Abstract
Introduction
Severe exacerbations of chronic obstructive pulmonary disease (COPD) are known to increase the risk of cardiovascular events. However, this association has not been investigated specifically in patients with COPD in Japan, whose characteristics may differ from those of Western patients (i.e., western Europe, the US, and Canada).
Methods
This longitudinal retrospective cohort study analyzed secondary claims data and included patients aged ≥ 40 years with COPD (International Classification of Diseases-10 codes J41–J44). All exacerbations occurring during follow-up were measured. Time-dependent Cox models were used to estimate hazard ratios (HRs) for the association between time periods following an exacerbation of COPD (vs. time prior to a first exacerbation) and occurrence of a first hospitalization for a severe fatal or non-fatal cardiovascular event.
Results
The analysis included 152,712 patients with COPD with a mean age of 73.8 years and 37.6% of whom were female. During a median follow-up of 37 months, 63,182 (41.4%) patients experienced ≥ 1 exacerbation and 13,314 (8.7%) patients experienced ≥ 1 severe cardiovascular event. Following an exacerbation of COPD, the risk of a severe cardiovascular event was increased in the first 30 days [adjusted HR (aHR) 1.44, 95% confidence interval (CI) 1.33–1.55] and remained elevated for 365 days post-exacerbation (aHR 1.13, 95% CI 1.04–1.23). Specifically, the risks of acute coronary syndrome or arrhythmias remained significantly increased for up to 180 days, and the risk of decompensated heart failure for 1 year.
Conclusion
Among Japanese patients with COPD, the risk of experiencing a severe cardiovascular event increased following a COPD exacerbation and remained elevated for 365 days, emphasizing the need to prevent exacerbations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Patients with chronic obstructive pulmonary disease (COPD) are at increased risk of cardiovascular events following an exacerbation; however, the risks have not been investigated in Japanese patients with COPD. |
A better understanding of the risks faced by Japanese patients with COPD, who have unique characteristics compared with populations studied overseas, could help inform patient care. |
What was learned from the study? |
In the multicountry EXACOS-CV Programme, Japanese patients with COPD tended to be older and were more likely to be male compared with Western populations (i.e., those from western Europe, the US, and Canada). |
The risk of a severe cardiovascular event was increased in the first 30 days after an exacerbation (adjusted hazard ratio: 1.44; 95% confidence interval 1.33–1.55) and the risk remained elevated for 365 days post-exacerbation (adjusted hazard ratio: 1.13; 95% confidence interval 1.04–1.23). |
Despite demographic differences and a generally lower baseline cardiovascular risk profile in Japanese versus Western patients with COPD, Japanese patients have a similarly elevated risk of severe cardiovascular events following moderate or severe COPD exacerbations as Western patients, illustrating the need for close post-exacerbation monitoring, regardless of ethnicity. |
Introduction
Exacerbations of chronic obstructive pulmonary disease (COPD) have a major impact on the health of patients and impose societal and economic burdens [1]. During an exacerbation, symptoms of COPD rapidly worsen and respiratory function declines. The effects of an exacerbation may be long lasting, such that a patient’s lung function and health-related quality of life may not fully recover [2]. Moreover, exacerbations are a risk factor for subsequent exacerbations [3] and can ultimately lead to death [4,5,6]. COPD and cardiovascular diseases frequently occur together and share common risk factors such as smoking, air pollution, sex, advanced age, and genetic factors [7, 8]. In addition, COPD is an independent risk factor for cardiovascular diseases [9,10,11]. Patients with COPD and cardiovascular diseases have a high risk of hospitalization and mortality [12,13,14].
An increased risk of cardiovascular events within the first 30 days following a COPD exacerbation was observed among patients with COPD and with cardiovascular risk factors in the SUMMIT post hoc analysis [15]. Recently, the multicountry EXAcerbations of COPD and their OutcomeS on CardioVascular diseases (EXACOS-CV) Programme, which includes real-world data gathered from claims databases, has reported associations between COPD exacerbations and the onset of cardiovascular events in the US, Canada, UK, Germany, and The Netherlands in patients with COPD and both with and without other baseline cardiovascular risk factors [16,17,18,19,20]. Compared with Western patients diagnosed with COPD, Japanese patients are more likely to be male, tend to be older, have a lower body mass index, and present with less frequent exacerbations and lower rates of cardiovascular disease and metabolic syndrome comorbidities [21,22,23,24,25,26]. Therefore, it is necessary to verify whether the associations between COPD exacerbations and the onset of cardiovascular events observed in other studies are likely to be applicable to Japanese patients, who have not been represented in earlier studies on this topic.
