46.
FDA approves osimertinib for first-line treatment of metastatic NSCLC with most common EGFR mutations [press release]. 2018. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-osimertinib-first-line-treatment-metastatic-nsclc-most-common-egfr-mutations.
Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–25.
Minari R, Bordi P, Tiseo M. Third-generation epidermal growth factor receptor-tyrosine kinase inhibitors in T790M-positive non-small cell lung cancer: review on emerged mechanisms of resistance. Transl Lung Cancer Res. 2016;5(6):695–708.
Hendriks LE, Kerr KM, Menis J, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):339–57.
Han B, Yang L, Wang X, Yao L. Efficacy of pemetrexed-based regimens in advanced non-small cell lung cancer patients with activating epidermal growth factor receptor mutations after tyrosine kinase inhibitor failure: a systematic review. Onco Targets Ther. 2018;11:2121–9.
Hayashi H, Sugawara S, Fukuda Y, et al. A Randomized phase II study comparing nivolumab with carboplatin-pemetrexed for EGFR-mutated NSCLC with resistance to EGFR tyrosine kinase inhibitors (WJOG8515L). Clin Cancer Res. 2022;28(5):893–902.
Passaro A, Wang J, Wang Y, et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA-2 study. Ann Oncol. 2024;35(1):77–90.
Gadgeel S, Wang E, Phani S, et al. EP12.01-64 metastatic non-small cell lung cancer with egfr mutations: treatment pattern and outcomes from a systematic literature review. J Thorac Oncol. 2023;18(11):S667.
Yang CJ, Hung JY, Tsai MJ, et al. The salvage therapy in lung adenocarcinoma initially harbored susceptible EGFR mutation and acquired resistance occurred to the first-line gefitinib and second-line cytotoxic chemotherapy. BMC Pharmacol Toxicol. 2017;18(1):21.
Yu HA, Goto Y, Hayashi H, et al. HERTHENA-Lung01, a phase II trial of patritumab deruxtecan (HER3-DXd) in epidermal growth factor receptor-mutated non-small-cell lung cancer after epidermal growth factor receptor tyrosine kinase inhibitor therapy and platinum-based chemotherapy. J Clin Oncol. 2023;41(35):5363–75.
Ma X, Long L, Moon S, Adamson BJS, Baxi SS. Comparison of population characteristics in real-world clinical oncology databases in the US: Flatiron Health, SEER, and NPCR. medRxiv. 2023:2020.03.16.20037143.
Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv pre-print server. 2020.
Ma X, Bellomo L, Magee K, et al. Characterization of a real-world response variable and comparison with RECIST-based response rates from clinical trials in advanced NSCLC. Adv Ther. 2021;38(4):1843–59.
Ton TGN, Pal N, Trinh H, et al. Replication of overall survival, progression-free survival, and overall response in chemotherapy arms of non-small cell lung cancer trials using real-world data. Clin Cancer Res. 2022;28(13):2844–53.
Zhang Q, Gossai A, Monroe S, Nussbaum NC, Parrinello CM. Validation analysis of a composite real-world mortality endpoint for patients with cancer in the United States. Health Serv Res. 2021;56(6):1281–7.
Alexander M, Wolfe R, Ball D, et al. Lung cancer prognostic index: a risk score to predict overall survival after the diagnosis of non-small-cell lung cancer. Br J Cancer. 2017;117(5):744–51.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–94.
Griffith SD, Miksad RA, Calkins G, et al. Characterizing the feasibility and performance of real-world tumor progression end points and their association with overall survival in a large advanced non-small-cell lung cancer data set. JCO Clin Cancer Inform. 2019;3:1–13.
Planchard D, Jänne PA, Cheng Y, et al. Osimertinib with or without chemotherapy in EGFR-mutated advanced NSCLC. N Engl J Med. 2023;389(21):1935–48.
