Abstract
Introduction
Asthma treatment guidelines classify inhaled corticosteroid (ICS) regimens as low, medium, or high dose. However, efficacy and safety are not independently assessed accordingly. Moreover, differences in ICS duration of action are not considered when a dose regimen is selected. We investigated the efficacy and safety implications of these limitations for available ICS molecules.
Methods
Published pharmacodynamic and pharmacokinetic parameters were used, alongside physiological and pharmacological principles, to estimate the efficacy and safety of available ICS molecules. Extent and duration of glucocorticoid receptor (GR) occupancy in the lung (efficacy) and cortisol suppression (systemic exposure and safety) were estimated.
Results
Some ICS regimens (e.g., fluticasone furoate, fluticasone propionate, and ciclesonide) rank high for efficacy but low for systemic exposure, contrary to how ICS dose equivalence is currently viewed. Differences in dose–response relationships for efficacy and systemic exposure were unique for each ICS regimen and reflected in their therapeutic indices. Notably, even low doses of most ICSs can generate high GR occupancy (≥ 90%) across the entire dose interval at steady state, which may explain previously reported difficulties in obtaining dose responses within the clinical dose range and observations that most clinical benefit typically occurs at low doses. The estimated post dose duration of lung GR occupancy for ICS molecules was categorized as 4–6 h (short), 14–16 h (medium), 25–40 h (long), or > 80 h (ultra-long), suggesting potentially large differences in anti-inflammatory duration of action.
Conclusion
In a real-world clinical setting where there may be poor adherence to prescribed therapy, our findings suggest a significant therapeutic advantage for longer-acting ICS molecules in patients with asthma.
Plain Language Summary
Patients with asthma often rely on inhaled corticosteroids to manage their symptoms by controlling lung inflammation. Inhaled corticosteroids can be used at low, medium, or high doses; however, the effectiveness, safety, and how long the effects last for a particular inhaled corticosteroid molecule are not considered when choosing them. This study investigated the safety and efficacy of different inhaled corticosteroid molecules. Leveraging published data on the mode of anti-inflammatory action and the rates these molecules are absorbed and eliminated from the body, we estimated their effectiveness and safety profiles, including duration of action in the lungs and systemic exposure levels. Some inhaled corticosteroid molecules such as fluticasone furoate, fluticasone propionate, and ciclesonide were found to exhibit high anti-inflammatory effectiveness in the lungs with minimal systemic exposure, contrasting the perceived similarities among currently used drug molecules. Anti-inflammatory duration of the unwanted systemic effect in the rest of the body was unique for each inhaled corticosteroid molecule. Notably, even the lowest doses of most inhaled corticosteroids were found to be effective in the lungs when taken as prescribed, supporting previous observations that clinical benefits are mostly realized at lower doses. Furthermore, estimated post dose durations of effectiveness for different inhaled corticosteroid molecules varied widely among different molecules, with some lasting a few hours and others lasting more than 80 h, suggesting significant differences in their duration of action. Overall, these findings demonstrate the potential advantage of using longer-acting inhaled corticosteroids, particularly for patients with asthma who may face challenges in adhering to prescribed regimens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
There is a lack of data on the relative efficacy, safety, and duration of efficacy in the context of the categorization of inhaled corticosteroid (ICS) therapies as low-, medium-, and high-dose regimens, for the treatment of patients with asthma |
The aim of this study was to examine the pharmacological basis for ICS dose responses, dose equivalence, and duration of action, and the implications of these factors not being appropriately considered by treatment guidelines and current clinical practice |
What was learned from the study? |
Our findings identify differences between the presumed and observed efficacy and systemic exposure across several commercially available ICS therapies, largely supporting the use of longer-acting ICS therapies in patients with asthma |
These data can support practicing physicians with optimizing treatment regimens for patients with asthma, particularly in those who have suboptimal adherence to their prescribed ICS therapy |
Introduction
Asthma treatment guidelines and the Global Initiative for Asthma strategy document classify inhaled corticosteroid (ICS) regimens via low-, medium-, and high-dose categories [1, 2], often with reference to the now discontinued beclomethasone dipropionate (BDP) chlorofluorocarbon (CFC) propellent inhaler (BECOTIDE circa 1972–2007). Low doses are assumed to be the lowest dose range required to achieve efficacy in mild-to-moderate asthma with a low risk of systemic exposure, whereas the high-dose category is assumed to be appropriate for moderate-to-severe asthma but inevitably associated with a high risk of systemic exposure. Although there is an underlying assumption that the dose responses for efficacy and systemic exposure are essentially the same for all ICSs after doses are adjusted for differences in glucocorticoid receptor (GR) binding affinity (potency) [3], the main concern for the high-dose ICS category is unwanted systemic exposure. Also, the possibility that different ICS molecules within the same dose category might differ in duration of action and therapeutic index is not taken into account. Furthermore, the rationale and pharmacological basis for using high-dose ICS regimens have not been robustly established. Indeed, evidence from clinical studies and meta-analyses support that most patients can achieve adequate efficacy with a low-dose ICS regimen. However, this may reflect the ideal scenario of high adherence to the treatment (i.e., a randomized controlled trial), where differences such as duration of action between ICS molecules may be less impactful. Conversely, in a real-world setting where patients miss doses and there is suboptimal adherence to therapy, ICS molecules with a longer duration of action may provide a significant therapeutic advantage. In this study, we examined the pharmacological basis for ICS dose responses, dose equivalence, and duration of action, and the implications of these factors not being appropriately considered by treatment guidelines and current clinical practice.
