Abstract
Introduction
This analysis evaluated the relative performance of vedolizumab and anti-tumor necrosis factor alpha (anti-TNFα) agents in subpopulations of biologic therapy-naive patients with Crohn’s disease (CD) and assessed whether patients in whom vedolizumab would have a larger treatment effect vs anti-TNFα agents could be identified.
Methods
Data were from EVOLVE, a real-world, multicountry, retrospective cohort study of patients with inflammatory bowel disease who initiated first-line biologic treatment with vedolizumab (n = 195) or anti-TNFα agents (n = 245). Prediction models for time to clinical remission were developed in vedolizumab- and anti-TNFα-treated patients and used to estimate effect scores, a metric of predicted comparative efficacy, for each patient. Patients were ranked by effect scores and potential subpopulations were investigated. Simplified rules to identify these subpopulations were also developed using classification tree analysis.
Results
Among all patients, median time to clinical remission was 7.8 months (vedolizumab) and 11.1 months (anti-TNFα) (P < 0.05). Among patients in the top 40% of the effect score distribution, the median time to clinical remission was 4.8 months (vedolizumab) vs 18.1 months (anti-TNFα) (adjusted hazard ratio 2.0, 95% confidence interval 1.3–2.9). A simplified rule for identifying a subpopulation more likely to benefit from vedolizumab was based on having an ongoing CD exacerbation, no prior emergency visits, and non-stricturing disease.
Conclusions
Subpopulations of biologic-naive patients with CD in whom vedolizumab appeared to have a larger effect relative to anti-TNFα agents for the outcome of clinical remission were identified. Validation of the identified subpopulations and simplified rules are warranted to confirm these findings.
ClinicalTrials.gov Identifier
NCT03710486.
Graphical Abstract available for this article.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Real-world data from the EVOLVE study were used to identify subpopulations of biologic-naïve patients with Crohn’s disease who would have a greater response to treatment with vedolizumab. | |
Three simplified rules were identified, with disease exacerbation, emergency department visits, and no fistulae prior to treatment initiation among the main predictors of clinical remission. | |
This set of simple rules identified subgroups of patients in whom vedolizumab appeared to have a larger effect on clinical remission relative to anti-TNF initiation. | |
Further validation may lead to better targeting of treatment and optimization of outcomes for patients with Crohn’s disease treated with vedolizumab. |
Digital Features
This article is published with digital features, including a graphical abstract to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.25450780.
Introduction
International guidelines on the management of patients with Crohn’s disease (CD) recommend initial treatment with immune modulator medications such as corticosteroids [1, 2]. Patients who do not respond to conventional therapy may be prescribed treatment with biologic anti-tumor necrosis factor alpha (anti-TNFα) agents; however, these drugs may pose some safety concerns and some patients either do not respond to treatment or lose response after experiencing a benefit [3].
Up to 30% of patients do not respond to anti-TNFα agents [4]. When patients develop non-response to anti-TNFα agents, switching to treatment with vedolizumab (α4β7 integrin inhibitor) or ustekinumab (interleukin-12/23 p40 inhibitor) is recommended [1]. However, clinical studies showed greater efficacy of vedolizumab in anti-TNFα-naive patients compared with patients who failed to respond to anti-TNFα [5], and exposure–efficacy analyses of clinical data indicated that prior use of anti-TNFα had a negative impact on response [6].
Real-world data on the use of vedolizumab in anti-TNFα-naive patients (i.e., as a first-line biologic) have thus far been limited. Previous analyses of real-world data from biologic-naive patients with CD from the EVOLVE study indicated that the effectiveness of vedolizumab was comparable with anti-TNFα [7]. An individualized approach, which may include first-line biologic treatment with vedolizumab instead of anti-TNFα agents, has been suggested [2]. Identifying patients with CD who are more likely to respond to vedolizumab relative to anti-TNFα as a first-line agent represents a gap in knowledge and would be an important step forward when developing a more personalized approach to CD therapy.
