Abstract
Introduction
For patients with chronic insomnia, conventional therapy may not always provide satisfactory efficacy and safety. Thus, switching to an alternative therapeutic agent can be explored. However, there is a lack of prospective studies evaluating the effectiveness of such changes. This prospective, non-randomized, open-label, interventional, multicenter study assessed whether Japanese patients with chronic insomnia dissatisfied with treatment could transition directly to lemborexant (LEM) from four cohorts—non-benzodiazepine sedative-hypnotic (zolpidem, zopiclone, or eszopiclone) monotherapy, dual orexin receptor antagonist (suvorexant) monotherapy, suvorexant + benzodiazepine receptor agonists (BZRAs), and melatonin receptor agonist (ramelteon) combination. We evaluated whether transitioning to LEM improved patient satisfaction based on efficacy and safety.
Methods
The primary endpoint was the proportion of successful transitions to LEM at 2 weeks (titration phase end), defined as the proportion of patients on LEM by the end of the 2-week titration phase who were willing to continue on LEM during the maintenance phase (Weeks 2–14). Patient satisfaction and safety (the incidence of treatment-emergent adverse events [TEAEs]) were assessed at 14 weeks (end of titration and maintenance phases).
Results
Among the 90 patients enrolled, 95.6% (95% confidence interval: 89.0–98.8%) successfully transitioned to LEM at 2 weeks. The proportions of patients who successfully continued on LEM were 97.8% and 82.2% at the end of the titration and maintenance phases (Weeks 2 and 14), respectively. The overall incidence of TEAEs was 47.8%; no serious TEAEs occurred. In all cohorts, the proportions of patients with positive responses were higher than the proportions with negative responses on the three scales of the Patient Global Impression-Insomnia version. During the maintenance phase, Insomnia Severity Index scores generally improved at Weeks 2, 6, and 14 of LEM transition.
Conclusions
Direct transition to LEM may be a valid treatment option for patients with insomnia who are dissatisfied with current treatment.
Trial Registration
ClinicalTrials.gov identifier, NCT04742699.
Similar content being viewed by others
Why carry out this study? |
For chronic insomnia patients, conventional therapy may not always lead to effective and safe results and switching to an alternative treatment may be an option. |
However, there is a shortage of prospective studies evaluating the efficacy and safety of such changes. |
This prospective, non-randomized, open-label, interventional, multicenter study assessed direct switching to lemborexant, a dual orexin receptor antagonist approved for insomnia treatment, from other insomnia treatments—non-benzodiazepine sedative-hypnotic (zolpidem, zopiclone, or eszopiclone) monotherapy, a different dual orexin receptor antagonist (suvorexant) monotherapy, suvorexant + benzodiazepine receptor agonists, and melatonin receptor agonist (ramelteon) + benzodiazepine receptor agonists combination. |
What was learned from the study? |
Findings highlight the successful transition to lemborexant from other treatments for patients with insomnia who were dissatisfied with, or anxious about, their current treatment and suggest improved patient satisfaction after switching to lemborexant based on the Patient Global Impression—Insomnia scale and Insomnia Severity Index. |
Direct transition to lemborexant may be a beneficial treatment option for patients with insomnia who are dissatisfied with their current treatment. |
Introduction
Insomnia is a common sleep disorder worldwide, with 30–40% of adults presenting with a symptom of the condition [1]. In Japan, a 2016 epidemiologic survey reported the prevalence of insomnia to be 12.2% in men and 14.6% in women [2]. The criteria for diagnosing chronic insomnia include difficulties in falling and/or staying asleep and daytime dysfunction [3, 4], with symptoms being acute, intermittent, short-term, or chronic [5]. Insomnia treatment aims to address nighttime sleep difficulties and reduce daytime dysfunction [6] and involves non-pharmacologic approaches (e.g., sleep hygiene and cognitive behavioral therapy) as first-line treatment and pharmacologic therapy [7].