Methods
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
ICS Lung Concentration–Time Profiles
The time profiles for steady-state ICS lung and plasma concentrations were based on published data describing dose fractions available in the lung, lung absorption rates, oral bioavailability, and systemic clearance for fluticasone furoate (FF), mometasone furoate (MF), fluticasone propionate (FP), budesonide (BUD), ciclesonide hydrofluoroalkane (CICHFA), beclomethasone dipropionate hydrofluoroalkane (BDPHFA), triamcinolone acetonide CFC (TAACFC), flunisolide hydrofluoroalkane (FLUHFA), and BDP CFC (BDPCFC) (Table 1) [3].
Airway Efficacy Based on Extent of Lung GR Occupancy
The extent of GR occupancy in the lung at any time post dose was calculated as a measure of efficacy using GR dissociation constants (Kd) and lung concentration–time profiles (Table 1). The amount of ICS absorbed from the lung into the systemic circulation was assumed to be equal to the amount deposited in the lung during the same interval at steady state. The absorbed ICS was assumed to be uniformly distributed throughout the lung tissue and potentially available for GR binding. For BDP and ciclesonide (CIC), the administered molecules are prodrugs that require conversion to the active moiety as a prerequisite for efficacy [3]. For BDP, this conversion was deemed essentially complete within the lung (97%) [3]. However, the conversion of CIC to the active desisobutyryl-CIC (des-CIC) is assumed to be less complete within the lung, so a correction was applied for CIC-active principle lung concentrations. The lung concentration required to achieve 50% GR occupancy was determined by the GR receptor Kd (e.g., when the concentration in the lung equals Kd, 50% of receptors are occupied) [3]. Other degrees of occupancy can be estimated from this relationship (e.g., at nine times the Kd, 90% of GR receptors are occupied).
The anti-inflammatory efficacy of the dose of currently available ICS regimens for patients with asthma was assumed to be directly correlated with the degree of GR occupancy in the lung. This was calculated from the ICS concentration–time profile in lung tissue and the GR dissociation constant. We assumed that the dose absorbed from the lung was potentially available to bind to GR, and if evenly distributed throughout lung tissue, this concentration should decline in line with the absorption rate constant from the lung. This gave an estimate of the theoretical extent of GR occupancy at any time post dose.
Duration of ICS Action Based on Duration of Airway GR Occupancy
The duration of GR occupancy maintenance was estimated, as described above, using the Kd or multiples thereof and the concentration in the airways at any time during and beyond the dose interval [4]. For example, the duration for maintaining 90% GR occupancy can be estimated using the formula:
where Kd is the GR dissociation constant, C0 is the lung concentration immediately post dose at steady state, and Ka is the ICS absorption rate constant from the lung. From this, the percentage of GR occupancy in the lung was calculated for various doses of BDP, BUD, FP, and FF immediately post dose at steady state, at the midpoint of the dose interval, at the end of the dose interval, and at 12, 24, 48, 72, 96, and 120 h after cessation of dosing.