In this study, we examined the association between the time following a COPD exacerbation and the onset of a first serious cardiovascular event, including death, in Japanese patients with COPD aged ≥ 40 years, regardless of pre-existing cardiovascular risk. Moreover, we investigated this association separately for moderate and severe exacerbations, and explored each cardiovascular event risk individually.
Methods
This longitudinal retrospective cohort study is part of the EXACOS-CV Programme being conducted in 10 countries and for which the methodology has been previously described [27]. The present report describes the results of EXACOS-CV in Japan. This study was conducted according to the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects. As the data for this study were extracted from a commercially available de-identified claims database, informed consent was not required. The protocol of this study and any amendments were approved in writing by the MINS Research Ethics Committee (MINS-REC220211; 7 April 2022). Permission was obtained to access and use the data from the Medical Data Vision (MDV) database.
Data Source
The MDV database, which combines anonymized claims data collected by multiple Diagnosis Procedure Combination (DPC) hospitals across Japan, was leveraged for this analysis [28]. At the time the study was conducted, the MDV database was the only resource that could be used to retrospectively evaluate the status of elderly patients diagnosed with COPD. Furthermore, the MDV database has been used previously to generate reports of real-world COPD data in Japan [29, 30]. DPC hospitals agree to be reimbursed by the government, based on detailed healthcare utilization by patients (and not on averaged costs). As of April 2019, the MDV database covered approximately 22% of the 1727 DPC hospitals in Japan, making it one of the largest databases of hospital medical information (hospitalizations, diagnoses, treatments, and procedures) available in Japan. The database contains information regarding inpatient and hospital outpatient visits, and hospital pharmacy drug dispensation.
Study Population
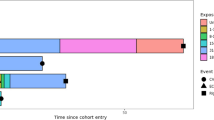
Individuals with a diagnosis of COPD were identified in the MDV database between 1 January 2015 and 31 December 2018. COPD diagnosis was approximated by the combination of (1) ≥ 2 diagnosis codes for COPD in the outpatient setting or ≥ 1 inpatient discharge diagnosis code for COPD [International Classification of Diseases (ICD)-10 codes: J41, simple and mucopurulent chronic bronchitis; J42, unspecified chronic bronchitis; J43, unilateral pulmonary emphysema; and J44, other chronic obstructive pulmonary diseases]; the first identified code defined cohort entry (start of follow-up), and (2) age ≥ 40 years upon cohort entry. Patients were required to have data available for ≥ 12 months prior to cohort entry (baseline) and at least 1 day of follow-up. Patients with a record of α1-antitrypsin deficiency (ICD-10 E88.0) during the baseline period were excluded. Follow-up lasted until the earliest of the outcome of interest or censoring (31 December 2019 or loss to follow-up/disenrollment). Deaths occurring outside the hospital are not captured in the MDV database. The study design and periods are shown in Fig. 1.