The individualized medicine approach developed by Cai et al. can be used to identify subpopulations for targeted treatment [8]. This approach identifies optimal subpopulations using a data-driven approach, which minimizes bias that may be associated with choosing specific subpopulations a priori. Conceptually, this approach is based on identification of patient characteristics predictive of response to each treatment, calculation of an individualized metric of predicted comparative efficacy reflecting expected benefit of a treatment relative to the comparator for each patient, stratification of patients into potential subpopulations based on this metric, and characterization of outcomes in these subpopulations. In this study, we used this approach to identify subpopulations of biologic-naive patients with CD who had an especially pronounced treatment effect on clinical remission with vedolizumab compared with anti-TNFα agents. After identifying subpopulations of patients with higher likelihood of clinical remission on vedolizumab, we proceeded to develop simplified rules based on a smaller set of patient characteristics, for easier applicability in clinical practice.
Methods
Study Design and Patients
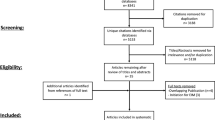
This analysis utilized data from patients with CD included in the EVOLVE study. EVOLVE is a real-world, multinational, multicenter, retrospective cohort study of adult patients (aged ≥ 18 years) with inflammatory bowel disease. These patients initiated first-line biologic treatments, either vedolizumab or anti-TNFα agents, between May 2014 and July 2017 [7]. Data were collected from patients’ medical charts between September 2017 and December 2018. Patients were included for analysis in the current study if they had available data on clinical remission and key baseline predictors. The “index” treatment was the initial administration of vedolizumab or anti-TNFα therapy. The study was approved by the local ethics committee at each participating site (Electronic Supplementary Material [ESM] Table S1). Patients alive at the time of chart abstraction (99% of patients) signed an informed consent form prior to participation in the study if there was no waiver of informed consent.
Outcome: Clinical Remission
In the real-world EVOLVE study, clinical remission was defined using a hierarchical approach, considering multiple measures, such as the Crohn’s Disease Activity Index (CDAI), the Harvey-Bradshaw Index (HBI), the modified HBI (mHBI), and a global assessment abstracted from medical notes. Patients were considered to be in clinical remission if they met any of the conditions outlined in the following algorithm: (i) CDAI of < 150; or (ii) if CDAI was unknown, HBI of ≤ 4 points; or (iii) if HBI was unknown, mHBI of ≤ 4 points; or (iv) if mHBI was unknown, if it was noted in the medical chart that the patient was in remission. The date of remission was determined as the date when patients were first documented as meeting any of these conditions in their medical records.
Development of Prediction Models
The approach to identify subpopulations of patients with higher rates of clinical remission is summarized in Fig. S1.
Two prediction models were developed for the time from the initiation of index treatment to clinical remission: one for patients treated with vedolizumab, and the other for patients treated with anti-TNFα agents. Several potential predictors were considered, including patient demographics, prior treatments, clinical characteristics at treatment initiation, Charlson Comorbidity Index, prior extraintestinal manifestations (EIMs), and measures of prior healthcare resource utilization, excluding routine visits or referrals. Potential predictors available for all patients were investigated in the models. Potential predictors available for ≥ 50% but < 100% of patients were also investigated in the models; however, imputation was performed when appropriate, such as when missing data on a particular characteristic could reasonably indicate the absence of the characteristic being assessed. Potential predictors with < 50% availability were not considered in the models. The lists of variables included and excluded in the prediction models are presented in Table S2 (see ESM).
Predictors of clinical remission in the two aforementioned models were selected using the least absolute shrinkage and selection operator (LASSO) method [9]. The predictive models were validated using fivefold cross-validation (i.e., the dataset was randomly divided into five approximately equal subsets; the LASSO-based variable selection approach was applied in four out of the five subsets [training dataset] and then evaluated in the remaining subset [validation dataset]). This step was repeated such that each subset served as the validation dataset only once. Model performance was evaluated using area under the curve (AUC) averaged across the five validation datasets.