Safety Based on ICS Systemic Exposure
Cortisol suppression was calculated as a measure of systemic exposure using a physiological model that relates the endogenous glucocorticoid (cortisol) production rate to the exogenous contributions (ICS) by converting them into cortisol-equivalent exposures [3]. The calculation takes into account the bioavailability, relative GR binding affinity, and systemic clearance of the exogenous and endogenous glucocorticoids to express the systemic exposure for each ICS as a cortisol-equivalent area under the plasma concentration–time curve and a corresponding steady-state cortisol suppression after 24 h [3] (Table 1).
Results
Dose Response
The dose response for efficacy in the lung for four ICS molecules was expressed as the percentage of GR occupancy in the lung at various timepoints post dose at steady state for a range of doses (2–2000 µg twice-daily [BID] for BDP, BUD, and FP, and 2–2000 µg once-daily [OD] for FF) (Fig. 1, Table 2). BDP 200 µg BID can produce 91.5% GR occupancy in the lung at the end of the 12-h dose interval, but only 33.0% GR occupancy at 24 h post dose and negligible (0.1%) GR occupancy at 48 h post dose. Similar values (93.4%, 41.9%, and 0.2% respectively) are seen for BUD. For FP, the duration of action is potentially much longer: for example, FP 100 µg BID can produce 98.2% and 94.6% GR occupancy in the lung 12 h and 24 h post dose, and 63.6% GR occupancy 48 h post dose, but only 14.8% GR occupancy 72 h post dose. In contrast, FF has potentially an even longer duration of action since FF 100 µg OD can achieve 92.8% GR occupancy in the lung 72 h post dose.
Dose response and duration of action for different ICS regimens in terms of GR occupancy in the lung. The percentage of GR occupancy in the lung over time for A BDP, B BUD, C FP, and D FF. The relationship between ICS dose and the percentage of GR occupancy in the lung at steady state is estimated at various times after cessation of dosing. BDP beclomethasone dipropionate, BID twice-daily, BUD budesonide, FF fluticasone furoate, FP fluticasone propionate, GR glucocorticoid receptor, ICS inhaled corticosteroid
Dose Equivalence
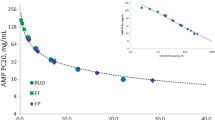
Cortisol suppression (24 h mean) and lung GR occupancy were calculated for various doses of each ICS molecule at steady state (Fig. 2, Table 3). The midpoint of the dose interval was used as the timepoint for lung GR occupancy (i.e., 6 h instead of 12 h post dose or 12 h instead of 24 h post dose) to avoid bias towards longer-acting ICS. These values were converted into the BDPCFC equivalent dose for cortisol suppression and lung GR occupancy (Fig. 1). FP 500 µg total daily dose (250 µg BID) was equivalent to 995 µg total daily dose of BDPCFC in terms of GR occupancy in the lung and 367 µg total daily dose of BDPCFC in terms of cortisol suppression, with corresponding dose ratios of 1.99 for efficacy and 0.73 for systemic exposure. BUD doses (400–1600 μg total daily dose) were similar to BDPCFC in terms of systemic exposure (dose ratio = 0.89), but less so in terms of efficacy (dose ratio = 0.62). FF 100 µg OD was equivalent to 831 µg total daily dose of BDPCFC in terms of GR occupancy in the lung (dose ratio = 8.31) but only 114 µg total daily dose of BDPCFC in terms of cortisol suppression (dose ratio = 1.14). The ICS dose regimens fell into four categories based on high or low efficacy and high or low systemic exposure, relative to 400 µg total daily dose (200 µg BID) of BDPCFC (Fig. 2). Accordingly, the calculated efficacy/systemic activity ratios varied widely from high (FF 7.29, CIC 3.98, FP 2.71) to low (MF 1.38, BDP 1, BUD 0.70) values (Table 3) and in line with their previously reported therapeutic indices [3, 5].