Study design. (1) Selection period: Selection period for patients with COPD (1 January 2015 to 31 December 2018; retrospective screening for patients with COPD in the database), (2) Study period end: 31 December 2019, (3) Cohort entry date: Date of the first COPD diagnosis and date of the start of the follow-up period (the cohort entry date is included in the follow-up period), (4) Baseline period: 12-month lookback period starting from the cohort entry date, defined as the start of follow-up; that is, the date of the first identified COPD diagnosis occurring after 1 January 2015 until 30 December 2018, (5) Follow-up period: Period from the date of cohort registration until the earliest of the following: a 31 December 2019 (end of follow-up), b loss to follow-up, including death, c occurrence of a first severe cardiovascular event. COPD chronic obstructive pulmonary disease, ICD International Classification of Diseases
Exposure
Exposures of interest were all moderate or severe exacerbations of COPD occurring during the study. A moderate exacerbation was defined as an outpatient visit for COPD (ICD-10 J41–J44) or acute bronchitis (ICD-10 J20–J22) with a dispensation of injectable steroid or oral corticosteroids or oral antibiotics within 5 days following the visit and for a duration of 5–15 days. A severe exacerbation was defined as a hospitalization with a main discharge code of COPD (ICD-10 J41–J44) (see Supplementary Methods in the electronic supplementary material for details). The exposure period started on the first day of the exacerbation and lasted up to 365 days post-exacerbation or until the occurrence of a cardiovascular event, whichever came first (Fig. S1 in the electronic supplementary material). The exposure period was divided a priori into subperiods of exposure (1–7 days, 8–14 days, 15–30 days, 31–180 days, 181–365 days, and > 365 days following the onset of an exacerbation), given that the incidence rates and hazard ratios (HRs) of the outcome were expected to change over the year following an exacerbation. Upon occurrence of a subsequent exacerbation, exposed time was reset to day 1. The unexposed period (used as reference in models) was the time prior to a first exacerbation, or prior to censoring in the absence thereof.
Outcomes
Severe cardiovascular events of interest were a hospitalization for acute coronary syndrome (acute myocardial infarction or unstable angina), decompensated heart failure (HF), arrhythmia (atrial fibrillation, flutter, or other cardiac arrhythmias, including cardiac arrest), cerebral ischemia (ischemic stroke or transient ischemic attack), or fatal cardiovascular event, i.e., any cardiac event that led to death within the same hospitalization (see Table S1 in the electronic supplementary material for a list of ICD-10 codes). The endpoints of interest were the time to a first severe cardiovascular event of any category, and the time to a first severe cardiovascular event of each category separately.
Statistical Analysis
Baseline characteristics were summarized in the entire population, as well as in patients who experienced at least one exacerbation of COPD during follow-up. Missing data were reported where possible and not imputed.
Crude incidence rates (per 100 person-years) of a first severe cardiovascular event of any category were computed in the unexposed period and the exposed periods following an exacerbation of any severity (moderate or severe), and following a moderate or a severe exacerbation separately. Time-dependent Cox proportional hazards models were used to estimate HRs and 95% confidence intervals (CIs) for the association between subperiods of time following a COPD exacerbation (vs. unexposed time) and time to a first severe cardiovascular event (of any category, then cardiovascular events of each category separately). In a first model exploring the risk of a cardiovascular event of any category following a moderate/severe exacerbation, the 95% CI of HRs for the 1 to 7-, 8 to 14-, and 15 to 30-day subperiods overlapped substantially; these adjacent exposure subperiods were combined in subsequent models. Potential time-fixed and time-varying confounders were selected a priori based on the literature and expert advice. Time-varying confounders such as cardiac, metabolic, and COPD medication use, number of exacerbations, and number of general practitioner visits were updated annually. All models were fitted without (unadjusted) and with all confounders (fully adjusted). Adjustments were made for use of any cardiac agent, metabolic agent, or COPD medication (described in Table S2 in the electronic supplementary material). No adjustments were made for multiple comparisons. The software used for the statistical analysis was SAS® Viya® version 03.05 (SAS Institute, Cary, NC, USA).
Results
A total of 152,712 patients with COPD were included (Fig. 2). Mean ± standard deviation age was 73.8 ± 11.2 years; 37.6% of patients were female (Table 1). The baseline frequencies of known cardiovascular risk factors including diabetes (20.9%), dyslipidemia (35.6%), hypertensive disease (54.3%), ischemic heart disease (26.0%), HF (26.2%), and cerebrovascular disease (21.5%) were prominent among the enrolled cohort. More than 50% of patients were prescribed cardiac-related treatment and considerably fewer were prescribed COPD maintenance inhaled therapy [6.4% used a long-acting muscarinic antagonist (LAMA) and 12.3% used a long-acting beta-2 agonist (LABA)].