Identification and Evaluation of Potential Higher-Efficacy Subpopulations
The prediction models developed within each group were subsequently used to calculate an “effect score” for each individual patient. Effect scores represent an individualized prediction of the potential benefit for each patient treated with vedolizumab. They were calculated as the difference between the predicted probability of clinical remission at 1 year with vedolizumab vs anti-TNFα. Patients were then ranked by their individual effect scores from highest to lowest, with higher effect scores representing a larger expected benefit on clinical remission when treated with vedolizumab relative to anti-TNFα. Potential subpopulations of patients were defined by sequentially grouping patients with the highest effect scores, including the top 20%, top 40%, top 60%, and top 80%.
Differences in the occurrences of clinical remission were then estimated between vedolizumab- and anti-TNFα-treated patients within each of these subpopulations. Propensity score adjustment was performed within each subpopulation to account for differences in baseline characteristics between vedolizumab- and anti-TNFα-treated patients within the subpopulations. Propensity scores were obtained from a logistic regression model of treatment with vedolizumab vs anti-TNFα within each subpopulation. Adjustment variables in the propensity score models included all the potential predictors considered in the LASSO models. Balance of baseline characteristics was assessed after propensity score adjustment. The adjusted treatment effect of vedolizumab vs anti-TNFα on clinical remission was estimated in each subpopulation using propensity score-weighted Kaplan–Meier analyses and Cox proportional hazards models adjusting for the propensity score.
Development and Evaluation of Simplified Decision Rules
Although the approach described above can help identify patient subpopulations that may benefit from vedolizumab, not all baseline characteristics used in the prediction models may be readily available in clinical practice. Furthermore, calculating effect scores for individual patients may be cumbersome in real-world settings. Therefore, it is valuable to develop simpler rules based on a reduced set of baseline characteristics that can accurately identify most, if not all, of the patients within the target subpopulations.
To that end, we sought to determine whether the previously identified vedolizumab subpopulations could be identified using simplified rule sets relying on fewer baseline characteristics. To achieve this, recursive partitioning and regression tree (RPART) classification analysis [8] was used to predict membership in the higher remission subpopulations previously identified for vedolizumab. The RPART algorithm generates decision trees aimed at classifying patients into the subpopulations of interest based on a set of predictors. In our pursuit of straightforward rules for patient classification, we imposed a depth constraint of three on the decision trees, meaning classifications were based on a maximum of three baseline characteristics. We then derived simplified rules for each subpopulation from the generated decision trees.
To evaluate the ability of the simplified rules to identify patients with a higher likelihood of achieving remission with vedolizumab compared with anti-TNFα, unadjusted and adjusted analyses of the treatment effect of vedolizumab vs anti-TNFα were conducted within the subsets of patients identified using the simplified rules.
P values < 0.05 were considered to be statistically significant. All analyses were conducted using R 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org).
Results
Patient Characteristics
A total of 195 patients treated with vedolizumab and 245 treated with anti-TNFα agents from the EVOLVE study met the current study inclusion criteria (Table 1). Most (195/440, 44.3%) patients had non-stricturing, non-penetrating disease with/without perianal disease, and ileal (136/440, 30.9%) or ileocolonic (148/440, 33.6%) location with/without upper gastrointestinal disease. At baseline, 160/440 (36.4%) had moderate CD and 62/440 (14.1%) had severe CD. A total of 44/440 (10.0%) patients had fistulae at their most recent assessment prior to receiving their index treatment, and 224/220 (50.9%) had ongoing disease exacerbation (defined as disease worsening of symptoms attributed to CD as noted in the medical record by clinician assessment) at index.
Predictors of Clinical Remission
The prediction model for vedolizumab identified the following 11 characteristics as predictors of time to clinical remission: location of intestinal involvement (colonic, ileal, or both), type of CD behavior (stricturing, penetrating, or neither), prior non-biologic therapy, use of immune modulators at treatment initiation, steroid dependency (dependent or refractory), fistulae at the most recent assessment prior to index, having one or more pre-existing EIMs at index, exacerbations at treatment initiation, prior emergency department/emergency room (ED/ER) visits, prior hospitalizations, and prior healthcare provider visits/referrals other than routine visits (Table 2).
The prediction model for anti-TNFα identified only age as a predictor of time to clinical remission (Table 2). The models were cross-validated and showed an AUC of 0.62 for the vedolizumab model and an AUC of 0.62 for the anti-TNFα model.