ICS dose equivalence expressed as BDP CFC MDI equivalent doses for efficacy in the lung and systemic exposure. Each ICS dose regimen is plotted in terms of the BDP dose with the same percent GR occupancy at the midpoint of the dose interval and the BDP dose with the same 24 h cortisol suppression. The figure is divided into four quadrants with 400 µg total daily dose (200 µg BID) of BDP as the central reference point. Relative to this, the other ICS dose regimens can be classified according to the quadrant in which they fall based on higher or lower efficacy and higher or lower systemic exposure relative to 400 µg total daily dose of BDP. This figure has been previously presented at the American Thoracic Society international conference, May 17–22, 2019, Dallas, TX, USA [4]. BDP beclomethasone dipropionate, BDPHFA beclomethasone dipropionate hydrofluoroalkane, BID twice-daily, BUDDPI budesonide dry powder inhaler, CIC ciclesonide, CFC chlorofluorocarbon, CICHFA ciclesonide hydrofluoroalkane, GR glucocorticoid receptor, DPI dry powder inhaler, FFDPI fluticasone furoate dry powder inhaler, FLUHFA flunisolide hydrofluoroalkane, FPDPI fluticasone propionate dry powder inhaler, HFA hydrofluoroalkane, ICS inhaled corticosteroid, MDI metered dose inhaler, MFDPI mometasone furoate dry powder inhaler, TAACFC triamcinolone acetonide chlorofluorocarbon
Duration of Action
The duration of action of each ICS molecule was calculated as the time post dose that 90% GR occupancy in the lung was maintained (Fig. 3). There were marked differences in the duration of action amongst the ICS regimens. The longest duration of action was found for FF (82.5 h), where high GR lung occupancy is potentially maintained for several days post dose (Table 4). FF also had the lowest GR dissociation constant and the slowest absorption rate from the lung (Table 1). The shortest duration was found for flunisolide (FLU; 4.5 h), which had the highest GR dissociation constant and the fastest absorption rate from the lung. ICS dose regimens fell into four categories for duration of action: short, 4–6 h (FLU, triamcinolone acetonide [TAA]); medium, 14–16 h (BUD, BDP); long, 25–40 h (MF, FP, CIC); and ultra-long, greater than 80 h (FF; Fig. 3, Table 4). Similar to the spread in efficacy/systemic activity ratios, we also identified differences in the duration of action/dose interval ratios for ICSs. Some ICSs provided duration of action well beyond the dosing interval (FF 3.44, FP 2.78) whilst others showed duration of action close to the dosing interval (BDP 1.28, BUD 1.15, CIC 1.55, MF 1.17).
Duration of action of ICS regimen. Time post dose during which 90% GR occupancy is maintained in the lung after cessation of dosing. BDPCFC beclomethasone dipropionate chlorofluorocarbon, BDPHFA beclomethasone dipropionate hydrofluoroalkane, BID, twice daily, BUD budesonide, CIC ciclesonide, FF fluticasone furoate, FLU flunisolide, FP fluticasone propionate, GR glucocorticoid receptor, ICS inhaled corticosteroid, MF mometasone furoate, OD once daily, QID four-times daily, TAA triamcinolone acetonide, TID three-times daily
Discussion
Dose Response
When a patient’s asthma is not controlled on ICS therapy, higher ICS doses may be prescribed to achieve the desired efficacy. However, there is weak pharmacological basis for expecting a dose response by increasing the ICS dose from low to medium and eventually to high. The findings from this analysis revealed that, theoretically, high levels of GR occupancy in the lung (≥ 90%) can be achieved even with low ICS doses and maintained during most of the dose interval [6]. For example, after dosing to steady state, a 200 µg BID dose of BDP will result in 98% GR occupancy at the midpoint of the dose interval (6 h post dose) and 91.5% GR occupancy at the end of the dose interval (12 h post dose). By 24 h post dose, GR occupancy falls to 33.0%. Although the degree of GR occupancy required for efficacy is not established, it is widely recognized that total daily doses of BDP 400 µg, BUD 400 µg, or FP 200 µg are sufficient to provide adequate efficacy in most patients with asthma, if they are adherent, as evident from clinical efficacy studies [7, 8], with little benefit gained from giving higher doses [9]. Lower degrees of GR occupancy are clearly associated with some efficacy, although the point at which occupancy is too low for adequate efficacy is unknown. Although higher ICS doses can produce a greater than 90% GR occupancy, there is a low likelihood of further clinical benefit, but an increased risk of systemic exposure [5, 7, 8, 10]. However, this rationale is largely based on data from clinical trials [7, 8, 11]. In a real-world setting with poor inhaler technique and/or adherence to therapy, the benefit/risk considerations of increasing doses may be different across the ICS dose range. Indeed, it has been experimentally demonstrated that all ICS molecules are not therapeutically similar and that across the approved doses for asthma, FF gave more protection against airway hyperresponsiveness with less systemic activity, and had a wider therapeutic index (systemic activity/airway potency ratio) than FP or BUD [5]. Furthermore, the real-world relevance of these differences, particularly in scenarios of poor adherence, has been explored more fully in another publication [6]. These findings are relevant to asthma treatment guidelines that currently assume that there is no difference in therapeutic index between ICS molecules and that for all ICS regimens a dose defined as “high dose” in these guidelines is unavoidably associated with a high risk of unwanted systemic side effects. Another reason for lack of response when increasing the dose of an ICS regimen might be the limited number of receptors available for ligand binding in the lung [12,13,14]. This would cause a saturation in GR occupancy by high-dose ICS regimens [12]. A possible exception proposed by the authors of this manuscript could be patients with what is regarded as “steroid-resistant” asthma or those perceived as requiring higher doses due to concomitant smoking or comorbidities, such as obesity. It is also important to highlight that in some patients with significant airflow limitation and obstruction, a low-dose ICS regimen might not provide 90% or higher GR occupancy and near maximal efficacy. Patients with moderate-to-severe asthma potentially require higher doses of ICS due to increasing underlying inflammation [15, 16]. Finally, changes to a patient’s lung architecture may mean that the ICS dose does not reach all desired regions of the lung in sufficient quantities, which may be further limited by their impediments to efficiently use an inhaler. These patients may require higher ICS doses and/or improved lung delivery (e.g., providing training on inhaler technique or using a spacer with metered dose inhaler).