In Japan, the main hypnotics used to treat insomnia before the introduction of lemborexant (LEM) included sedative-hypnotic benzodiazepine receptor agonists (BZRAs), comprising benzodiazepines and non-benzodiazepine sleeping pills (Z-drugs), the melatonin receptor agonist ramelteon (RMT), and the dual orexin receptor antagonist (DORA) suvorexant (SUV). Another DORA, daridorexant, has not been approved for use in Japan. Clinically, hypnotics are prescribed as monotherapy and concomitantly, particularly BZRAs with SUV or RMT.
Regarding efficacy, the American Academy of Sleep Medicine guidelines [8] recommend RMT only for sleep onset latency and SUV only for sleep maintenance. In contrast, BZRAs such as temazepam, zolpidem (ZOL), and eszopiclone (ESZ) are recommended for both sleep onset and maintenance. However, regarding safety, BZRAs have various adverse effects, including risk of falls due to their muscle relaxant effects, memory impairment, daytime impairment due to daytime sedation, rebound insomnia, and paradoxical reactions owing to long-term continuous use and tolerance [9]. Furthermore, BZRAs have withdrawal symptoms resulting from physical dependence [9]. The risk of dependence on BZRAs tends to increase with prolonged use or use of multiple drugs and high dosages [10], and safe reduction or withdrawal of BZRA hypnotics is a crucial issue. RMT and SUV are said to have a relatively low risks of these effects [11].
Insomnia can be chronic and require long-term drug treatment. However, not all medications are effective, and there are issues with side effects, interactions with other medications, time to onset of efficacy, and reduced efficacy due to prolonged symptoms or tolerance [12, 13]. Thus, the need arises for physicians to consider switching between different insomnia medications to find the optimal treatment. Furthermore, discontinuing BZRA prescriptions has been advocated by several clinical practice guidelines and professional medical societies, especially in the elderly [8, 14,15,16]. However, the most effective and safe method for switching between these medications has not been well studied.
When treating insomnia in Japan, healthcare providers often determine whether to switch or combine treatments based on various factors. These include the effectiveness and tolerability of previous treatments, the cost of medical care, the provider’s treatment plan, patient feedback, and overall treatment satisfaction. However, each drug has different characteristics; thus, it is unclear whether patients should switch to a new drug or return to the original treatment when there is dissatisfaction or concern about their current treatment. A phase 4 study observed that only 28.8% of participants with long-term BZRA administration could successfully transition to RMT (NCT00492232) [17], a drug approved only to treat sleep onset. Therefore, drugs that ameliorate both difficulty falling asleep and difficulty staying asleep may be able to succeed for that purpose.
LEM is a DORA approved in the US in 2019 and approved and commercialized in Japan in 2020 to treat adult patients with insomnia. DORAs are well tolerated without rebound insomnia, withdrawal symptoms, or the potential for dependence [18]. Phase 3 trials of LEM in patients with insomnia disorder [19,20,21] showed that LEM improved both sleep onset and sleep maintenance compared with placebo and zolpidem tartrate extended-release (ZOL-ER). LEM was superior in terms of both sleep onset latency and sleep maintenance (in addition to no carryover effect) vs placebo or ZOL-ER [19, 20]. Furthermore, a single-center retrospective analysis in Japan suggested that a low-dose BZRA may be entirely replaced by LEM using a tapering method [22]. Another prospective study reported that directly switching to LEM or LEM used as add-on treatment had a low discontinuation rate for LEM (16.07%), indicating the retention rate was high [23]. Thus, LEM may be useful as an alternative treatment for patients dissatisfied with their medications and improve sleep onset and/or sleep maintenance and quality, with a low potential for next-day residual effects.
There are no multicenter prospective study reports on the effects of directly switching to LEM from monotherapy with Z-drugs or SUV or switching from SUV or RMT in combination with BZRA. Furthermore, previous study designs involved starting LEM after a certain period of initial treatment or washout of the last insomnia therapy. Therefore, this study (Study 401) was designed based on actual clinical practice as the first study to assess the efficacy and safety of direct transitioning to LEM from Z-drug (ZOL, zopiclone [ZOP] or ESZ), SUV, SUV + BZRA, and RMT + BZRA.