Description of Higher-Efficacy Vedolizumab Subpopulations
In the full population of included patients, clinical remission rates at 12 and 24 months based on unadjusted Kaplan–Meier analyses were 60.9% and 75.7% in patients treated with vedolizumab and 56.1% and 68.8% in patients treated with anti-TNFα agents. Unadjusted median time to clinical remission (95% confidence interval [CI]) was 9.0 (7.2–11.0) months for patients treated with vedolizumab and 10.1 (7.6–12.7) months for patients treated with anti-TNFα agents (unadjusted log-rank P = 0.35). After propensity score adjustment, clinical remission rates at 12 and 24 months were 63.2% and 75.0% in patients treated with vedolizumab and 51.2% and 64.9% in patients treated with anti-TNFα agents. Adjusted median time to clinical remission (95% CI) was 7.8 (6.3–10.9) months for patients treated with vedolizumab and 11.1 (9.6–18.1) months for patients treated with anti-TNFα agents (weighted log-rank P < 0.05).
Differences in the rates of remission with vedolizumab relative to anti-TNFα agents in propensity score adjusted analyses within each of the studied subpopulations are shown in Fig. 1. Tables S3–7 (see ESM) show baseline characteristics after adjustment in each subpopulation. The weighted median time to clinical remission for patients treated with vedolizumab relative to anti-TNFα agents in the subpopulation of patients with the top 20% of effect scores was 4.0 months for vedolizumab vs 14.1 months for anti-TNFα agents (adjusted hazard ratio [HR] 3.3, 95% CI 1.9–5.8). Corresponding median times to remission for vedolizumab vs anti-TNFα agents and adjusted HR estimates in the top 40%, top 60%, and top 80% subpopulations were 4.8 vs 18.1 months (HR 2.0, 95% CI 1.3–2.9), 4.8 vs 12.7 months (HR 1.7, 95% CI 1.2–2.2), and 4.8 vs 11.7 months (HR 1.4, 95% CI 1.0–1.8), respectively.
In each subpopulation, clinical remission by 1 year after index treatment was achieved in the majority of patients treated with vedolizumab compared with approximately half of the patients treated with anti-TNFα agents (Fig. 2). In the top 20% subpopulation, the estimated proportions of vedolizumab- and anti-TNFα-treated patients who achieved clinical remission by 1 year were 91.5% and 47.2%, respectively; in the top 80% subpopulation, the proportions were 73.5% and 50.6%, respectively.
Compared with the full sample of patients treated with vedolizumab, patients in the top 20% and 40% subpopulations treated with vedolizumab were older (mean ages of 58.9 years and 55.1 years for the subpopulations, respectively, vs 51.8 years for the full sample), more likely to have non-stricturing and non-penetrating disease behaviors (62.2% and 60.7%, respectively, vs 43.6%), less likely to have fistulae (0.0% and 0.0%, respectively, vs 3.6%), less likely to have ongoing exacerbation of disease at index (75.6% and 67.4%, respectively, vs 45.6%), more likely to have EIMs (37.8% and 28.1%, respectively, vs 17.9%), and less likely to have healthcare provider visit/referrals other than routine visits (22.2% and 36.0%, respectively vs 50.8%), or ED/ER visits (0.0% and 0.0%, respectively vs 7.7%) (Table 3).
Description and Evaluation of Simplified Decision Rules
Simplified rules for the top 40% (rule A), top 60% (rule B), and top 80% (rule C) subpopulations are summarized (Fig. 3 and Table 4).