Another dose response question worth consideration is whether ICS with high systemic bioavailability, when administered at high doses, can have systemic activity sufficient to contribute to efficacy in the airways. In this regard, it has been shown that high doses of some ICS have similar systemic activity to low-dose orally administered prednisolone [17], hence it seems reasonable to assume that there would be a systemic contribution to their pharmacological effect at high doses. However, it has also been demonstrated that administering large systemic doses of an ICS to achieve higher systemic concentrations than those that occur during inhaled administration does not result in efficacy in the airways. Together these observations suggest that the systemic contribution to airways efficacy for ICS when used at recommended doses is likely to be insignificant, although it would not rule out a systemic effect if there is such a mechanism of action.
Dose Equivalence
We demonstrate that the relationship between topical efficacy in the lung and systemic exposure (sometimes referred to as the therapeutic index) was different for each ICS. This is critical when deciding whether alternative ICS regimens are dose equivalent. Notably, some regimens (i.e., FF) could be classified as high for efficacy but low for systemic exposure, which is contrary to how ICS dose equivalence is currently viewed. The use of relative percentages of GR occupancy in the lung and relative cortisol suppression, rather than absolute values, circumvents the problem of not knowing the exact degree of GR occupancy that is required for efficacy or associated with systemic exposure. We used BDPCFC 200 µg BID as the benchmark to make dose equivalence comparisons with the other ICS dose regimens (Fig. 2). However, it is known that this low dose of BDP (200 µg BID) is sufficient for adequate efficacy in most patients with asthma [1, 2, 18, 19]. Our results demonstrate that this dose maintains at least 90% GR occupancy for the entire 12-h dose interval, although lower doses or wider dose intervals do not achieve this and are therefore likely to be suboptimal for efficacy.
Duration of Action
The hypothesis of different ICS molecules varying in their duration of action has not been fully explored either theoretically or clinically. The extent of the ICS molecules’ retention in the airway is determined by the dose delivered to the lung, solubility and dissolution rate, lung tissue binding affinity, and absorption rate from the lung [3]. Post dose, the lung concentration then declines in line with the absorption rate from the lung [4]. This concentration, together with the GR dissociation constant, determines the degree of GR occupancy and how long it is maintained. It also forms the theoretical basis for ICS molecules differing in their durations of action. The latter also influences the dose interval that may be feasible with different ICS regimens [3]. Indeed, the recommended dose intervals for ICS regimens used in clinical practice vary from three to four times daily for short-acting ICS (e.g., TAA, FLU) to BID for medium-acting ICS (e.g., BDP, BUD) and the long-acting ICS FP, and OD for some long-acting (e.g., CIS, MF) and ultra-long-acting ICS (e.g., FF). A recent clinical trial in patients with asthma provided evidence that the duration of anti-inflammatory action of FF, as measured by fractional exhaled nitric oxide (FeNO) suppression, is potentially longer than that reported for BUD [20]. These data for FF are shown in Fig. 4, which highlights that the time course for the estimated duration of GR occupancy in the lung corresponds to the duration of anti-inflammatory action observed in the aforementioned clinical trial using FeNO suppression as a measure of efficacy [20].