During a median follow-up duration of 37 months (interquartile range, 21–58), 63,182 (41.4%) patients experienced at least one moderate or severe COPD exacerbation, and 13,314 (8.7%) patients experienced at least one fatal or non-fatal cardiovascular event, among whom 2752 (1.8%) experienced the event within 365 days after an exacerbation (Table 2). The most common event was decompensated HF (6212 patients, 46.7%). Of 378 cardiac-related deaths occurring within a year post-exacerbation, the most common events associated with death were cardiac arrest in 165 (43.7%) patients, and congestive HF in 151 (39.9%) patients.
Crude incidence rates of a first severe cardiovascular event of any category were higher during the 1 to 7-, 8 to 14-, and 15 to 30-day subperiods following a moderate exacerbation, and during the 1 to 7-, 8 to 14-, 15 to 30- and 31 to 180-day subperiods following a severe exacerbation, compared with the unexposed period (Table 3). The risk of a first fatal or non-fatal severe cardiovascular event of any category increased in the first 30 days following the onset of an exacerbation (any severity) with adjusted HR (aHR): 1.44; 95% CI 1.33–1.55 and then decreased gradually, but remained elevated for up to 365 days (181 to 365-day subperiod aHR: 1.13; 95% CI 1.04–1.23) (Fig. 3). Following a moderate exacerbation, the risk of a first severe cardiovascular event remained increased for up to 365 days (181 to 365-day subperiod aHR: 1.13; 95% CI 1.04–1.23). Similar HRs were observed following a severe COPD exacerbation; however, the elevated HR was significantly increased for up to 180 days. Regarding each category of cardiovascular event (Table 4), the risk of decompensated HF was increased by more than 40% within the first 180 days following the onset of a moderate or severe COPD exacerbation and remained increased for up to 365 days. The risks of acute coronary syndrome and arrhythmias also increased following an exacerbation and remained significantly elevated for up to 180 days. A 20% increase in the risk of cerebral ischemia was observed in the 31 to 180-day post-exacerbation period. The risk of a fatal cardiovascular event was the highest within the first 30 days post-exacerbation onset and remained elevated for up to 365 days.
Risk of a cardiovascular event by period of exposure in the full analysis population. Hazard ratios with 95% CIs are shown for time to first cardiovascular event of any type (including in-hospital death), comparing exposure periods in the 365 days following a COPD exacerbation of any severity by four exposure categories. CI confidence interval, COPD chronic obstructive pulmonary disease
Discussion
In this large cohort including over 152,000 patients diagnosed with COPD and identified in the MDV database in Japan, we explored the incidence and risk of hospitalization for a fatal or non-fatal cardiovascular event in specific time periods following an exacerbation.
Increased Risk of Cardiopulmonary Events in COPD
To our knowledge, this study is the first in Japan to demonstrate the association between exacerbations of COPD and the increased risk of severe cardiovascular events. We showed that the risk of a fatal or non-fatal severe cardiovascular event is significantly increased in the year, and particularly in the first 30 days, following a moderate or severe exacerbation in patients with COPD, and was not limited to those with baseline cardiovascular risk factors. In addition, we found a 43% increased risk of fatal or non-fatal cardiovascular events in the first 30 days following the onset of a moderate exacerbation. Although there are few reports that have evaluated cardiovascular event risk following a moderate exacerbation, the HRs in our study were slightly lower than those of a previous study (IMPACT trial, HR: 2.00; 95% CI 1.32–3.05) [31]. Regarding risks following a severe exacerbation, we found a 59% increased risk in the first 30 days, which is lower than expected based on previous findings [15, 32, 33]. There are several possible and non-mutually exclusive reasons for the smaller HRs found in our study. First, in some studies, patient populations have been restricted to patients with moderate to severe COPD, or those with baseline cardiovascular risk factors. A second possibility involves characteristics of Japanese patients (e.g., body mass index) and the standard practices/approaches to primary prevention typical of the healthcare system in Japan [33]. In fact, our study showed that many patients diagnosed with cardiovascular disease in this population had received prophylactic cardiac (51.8%) and metabolic agents (30.0%). Another possibility is the underestimation of the risk of events due to the absence of information on cardiovascular events or deaths occurring outside of the hospital. Patients who had a cardiovascular event or died outside the hospital contributed erroneously to the number of person-years at risk and overestimated it, which likely led to underestimating the incidence rate of events. Exposed patients (i.e., those with exacerbations) are more likely to die than unexposed ones, thus leading to differential misclassification and underestimation of risks of cardiovascular events overall. In addition, the small number of severe exacerbations led to a lack of statistical power.