Choudhury NJ, Marra A, Sui JSY, et al. Molecular biomarkers of disease outcomes and mechanisms of acquired resistance to first-line osimertinib in advanced EGFR-mutant lung cancers. J Thorac Oncol. 2023;18(4):463–75.
Acknowledgements
Medical Writing, Editorial, and Other Assistance
The authors thank Alexandra Vasile for their contribution to the conception and design of this work. Medical writing assistance was provided by Henry Blanton and Kristen Downs (Genesis Research Group) and by Amos Race, PhD, CMPP (Nucleus Global), funded by Daiichi Sankyo.
Funding
This study and the journal’s Rapid Service and Open Access Fees were funded by Daiichi Sankyo, Inc, Basking Ridge, NJ, USA.
Author information
Authors and Affiliations
Contributions
Jyoti Patel, Jie Meng, Hoa Le, Yoko Tanaka, Sudarshan Phani, Maribel Salas, Chuntao Wu, David Sternberg, Stephen Esker, Jeffrey P. Anderson, Aaron Crowley, Summera Q. Zhou, Camryn Lieb, Haiyan Sun, Quan V. Doan, Anu Santhanagopal, Karen L. Reckamp were involved in study design, analysis, and interpretation. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors meet ICMJE criteria, and all those who fulfilled those criteria are listed as authors. All authors provided direction and comments on the manuscript, made the final decision about where to publish these data, and approved submission to this journal.
Corresponding author
Ethics declarations
Conflict of Interest
Jyoti Patel is an employee of Northwestern University and reports an advisory role for AstraZeneca, AbbVie, Bristol Myers Squibb, Takeda, Genentech, and Daiichi Sankyo. Jie Meng, Hoa Le, Yoko Tanaka, Sudarshan Phani, Chuntao Wu, David Sternberg, and Stephen Esker are employees of Daiichi Sankyo and hold stock in Daiichi Sankyo. Maribel Salas is an employee of Bayer Inc. and was an employee of Daiichi Sankyo at the time of the study. Anu Santhanagopal is an employee of Boehringer Ingelheim and was an employee of Daiichi Sankyo at the time of the study. Summera Q. Zhou is an employee of Daiichi Sankyo and was an employee of Genesis Research Group at the time of the study. Jeffrey P. Anderson, Aaron Crowley, Camryn Lieb, and Haiyan Sun are employees of Genesis Research Group, whose company received research funding from Daiichi Sankyo to complete this study. Quan V. Doan was an employee of Genesis Research Group at the time of the study and has no additional affiliations to declare. Karen L. Reckamp is an employee of Cedars-Sinai Medical Center and reports receiving research funding to her institution from Mirati, Genentech, Blueprint, Calithera, Daiichi Sankyo, Elevation Oncology, and Janssen and reports receiving honoraria as a consultant for Amgen, AstraZeneca, Blueprint, Daiichi Sankyo, EMD Serono, Genentech, GlaxoSmithKline, Janssen, Lilly, Merck KGaA, Mirati, Seagen, and Takeda.
Ethical Approval
This study was exempt from review by an institutional review board because the database was compliant with the Health Insurance Portability and Accountability Act, the data did not include any identifiable patient information, and the study did not involve human subjects, as defined by 45 CFR 46.102(f)(2). Permission was granted from the database owner (Flatiron Health) to access the database.
Additional information
Affiliations for authors are reflective of the time of the study. Since the time of the study the affiliations for the following authors have changed and are reflected in the Conflict of Interest section: Maribel Salas, Summera Q. Zhou, Quan V. Doan, and Anu Santhanagopal.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Patel, J., Meng, J., Le, H. et al. Real-World Treatment Patterns and Clinical Outcomes Among Patients with Metastatic or Unresectable EGFR-Mutated Non-Small Cell Lung Cancer Previously Treated with Osimertinib and Platinum-Based Chemotherapy. Adv Ther 41, 3299–3315 (2024). https://doi.org/10.1007/s12325-024-02936-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02936-4