Time course for the estimated duration of GR occupancy in the lung corresponds to duration of anti-inflammatory action measured in a clinical trial. Concentration of FF in the lung and plasma, their relationship to estimated percentage of GR occupancy in the lung and observed percentage of FeNO suppression following cessation of 100 μg OD dosing at steady state in patients with asthma [19]. FeNO fractional exhaled nitric oxide, FF fluticasone furoate, GR glucocorticoid receptor, NO nitric oxide, OD once-daily
As discussed above, all ICS doses within the clinical dose range can potentially result in high levels of GR occupancy (≥ 90%) in the lung during most of the dose interval. This ought to correspond with near maximum clinical efficacy. However, the various ICS molecules differ vastly, from 4.5 h for FLU to 82.5 h for FF, in the duration for which these high levels of GR occupancy can be maintained (Table 4, Fig. 3). This depends on (i) how much and how long the molecule is retained in the lung and (ii) the concentration needed to achieve high levels of GR occupancy reflected by the GR Kd [3]. There may be little advantage to giving larger doses due to cases where even low ICS doses can result in high GR occupancy in the lung during most of the dose interval. However, it is possible that increasing the ICS dose might increase GR occupancy maintenance and subsequently extend the duration of anti-inflammatory action. If the ICS dose is doubled, the duration of action will be increased by one half-life [21]. This half-life is associated with lung absorption and hence retention. Unfortunately, for most ICSs, the half-life is relatively short (3 h or less for FLU, TAA, BDP, and BUD; 5–7 h for MF, CIC, and FP; Table 1). Therefore, increasing the dose would only result in a correspondingly small increase in the duration of action for these ICS regimens, except for FF, which has a notably longer half-life of approximately 20 h (Table 1). Thus, doubling the FF dose would increase its duration of action by close to 1 day. Even at a low dose of 100 µg, FF has the longest duration of action since its longer lung retention is coupled with a higher GR affinity, allowing for a OD dosing regimen. The identified differences in duration of action/dose interval ratio may have important clinical implications. For FF and FP, where the duration of action is several times that of the dosing interval, the anti-inflammatory effect may be maintained, even if one or two doses are missed. For ICS (BDP, BUD, CIC, MF), where the duration of action is close to the dosing interval, the anti-inflammatory effect may wane if a dose was to be missed. This finding may help explain observed real-world benefits for FF and FP where adherence is suboptimal. It may further be feasible to extend the dosing interval for these ICS whilst maintaining efficacy (i.e., alternate-day dosing for FF).
When GR occupancy in the lung is calculated as defined, it represents the maximum theoretical GR occupancy achievable if all the bioavailable drug in the lung reaches the pharmacological target in the biologically relevant cells. However, in practice, the extent of GR occupancy achieved will vary for various ICS molecules, driven not only by their GR binding affinities but also their physicochemical properties. Lipophilicity and permeability are the physicochemical properties most likely to influence drug availability for GR binding as they determine dissolution, solubility, and partitioning into target cells and tissues within the airway [6]. Although these factors are independently quantifiable in vitro, it is not known how they act together in vivo within the airways. It has been reported that differences in permeability observed for steroids have a weak inverse correlation with lipophilicity (ClogP) [22]. This is because hydrophilic steroids tend to diffuse faster over the cell monolayers in comparison to the hydrophobic steroids, which diffuse more slowly [22]. The relationship with ClogP suggests that partitioning of the steroids between the biological membrane and the surrounding aqueous phase is one of the main mechanisms for absorption, indicating passive diffusion. Therefore, it is expected that ICSs with lower lipophilicity may have higher permeability compared with ICSs with higher lipophilicity. Since these physicochemical properties significantly influence the effect of ICS molecules, they should be considered when trying to determine a molecule’s dose response, equivalence, and duration of action. Additionally, such assumptions about GR occupancy calculated in this way are only achievable in patients with asthma who have good inhaler technique and are adherent to the prescribed dose regimen [6].