Kaplan–Meier curves for adjusted time to clinical remission, by subpopulation. The red line shows the anti-TNFα treatment group and the green line shows the vedolizumab treatment group. ED/ER emergency department/emergency room, TNFα tumor necrosis factor alpha. aBased on prediction models, an effect score was estimated for each patient as the difference in the predicted 1-year rate of clinical remission associated with vedolizumab vs anti-TNFα treatment
Rule A for the top 40% subpopulation was based on the following criteria: patients (i) had an exacerbation ongoing at index; (ii) did not have ED/ER visits prior to index; and (iii) had pre-index disease behavior classified as other than stricturing with/without perianal disease. Patients meeting these three criteria made up 32% of the patients included in our EVOLVE analysis. Among these patients, median time to clinical remission was 6.7 months for vedolizumab-treated patients (n = 55) and 18.1 months for anti-TNFα-treated patients (n = 86) (unadjusted log-rank P < 0.001), and the adjusted HR of clinical remission for vedolizumab vs anti-TNFα was 2.9 (95% CI 1.7–5.0).
Rule B for the top 60% subpopulation differs with respect to one condition compared with rule A above. Patients identified by this rule (i) had an exacerbation ongoing at index; (ii) did not have ED/ER visits prior to index; and (iii) did not have fistulae at the most recent assessment prior to index. Among patients meeting rule B criteria, median time to clinical remission was 7.2 months for vedolizumab-treated patients (n = 81) and 14.1 months for anti-TNFα-treated patients (n = 99) (unadjusted log-rank P < 0.01), and the adjusted HR of clinical remission for vedolizumab vs anti-TNFα was 2.1 (95% CI 1.3–3.4).
Rule C for the top 80% subpopulation was based on the following criteria: (i) did not have ED/ER visits prior to index; and (ii) did not have fistulae at the most recent assessment prior to index. Patients meeting these criteria encompassed 81% of the patients with CD included in our EVOLVE analysis. Among these patients, median time to clinical remission was 8.5 months for vedolizumab-treated patients (n = 174) and 11.1 months for anti-TNFα-treated patients (n = 182) (unadjusted log-rank P < 0.05), and the adjusted HR of clinical remission for vedolizumab vs anti-TNFα was 1.7 (95% CI 1.2–2.3).
Discussion
Given the multitude of treatment options available for inflammatory bowel diseases, the identification of patient characteristics that enhance the likelihood of a therapeutic response to specific medications could significantly inform treatment decisions. However, achieving this requires a method of identifying such patients. In this study, we used real-world data from patients with CD from the EVOLVE real-world study to ascertain whether we could identify subpopulations of patients with higher rates of clinical remission when treated with vedolizumab vs anti-TNFα on the basis of differences in predictors of clinical remission between these two groups. Following the identification of potential subpopulations, we characterized differences in remission outcomes within the subpopulations. Finally, we derived a simplified set of rules, which could be readily implemented in real-world clinical practice, to identify patients more likely to benefit from vedolizumab.
Other studies have examined predictors of response in patients with CD treated with vedolizumab. A tool by Dulai et al. developed using vedolizumab clinical trial data found that prior bowel surgery, prior anti-TNF exposure, fistulizing disease, albumin, and C-reactive protein (CRP) were associated with response to vedolizumab in a mixed population of biologic-experienced and biologic-naive patients [10]. A study by Baumgart et al. using a Spanish cohort of patients with CD treated with vedolizumab found that a low HBI score and no hospitalizations were predictors of remission [11]. Another study by Shelton et al. using a US cohort of patients with CD treated with vedolizumab found that baseline CRP ≥ 8 mg/l was associated with lower odds of response [12]. Although these studies offer valuable insights, their primary aim was to predict which patients were more likely to achieve favorable outcomes when treated with vedolizumab, rather than to specifically identify those who might have better outcomes with vedolizumab vs an anti-TNFα. It is possible that a subset of patients will experience remission, regardless of the treatment they receive. However, our objective was to investigate whether we can discern patients who are more likely to benefit from vedolizumab compared with anti-TNFα agents.
In the current study, patient characteristics associated with higher rates of clinical remission on vedolizumab relative to anti-TNFα agents included having an exacerbation at treatment initiation, having no ED/ER visits prior to treatment initiation, and having no fistulae at the most recent assessment prior to treatment initiation. Classifying patients solely on the basis of these patient characteristics was largely successful in replicating the subpopulation classifications initially derived from a broader set of identified predictors. Nevertheless, external validation of the simplified rules presented in this study is needed to understand their reproducibility in different populations, and to provide further guidance on their application in clinical practice.