Our results showed similar trends to those of other EXACOS-CV studies [16,17,18,19,20], but the HRs for cardiovascular events in the first 30 days post-COPD exacerbation were lower in our study, especially following a severe exacerbation. The Japanese population was consistently older (mean age, 74 vs. 63–69 years) with a higher percentage of male patients (62% vs. 45%–60%) than any other EXACOS-CV population. There were also important differences in the baseline characteristics, such as lower rates of hypertension (54% vs. 68%–71%) and hyperlipidemia (36% vs. 67%–71%) compared with the US population [16], although variables such as smoking and obesity were not taken into account in the present study.
Regarding individual categories of cardiovascular events, the strength and temporality of associations differed across categories in our study. Acute coronary syndrome and fatal cardiovascular events were the outcomes with the highest levels of risk in the first 30 days, with 80% and 86% risk increases, respectively. The risks, except for cerebral ischemia, were significantly elevated more than 180 days following an exacerbation. We did not find evidence of a clear association between an exacerbation and the occurrence of cerebral ischemia, with an increased risk of 20% in the 31 to 180-day period only. These results, except for cerebral ischemia, were similar to those of the other EXACOS-CV studies, although the magnitudes of the HRs differed.
A second important finding is the low use of COPD maintenance therapy: approximately 6%–12% of patients were using at least one class of maintenance inhaled therapy at baseline in this study. Previous observational studies conducted for patients managed by pulmonologists in Japan showed that at least 90% of patients received COPD maintenance therapy [34,35,36]. Our results suggest that many patients diagnosed with COPD (ICD-10 J41–J44) in real-world practice in Japan, including those not using spirometry, may not be managed by pulmonologists and have not initiated COPD maintenance inhaled treatment. Recent studies showed that delayed initiation of treatment is associated with worse clinical outcomes, thus demonstrating the benefit of maintenance therapy [37, 38]. Physicians who are not specialists in respiratory diseases should be educated about the potential importance of COPD treatment in helping to prevent COPD exacerbations and reduce corresponding cardiovascular event risk. Similarly, respiratory specialists should appropriately assess patients with COPD for cardiovascular risk factors and intervene where necessary with suitable treatments for cardiovascular disease [39, 40].
Taken together, the above suggests that Japanese patients with COPD, who tend to be older, have a similarly high risk of cardiovascular events after an exacerbation, despite having fewer risk factors for cardiovascular events than their Western counterparts. This is a critical finding because it indicates that physicians in Japan need to remain vigilant in monitoring patients for post-exacerbation cardiovascular events, despite there being a perceived lower risk because of a relative lack of baseline cardiovascular risk factors.
Strengths and Limitations
The present study used the large MDV database, which enabled us to identify a large cohort of patients with COPD in Japan. Contrary to most previously published studies, patients were included irrespective of COPD disease severity or having a cardiovascular event. Therefore, our results are more generalizable to a real-world population of patients living with COPD in Japan. The study has some limitations, one of which is the absence of reliable information on smoking or obesity, which are two important risk factors [41]. The MDV database only captures information on diseases resulting in reimbursement or claims; therefore, information on diseases that did not result in a claim would be overlooked. In addition, as in previous database studies [16,17,18,19,20, 42, 43], a wide range of diagnostic codes including emphysema and chronic bronchitis were used to select patients. The higher percentage of patients using mucolytics compared with maintenance bronchodilator therapy in our study suggests that the study population was a mix of patients with COPD and those with chronic bronchitis. However, in a subgroup analysis comparing the frequency of cardiovascular events between patients with COPD (ICD-10 J44) and other diagnostic entities for COPD (ICD-10 J41–J43), the time since COPD exacerbation and the frequency of cardiovascular events showed a consistent trend (data not shown). Also, residual confounding is possible despite adjustment for multiple confounders. Exposure or outcome events could have been missed: e.g., if patients were transferred to different hospitals, received treatment or died outside the hospital, such data would not be provided to the MDV. The rate of moderate exacerbations could have been underestimated if patients were treated in primary care settings and thus misclassified as having no exposure. Additionally, the resulting HRs for cardiovascular risk could have also been underestimated. In the future, some limitations could be addressed by using a more comprehensive database including inter-hospital/clinic data and data related to cardiovascular risk factors (e.g., smoking or obesity).