Limitations of the Investigation
This analysis has some limitations, which should be considered. The fraction of the dose absorbed from the lung was assumed to be equal to the drug available for GR binding in the lung. Although not all drug inhaled or deposited in the lung is necessarily absorbed into systemic circulation, the amount that is will be the dissolved drug fraction that is able to cross the barriers of the lung epithelium, enter the bloodstream, and hence potentially bind to GR within lung cells. This fraction of the dose was assumed to be evenly distributed throughout the total lung tissue of 1 kg at steady state. Although the inhaled drug particles are unlikely to be deposited evenly immediately after a single dose, after repeated dosing and allowing for diffusion and redistribution of the dissolved drug, this is a more reasonable assumption once steady state is reached. In patients with asthma who have significant ventilation and perfusion mismatch in the lungs, there may be greater differences in local drug deposition, but the assumptions based on the average lung concentration of drug may not be impacted and variability in GR occupancy drug response might be higher. Then, even if there are areas of higher and lower drug concentration throughout the lung, the average concentration should equate to the assumption of even drug distribution. It was deemed appropriate to assume that this average lung concentration can be used to calculate the percent GR occupancy in the lung. Although there will be some non-specific binding to lung tissue, the GR dissociation constants for the various ICS molecules were assumed to be appropriate for this analysis since they were derived using measurements from human lung cytosol, instead of animal tissue/cells, to estimate the GR affinities in vitro.
Considering the assumptions made in the data analyses, it is important to approach the applicability to real-world patients cautiously until validated by real-world studies.
Conclusions
Here, we show that all ICS molecules, even the lowest dose regimens, have the potential to generate high levels of GR occupancy in the lung for most of the dose interval under ideal conditions of adherence to therapy. On cessation of dosing, after dosing to steady state, ICSs with longer duration of action provided greater than 90% GR occupancy well beyond the dosing interval; FF (100 µg OD) can achieve 92.8% GR occupancy in the lung 72 h post dose, versus 94.6% GR occupancy 24 h post dose for FP (200 µg BID), whilst ICSs with shorter duration of action provided greater than 90% GR occupancy for the first 12 h but not beyond 24 h (93.4% and 41.9% GR occupancy at 12 and 24 h post dose, respectively, for BUD [400 µg BID]). The relationship between efficacy in the lung and systemic exposure was different for each ICS. This is particularly important when deciding whether alternative ICS regimens are dose equivalent. Notably, some regimens could be classified as high for efficacy but low for systemic exposure, which is contrary to how ICS dose equivalence is currently viewed. Duration of GR occupancy in the lung and its implications for efficacy maintenance showed the largest potential for differentiating between ICS molecules. ICS dose regimens were categorized as follows for duration of action: short, 4–6 h (FLU, TAA); medium, 14–16 h (BUD, BDP); long, 25–40 h (MF, FP, CIC); and ultra-long, greater than 80 h (FF). This feature is likely to confer a therapeutic advantage for longer-acting ICS regimens, particularly in patients with asthma who are poorly adherent to their prescribed ICS therapy.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Global Initiative for Asthma. Global strategy for asthma management and prevention 2014. https://ginasthma.org/. Accessed 20 Oct 2023.
British Thoracic Society, Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. Thorax. 2003;58 Suppl 1(Suppl 1):i1–94.
Daley-Yates PT. Inhaled corticosteroids: potency, dose equivalence and therapeutic index. Br J Clin Pharmacol. 2015;80(3):372–80.
Daley-Yates PT. Pharmacological basis of inhaled corticosteroid (ICS) dose equivalence and duration of action. Thorax. 2019;74:A215–6.
Daley-Yates PT, Brealey N, Thomas S, et al. Therapeutic index of inhaled corticosteroids in asthma: a dose-response comparison on airway hyperresponsiveness and adrenal axis suppression. Br J Clin Pharmacol. 2021;87(2):483–93.
Daley-Yates PT, Aggarwal B, Lulic Z, Fulmali S, Cruz AA, Singh D. Pharmacology versus convenience: a benefit/risk analysis of regular maintenance versus infrequent or as-needed inhaled corticosteroid use in mild asthma. Adv Ther. 2022;39(1):706–26.
Masoli M, Holt S, Weatherall M, Beasley R. Dose-response relationship of inhaled budesonide in adult asthma: a meta-analysis. Eur Respir J. 2004;23(4):552–8.
Holt S, Suder A, Weatherall M, Cheng S, Shirtcliffe P, Beasley R. Dose-response relation of inhaled fluticasone propionate in adolescents and adults with asthma: meta-analysis. BMJ. 2001;323(7307):253–6.
Beasley R, Harper J, Bird G, Maijers I, Weatherall M, Pavord ID. Inhaled corticosteroid therapy in adult asthma. Time for a new therapeutic dose terminology. Am J Respir Crit Care Med. 2019;199(12):1471–7.