In addition to treatment efficacy, other factors considered to inform treatment decisions include safety, patient preferences, and cost. Previous studies conducted within the EVOLVE population have shown that biologic-naive patients treated with vedolizumab were less likely to experience serious side effects compared with patients treated with anti-TNFα [13]. Although not examined in this study because of a lack of available data, exploring whether the patient subpopulations identified here may have a lower risk of adverse events would be helpful in future research. Further, cost is an important consideration during treatment selection. The current study did not assess cost, as it will vary among patients according to geographical location and insurance coverage, as well as recently available biosimilars.
The current study is subject to several limitations. First, patient characteristics considered as potential predictors of clinical remission were limited to those that were available for ≥ 50% of patients in the real-world EVOLVE study. Variables not available in this real-world data source, such as magnetic resonance imaging or endoscopy results, as well as variables with large portions of missing data, such as CRP, were not included. It is possible that additional variables not included in the model could impact relative efficacy of vedolizumab vs anti-TNFα. For example, age at diagnosis and initial use of steroids were not available and are known to be important prognostic factors in patients with CD [14, 15]. Subpopulations identified may have differed had these other predictors also been available. In addition, the model AUC was 0.62 for both models, which may have been improved if other predictors had been available.
Further, EVOLVE data reflect real-world clinical practice across multiple countries and centers. As such, in contrast with clinical trials with prospective, regimented assessments of key disease features, some variables included in this study were only recorded in the notes when assessed during visits (such as assessment if exacerbation was ongoing or the notation of when one of the components of the clinical remission hierarchical definition was achieved). However, these limitations would apply to both treatment groups equally, and would not be expected to bias comparisons across groups.
An additional limitation is that anti-TNF agents were collectively studied as a group and differences between individual anti-TNFα agents were not considered. Although additional treatments such as biosimilars are now also treatment options for patients, the EVOLVE study only collected information on patients treated with vedolizumab or anti-TNFα. Finally, although adjustments for baseline characteristics were performed to reduce bias, this was not a head-to-head, prospective randomized controlled trial; therefore, some residual bias may exist.
Conclusion
In this study, we have described a simple set of rules that can identify subgroups of patients with CD in whom vedolizumab appeared to have a larger effect relative to anti-TNFα agents for the outcome of clinical remission. The major strength of this study was that it included real-world, biologic-naive patients with CD and, therefore, mirrors routine worldwide clinical practice. Prospective validation of the identified subpopulations in other data sources is needed to confirm these findings. If validated, these rules can inform targeting of treatment and optimization of outcomes for patients with CD treated with vedolizumab.
Data Availability
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants data supporting the results reported in this article, will be made available within 3 months from initial request to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
References
Torres J, Bonovas S, Doherty G, et al. ECCO guidelines on therapeutics in Crohn’s disease: medical treatment. J Crohns Colitis. 2019;14(1):4–22.
Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(suppl 3):s1-106.
Cohen BL, Sachar DB. Update on anti-tumor necrosis factor agents and other new drugs for inflammatory bowel disease. BMJ. 2017;357:j2505.
Adegbola SO, Sahnan K, Warusavitarne J, Hart A, Tozer P. Anti-TNF therapy in Crohn’s disease. Int J Mol Sci. 2018;19(8):2244.
Sands BE, Sandborn WJ, Van Assche G, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease in patients naïve to or who have failed tumor necrosis factor antagonist therapy. Inflamm Bowel Dis. 2017;23(1):97–106.
Rosario M, French JL, Dirks NL, et al. Exposure-efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn’s disease. J Crohns Colitis. 2017;11(8):921–9.
Bressler B, Yarur A, Silverberg MS, et al. Vedolizumab and anti-tumour necrosis factor α real-world outcomes in biologic-naive inflammatory bowel disease patients: results from the EVOLVE study. J Crohns Colitis. 2021;15(10):1694–706.
Cai T, Tian L, Wong PH, Wei LJ. Analysis of randomized comparative clinical trial data for personalized treatment selections. Biostatistics. 2011;12(2):270–82.