Conclusions
In Japanese patients, the first year following a COPD exacerbation carries increased risk of being hospitalized for, or dying from, a cardiovascular event. Our study revealed that Japanese patients with COPD, despite having a generally lower proportion of cardiovascular risk factors than Western patients, remained at a similar level of risk of onset of post-exacerbation cardiovascular events. The cardiopulmonary risk of patients with COPD requires multidisciplinary care to optimize the management of both COPD and comorbid cardiovascular diseases. Furthermore, patients should be closely monitored for cardiovascular risk following either a moderate or severe exacerbation.
Data Availability
The datasets generated during and/or analyzed during the current study may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
References
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–858.
Watz H, Tetzlaff K, Magnussen H, et al. Spirometric changes during exacerbations of COPD: a post hoc analysis of the WISDOM trial. Respir Res. 2018;19:251.
Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38.
Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67:957–63.
Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–31.
Hurst JR, Skolnik N, Hansen GJ, et al. Understanding the impact of chronic obstructive pulmonary disease exacerbations on patient health and quality of life. Eur J Intern Med. 2020;73:1–6.
Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Ther Adv Respir Dis. 2018;12:1753465817750524.
Zhu Z, Wang X, Li X, et al. Genetic overlap of chronic obstructive pulmonary disease and cardiovascular disease-related traits: a large-scale genome-wide cross-trait analysis. Respir Res. 2019;20:64.
Onishi K. Total management of chronic obstructive pulmonary disease (COPD) as an independent risk factor for cardiovascular disease. J Cardiol. 2017;70:128–34.
Chetty U, McLean G, Morrison D, Agur K, Guthrie B, Mercer SW. Chronic obstructive pulmonary disease and comorbidities: a large cross-sectional study in primary care. Br J Gen Pract. 2017;67:e321–3.
Yoshihisa A, Takiguchi M, Shimizu T, et al. Cardiovascular function and prognosis of patients with heart failure coexistent with chronic obstructive pulmonary disease. J Cardiol. 2014;64:256–64.
Andell P, Koul S, Martinsson A, et al. Impact of chronic obstructive pulmonary disease on morbidity and mortality after myocardial infarction. Open Heart. 2014;1:e000002.
Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137:1091–7.
Nishiyama K, Morimoto T, Furukawa Y, et al. Chronic obstructive pulmonary disease–an independent risk factor for long-term cardiac and cardiovascular mortality in patients with ischemic heart disease. Int J Cardiol. 2010;143:178–83.
Kunisaki KM, Dransfield MT, Anderson JA, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med. 2018;198:51–7.
Daniels K, Lanes S, Tave A, et al. Risk of death and cardiovascular events following an exacerbation of COPD: the EXACOS-CV US Study. Int J Chron Obstruct Pulmon Dis. 2024;19:225–41.
Hawkins NM, Nordon C, Rhodes K, et al. Heightened long-term cardiovascular risks after exacerbation of chronic obstructive pulmonary disease. Heart. 2024;110:702–9.
Graul EL, Nordon C, Rhodes K, et al. Temporal risk of non-fatal cardiovascular events post COPD exacerbation: a population-based study. Am J Respir Crit Care Med. 2024;209:960–72.
Vogelmeier CF, Rhodes K, Garbe E, et al. Elucidating the risk of cardiopulmonary consequences of an exacerbation of COPD: results of the EXACOS-CV study in Germany. BMJ Open Respir Res. 2024;11:e002153.
Swart KMA, Baak BN, Lemmens L, et al. Risk of cardiovascular events after an exacerbation of chronic obstructive pulmonary disease: results from the EXACOS-CV cohort study using the PHARMO Data Network in the Netherlands. Respir Res. 2023;24:293.