Methods
Study Design
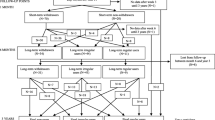
This was a prospective, non-randomized, open-label, multicenter (nine institutions; see Table S1 for details) study conducted between March 24, 2021 (enrollment date of the first study patient) and June 20, 2022. We evaluated the efficacy and safety of four treatment arms: Z-drug monotherapy, SUV monotherapy, SUV combination therapy with a BZRA, and RMT combination therapy with a BZRA. This study consisted of three phases: a pretreatment phase (2 weeks), a titration phase (treatment transition phase comprising the 2 weeks immediately after direct switching from other drugs to LEM), and a maintenance phase (12 weeks), which followed the titration phase (Fig. 1).
The study protocol was approved by the NPO Clinical Research Network Fukuoka Certified Review Board and was registered at ClinicalTrials.gov under the identifier NCT04742699. The study adhered to the Declaration of Helsinki of 1964 and its later amendments, the Clinical Trials Act (Japanese law), and local regulations. All patients provided informed consent to participate in this study. At the beginning of the treatment transition period and at the end of the treatment continuation period, participants received a burden reduction allowance (¥5000 yen QUO card each time).
Prior to obtaining informed consent, each patient was informed according to the consent document, which explained about lemborexant according to the Japanese package insert, the overview and purpose of the study, the reasons why the patient was selected as a study subject, and the inclusion/exclusion criteria.
Patients
Patients included in this study were those referred by their physician at the time of the visit who met the following inclusion criteria: males or females aged ≥ 20 years; who provided written informed consent to participate in the study; were dissatisfied with the efficacy or tolerability of prior medications and wished to transition from them; were receiving Z-drug monotherapy or SUV monotherapy or SUV combination or RMT combination at least five nights per week in the month before the start of the pretreatment phase; met the Diagnostic and Statistical Manual of Mental Disorders, fifth edition [24] criteria for insomnia disorder (i.e., complaints about nighttime sleep accompanied by one [or more] of the following symptoms: difficulty falling asleep, difficulty staying asleep, and early morning awakenings, despite adequate sleep opportunities; sleep difficulty occurring at least three nights a week; sleep difficulties lasting for at least 3 months; and sleep difficulty causing daytime dysfunction); were able to get at least 7 h of time in bed; confirmed use of a previous medication for the treatment of insomnia for at least five nights per week during the pretreatment phase; and were able to comply with the provisions of the research protocol for this study. Regarding the setting of ≥ 20 years of age as an inclusion criterion, Japan lowered the age of adulthood from 20 to 18 on April 1, 2022. Before this law went into effect, this study was initiated, and the last patient was included (February 28, 2022). Therefore, younger participants could not have been included. Regarding the evaluation of “Dissatisfied,” the physician asked the patient if they were dissatisfied with their current insomnia medication/s and would like to change at the time of informed consent acquisition: for example, dissatisfied with Z-drug monotherapy or RMT + BZRA for sleep maintenance; dissatisfied with sleep onset with SUV monotherapy or SUV + BZRA; patients were included if they agreed with these or agreed that they wished to reduce or discontinue their current treatment.
The exclusion criteria were as follows: breastfeeding or pregnant during the pretreatment period or wishing to become pregnant during the study period; moderate or severe obstructive sleep apnea; severe cardiac, respiratory, gastrointestinal, kidney, neurologic or mental disorders, chronic pain, or malignancy; specific sleep disorders other than insomnia, such as periodic limb movement disorder, restless leg syndrome, or circadian rhythm sleep disorder (except for patients diagnosed with mild obstructive sleep apnea, who could be included), narcolepsy or cataplexy, or who take more than three prolonged naps per day; using pretreatment drugs for insomnia at doses other than the approved doses in Japan; receiving two or more concomitant BZRAs; receiving sedatives; starting a new non-pharmacologic treatment for insomnia (e.g., cognitive behavioral therapy) within 1 week before the start of the pretreatment period; unable to abstain from excessive alcohol consumption during the study participation period; hypersensitivity to additives in LEM; hepatic dysfunction of moderate severity or higher (aspartate aminotransferase, alanine aminotransferase, or γ-gamma-glutamyl transpeptidase at or above three times the upper limit of the institutional reference value); use of antipsychotic medications or suicide attempts within the past approximately 2 years judged by the investigator to have the potential to affect safety or any of the study endpoints; previously treated with LEM (including patients who have participated in LEM clinical trials); or were deemed ineligible by the investigator for study participation.