Tibshirani R. Regression shrinkage and selection via the lasso. J Roy Stat Soc: Ser B (Methodol). 1996;58(1):267–88.
Dulai PS, Boland BS, Singh S, et al. Development and validation of a scoring system to predict outcomes of vedolizumab treatment in patients with Crohn's disease. Gastroenterology. 2018;155(3):687–95.e10.
Baumgart DC, Bokemeyer B, Drabik A, Stallmach A, Schreiber S, Vedolizumab GC. Vedolizumab induction therapy for inflammatory bowel disease in clinical practice—a nationwide consecutive German cohort study. Aliment Pharmacol Ther. 2016;43(10):1090–102.
Shelton E, Allegretti JR, Stevens B, et al. Efficacy of vedolizumab as induction therapy in refractory IBD patients: a multicenter cohort. Inflamm Bowel Dis. 2015;21(12):2879–85.
Yarur A, Mantzaris GJ, Kopylov U, et al. 795 real world safety of vedolizumab and anti-TNF therapies in biologic-naïve ulcerative colitis and Crohn’s disease patients: results from the EVOLVE study. Am J Gastroenterol. 2019;114:S460.
Dias CC, Rodrigues PP, da Costa-Pereira A, Magro F. Clinical prognostic factors for disabling Crohn’s disease: a systematic review and meta-analysis. World J Gastroenterol. 2013;19(24):3866–71.
Karoui S, Serghini M, Dachraoui A, Boubaker J, Filali A. Prognostic factors in Crohn’s disease: a systematic review. Tunis Med. 2013;91(4):230–3.
Acknowledgements
The authors are grateful to the patients and investigators who participated in the EVOLVE study. The authors would also like to thank Ibou Dieye, Emma Billmyer, and Ha Nguyen of Analysis Group (Boston, MA, USA) for assistance with data modeling and analysis.
Medical Writing/Editorial Assistance
Medical writing assistance was provided by Paul Hassan, PhD, CMPP, of Excel Scientific Solutions (Horsham, UK) and was funded by Takeda, Zurich, Switzerland.
Funding
Open access funding provided by SCELC, Statewide California Electronic Library Consortium. This study and the open access fee for this publication were funded by Takeda, Zurich, Switzerland.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design and implementation of the study, the acquisition and analysis of the data, and reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Andres Yarur reports serving on advisory boards for Arena, Bristol Myers Squibb, Prometheus Laboratories, and Takeda. Gerassimos J. Mantzaris reports research grants from AbbVie, Genesis, MSD, and Takeda, and being an adviser/speaker for AbbVie, Aenorasis, Dr Falk Pharma, Ferring, Hospira, Janssen, MSD, MYLAN, Pfizer, Takeda, and Vianex. Song Wang, Shashi Adsul, and Pravin Kamble are employees of the study sponsor, Takeda, and hold stock or stock options in Takeda. Erin Cook, Gautam Sajeev, and Annie Guerin are employees of Analysis Group, who were contracted by Takeda to perform this analysis. Brian Bressler reports being an adviser/speaker for AbbVie, Bristol Myers Squibb, Ferring, Janssen, Merck, Novartis, Pfizer, and Takeda; adviser for Alimentiv, Allergan, Amgen, AMT, Bristol Myers Squibb, Celgene, Fresenius Kabi, Genentech, Gilead, Iterative Scopes, Merck, Microbiome Insights, Mylan, Pendopharm, and Protagonist; research support from AbbVie, Alvine, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Genentech, GlaxoSmithKline, Janssen, Merck, and Qu Biologic; and stock options in Qu Biologic.
Ethical Approval
The study was approved by the local ethics committee at each participating site. Patients alive at the time of chart abstraction (99% of patients) signed an informed consent form prior to participation in the study if there was no waiver of informed consent.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yarur, A., Mantzaris, G.J., Wang, S. et al. Stratified Patient Profiling for Vedolizumab Effectiveness in Crohn’s Disease: Identifying Optimal Subgroups for Enhanced Treatment Response in the EVOLVE Study. Adv Ther 41, 2324–2341 (2024). https://doi.org/10.1007/s12325-024-02825-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02825-w