Ishii T, Nishimura M, Akimoto A, James MH, Jones P. Understanding low COPD exacerbation rates in Japan: a review and comparison with other countries. Int J Chron Obstruct Pulmon Dis. 2018;13:3459–71.
Makita H, Suzuki M, Konno S, et al. Unique mortality profile in Japanese patients with COPD: an analysis from the Hokkaido COPD Cohort Study. Int J Chron Obstruct Pulmon Dis. 2020;15:2081–90.
Kawayama T, Takahashi K, Ikeda T, et al. Exacerbation rates in Japanese patients with obstructive lung disease: a subanalysis of the prospective, observational NOVELTY study. Allergol Int. 2024;73:71–80.
Miyazaki M, Nakamura H, Chubachi S, et al. Analysis of comorbid factors that increase the COPD assessment test scores. Respir Res. 2014;15:13.
Suzuki M, Makita H, Ito YM, et al. Clinical features and determinants of COPD exacerbation in the Hokkaido COPD cohort study. Eur Respir J. 2014;43:1289–97.
Takahashi S, Betsuyaku T. The chronic obstructive pulmonary disease comorbidity spectrum in Japan differs from that in western countries. Respir Investig. 2015;53:259–70.
Nordon C, Rhodes K, Quint JK, et al. EXAcerbations of COPD and their OutcomeS on CardioVascular diseases (EXACOS-CV) Programme: protocol of multicountry observational cohort studies. BMJ Open. 2023;13:e070022.
Hayashida K, Murakami G, Matsuda S, Fushimi K. History and profile of Diagnosis Procedure Combination (DPC): development of a real data collection system for acute inpatient care in Japan. J Epidemiol. 2021;31:1–11.
Muro S, Suzuki M, Nakamura S, et al. Real-world effectiveness of early intervention with fixed-dose tiotropium/olodaterol vs tiotropium in Japanese patients with COPD: a high-dimensional propensity score-matched cohort analysis. Respir Res. 2021;22:180.
Czira A, Akiyama S, Ishii T, et al. Benefit of prompt vs delayed initiation of triple therapy following an exacerbation in patients with COPD in Japan: a retrospective cohort study. Int J Chron Obstruct Pulmon Dis. 2023;18:2933–53.
Dransfield MT, Criner GJ, Halpin DMG, et al. Time-dependent risk of cardiovascular events following an exacerbation in patients with chronic obstructive pulmonary disease: post hoc analysis from the IMPACT trial. J Am Heart Assoc. 2022;11: e024350.
Reilev M, Pottegård A, Lykkegaard J, Søndergaard J, Ingebrigtsen TS, Hallas J. Increased risk of major adverse cardiac events following the onset of acute exacerbations of COPD. Respirology. 2019;24:1183–90.
Kuwabara M, Mori M, Komoto S. Japanese national plan for promotion of measures against cerebrovascular and cardiovascular disease. Circulation. 2021;143:1921–31.
Oishi K, Hirano T, Hamada K, et al. Characteristics of 2017 GOLD COPD group A: a multicenter cross-sectional CAP study in Japan. Int J Chron Obstruct Pulmon Dis. 2018;13:3901–7.
Ichinose M, Minakata Y, Motegi T, et al. A non-interventional, cross-sectional study to evaluate factors relating to daily step counts and physical activity in Japanese patients with chronic obstructive pulmonary disease: STEP COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:3385–96.
Hashimoto S, Yoshida Y, Makita N, et al. Real-world evidence on the diagnostic and clinical characteristics of asthma in Japanese patients with COPD: the ACO Japan cohort study. Int J Chron Obstruct Pulmon Dis. 2023;18:37–46.
Yamada H, Matsumoto I, Makita N, et al. Effect of timing of bronchodilator therapy initiation on exacerbations in patients with chronic obstructive pulmonary disease: a retrospective cohort study. Respir Res. 2022;23:255.