Chanez P, Karlstrom R, Godard P. High or standard initial dose of budesonide to control mild-to-moderate asthma? Eur Respir J. 2001;17(5):856–62.
Masoli M, Weatherall M, Holt S, Beasley R. Clinical dose-response relationship of fluticasone propionate in adults with asthma. Thorax. 2004;59(1):16–20.
Esmailpour N, Högger P, Rabe KF, Heitmann U, Nakashima M, Rohdewald P. Distribution of inhaled fluticasone propionate between human lung tissue and serum in vivo. Eur Respir J. 1997;10(7):1496–9.
Hochhaus G, Rohdewald P, Mollmann H, Greschuchna D. Identification of glucocorticoid receptors in normal and neoplastic adult human lung. Res Exp Med (Berl). 1983;182(1):71–8.
Rousseau GG, Baxter JD. Glucocorticoid receptors. Monogr Endocrinol. 1979;12:49–77.
Lotvall J, Inman M, O’Byrne P. Measurement of airway hyperresponsiveness: new considerations. Thorax. 1998;53(5):419–24.
Singh D, Garcia G, Maneechotesuwan K, et al. New versus old: the impact of changing patterns of inhaled corticosteroid prescribing and dosing regimens in asthma management. Adv Ther. 2022;39(5):1895–914.
Lawrence M, Wolfe J, Webb DR, et al. Efficacy of inhaled fluticasone propionate in asthma results from topical and not from systemic activity. Am J Respir Crit Care Med. 1997;156:744–51.
Gustafsson P, Tsanakas J, Gold M, Primhak R, Radford M, Gillies E. Comparison of the efficacy and safety of inhaled fluticasone propionate 200 micrograms/day with inhaled beclomethasone dipropionate 400 micrograms/day in mild and moderate asthma. Arch Dis Child. 1993;69(2):206–11.
Leblanc P, Mink S, Keistinen T, Saarelainen PA, Ringdal N, Payne SL. A comparison of fluticasone propionate 200 μg/day with beclomethasone dipropionate 400 μg/day in adult asthma. Allergy. 1994;49(5):380–5.
Bardsley G, Daley-Yates PT, Baines A, et al. Anti-inflammatory duration of action of fluticasone furoate/vilanterol trifenatate in asthma: a cross-over randomised controlled trial. Respir Res. 2018;19(1):133.
Rowland M, Tozer TN. Clinical pharmacokinetics: concepts and applications. 3rd ed. Philadelphia: Lea & Febiger; 1995.
Faassen F, Kelder J, Lenders J, Onderwater R, Vromans H. Physicochemical properties and transport of steroids across Caco-2 cells. Pharm Res. 2003;20(2):177–86.
Medical Writing and/or Editorial Assistance.
Medical writing support, under the guidance of the authors, was provided by Fiona Scott, PhD, and Alexia Tsakaneli, PhD, of Ashfield MedComms (Glasgow, UK), an Inizio company, and was funded by GSK.
Funding
This study was funded by GSK. Open Access and Rapid Service publication charges were funded by GSK. Trademarks are the property of their respective owners (BECOTIDE [GSK]).
Author information
Authors and Affiliations
Contributions
Peter T. Daley-Yates conceived the investigation, designed the study, performed the data analysis and simulations, and prepared the manuscript. Bhumika Aggarwal and Maximilian Plank provided critical analysis of the study and contributed to manuscript preparation. All authors met the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Corresponding author
Ethics declarations
Conflict of Interest
Peter T. Daley-Yates was an employee and a shareholder of GSK at the time of the study. Bhumika Aggarwal and Maximilian Plank are employees and shareholders of GSK.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Peter T. Daley-Yates’ affiliation was correct at the time of study.
Prior Presentation: Some of the material discussed in this manuscript was presented at the American Thoracic Society international conference, May 17–22, 2019, Dallas, TX, USA [4] and at the British Thoracic Society meeting, December 4–6, 2019, London, UK: Daley-Yates P, P231 Pharmacological basis of inhaled corticosteroid (ICS) dose equivalence and duration of action. Thorax 2019;74:A215–A216.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Daley-Yates, P.T., Aggarwal, B. & Plank, M. Pharmacological Basis of Differences in Dose Response, Dose Equivalence, and Duration of Action of Inhaled Corticosteroids. Adv Ther 41, 1995–2009 (2024). https://doi.org/10.1007/s12325-024-02823-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02823-y