Tkacz J, Evans KA, Touchette DR, et al. PRIMUS - Prompt initiation of maintenance therapy in the US: a real-world analysis of clinical and economic outcomes among patients initiating triple therapy following a COPD exacerbation. Int J Chron Obstruct Pulmon Dis. 2022;17:329–42.
Matsunaga K, Harada M, Suizu J, Oishi K, Asami-Noyama M, Hirano T. Comorbid conditions in chronic obstructive pulmonary disease: potential therapeutic targets for unmet needs. J Clin Med. 2020;9:3078.
Rothnie KJ, Smeeth L, Herrett E, et al. Closing the mortality gap after a myocardial infarction in people with and without chronic obstructive pulmonary disease. Heart. 2015;101:1103–10.
Hawkins NM, Peterson S, Ezzat AM, et al. Control of cardiovascular risk factors in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2022;19:1102–11.
Wang M, Lin EPY, Huang LC, Li CY, Shyr Y, Lai CH. Mortality of cardiovascular events in patients with COPD and preceding hospitalization for acute exacerbation. Chest. 2020;158:973–85.
Sivakumaran S, Alsallakh MA, Lyons RA, Quint JK, Davies GA. Identifying COPD in routinely collected electronic health records: a systematic scoping review. ERJ Open Res. 2021;7:00167–2021.
Medical Writing/Editorial Assistance
The authors wish to thank Keyra Martinez Dunn, MD, and Michelle Belanger, MD, of Edanz, Japan, for providing medical writing support, which was funded by AstraZeneca K.K., Japan, through EMC K.K., Japan, in accordance with Good Publication Practice guidelines (https://www.ismpp.org/gpp-2022).
Authorship
All authors have made substantial contributions to the conception or design of the work or the acquisition, analysis, or interpretation of the data; have drafted the work or revised it critically for important intellectual content; approve the version to be published; and have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This study was funded by AstraZeneca K.K. The journal’s Rapid Service Fee and Open Access Fee were also funded by AstraZeneca K.K.
Author information
Authors and Affiliations
Contributions
Conceptualization: Kazuto Matsunaga, Yuri Yoshida, Naoyuki Makita, Kirsty Rhodes, Clementine Nordon. Data curation: Kenichiro Nishida, Kirsty Rhodes. Formal analysis: Kenichiro Nishida, Kirsty Rhodes. Methodology: Yuri Yoshida, Naoyuki Makita, Kirsty Rhodes, Clementine Nordon. Project administration: Yuri Yoshida, Clementine Nordon. Software: Kenichiro Nishida, Kirsty Rhodes. Supervision: Yuri Yoshida, Naoyuki Makita, Kirsty Rhodes, Clementine Nordon. Visualization: Kazuto Matsunaga, Yuri Yoshida, Naoyuki Makita, Clementine Nordon. Writing – original draft: all authors. Writing – review and editing: all authors.
Corresponding author
Ethics declarations
Conflict of Interest
Kazuto Matsunaga received lecture fees from AstraZeneca, Sanofi, Chugai, GSK, Novartis Pharma, Kyorin Pharmaceutical, and Boehringer Ingelheim. Yuri Yoshida is an employee of AstraZeneca. Naoyuki Makita is an employee of AstraZeneca. Kenichiro Nishida is an employee of AstraZeneca. Kirsty Rhodes is an employee of AstraZeneca and holds shares or stock/stock options in AstraZeneca. Clementine Nordon is an employee of AstraZeneca and holds shares or stock/stock options in AstraZeneca.
Ethical Approval
This study was conducted according to the Declaration of Helsinki and the Ethical Guidelines for Medical and Health Research Involving Human Subjects. As the data for this study were extracted from a commercially available de-identified claims database, informed consent was not required. The protocol of this study and any amendments were approved in writing by the MINS Research Ethics Committee (MINS-REC220211; 7 April 2022). Permission was obtained to access and use the data from the MDV database.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Matsunaga, K., Yoshida, Y., Makita, N. et al. Increased Risk of Severe Cardiovascular Events Following Exacerbations of Chronic Obstructive Pulmonary Disease: Results of the EXACOS-CV Study in Japan. Adv Ther (2024). https://doi.org/10.1007/s12325-024-02920-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12325-024-02920-y