Intervention
Patients were assigned to one of four treatment cohorts (Z-drug monotherapy, SUV monotherapy, SUV combination, and RMT combination) according to their pretreatment status. During the pretreatment phase, existing treatment was continued without any change. At the end of the pretreatment phase (Week 0), the Z-drug, SUV, and RMT were discontinued and LEM treatment (administered orally, just before bedtime) was started and continued during the titration phase (2 weeks) and maintenance phase (12 weeks). In the titration phase, LEM 5 mg/day was prescribed and recommended to be maintained for 7 days, after which the dose could be increased to LEM up to 10 mg/day per the Japanese package insert. In the maintenance phase (12 weeks), LEM dosage could be changed after consultation with the physician upon patient request.
In the SUV and RMT combination cohorts, BZRA was administered unchanged during the titration phase (up to Week 2) and allowed to be changed (discontinued, reduced, or increased to a dose not exceeding that of the pretreatment phase) during the maintenance phase. Additional intermittent doses of RMT were allowed as a rescue dose during treatment. Other sedating drugs were not allowed in combination with Z-drugs, as either an increase in dose or a new addition.
In this study, Z-drugs included ZOL, ZOP, and ESZ, while BZRAs included the following GABAA receptor agonists indicated for insomnia or sleep disorders in Japan: brotizolam, triazolam, lormetazepam, rilmazafone, fulnitrazepam, nitrazepam, quazepam, etizolam, estazolam, fulrazepam, and alprazolam. The study participants recorded their sleep status and the status of LEM and concomitant insomnia medications in a logbook (see Supplementary Method 1 for more details).
Study Endpoints
Primary Endpoints
The primary endpoint was the proportion of successful cases of continuous transition to LEM by the end of the titration phase (at 2 weeks), as a comprehensive measure of both efficacy and safety responses in the early stages of treatment and patient satisfaction with the treatment, which was the same endpoint as in a previous study [21]. This was defined as the proportion of patients who remained on LEM by the end of the 2-week titration phase and were willing to continue on LEM treatment in the maintenance phase in each treatment cohort. Patients who were willing to continue LEM treatment but could not participate in the maintenance phase for reasons unrelated to LEM treatment (such as change of address, financial burden of commuting to the site, or moving to another medical institution) were also included in the proportion of successful cases (the primary endpoint).
Secondary Endpoints
Secondary endpoints were as follows: proportion of patients who remained on LEM in each treatment cohort and the proportion of patients who remained on LEM monotherapy at the end of the 2-week titration phase and 12-week maintenance phase regardless of their willingness to continue treatment with LEM, patient satisfaction assessed by change from baseline in the Patient Global Impression of Insomnia (PGI-I) for 14 weeks (at the end of the titration phase + maintenance phase overall and for each treatment cohort), proportion of patients with a dose increase from LEM 5 mg/day to LEM 10 mg/day during the titration phase and the maintenance phase. In PGI-I, items 1–3 assessed perceptions of study medication effects measured on a 3-point scale (1 = positive, 2 = neutral, 3 = negative): item 1: study medication helped/worsened sleep; item 2: study medication decreased/increased time to fall asleep; item 3: study medication increased/decreased total sleep time. Item 4 assessed the appropriateness of study drug strength measured on a different 3-point scale (1 = too strong, 2 = just right, 3 = too weak).
As safety endpoints, incidence of treatment-emergent adverse events (TEAEs) and tolerability of LEM were objectively assessed and tabulated by the physician in charge during 14 weeks (titration phase + maintenance phase) of treatment with LEM. The severity of TEAEs was determined as: mild (discomfort is present but does not interfere with normal daily activities), moderate (discomfort that interferes with normal daily activities), or severe (inability to work or to lead a normal daily life).
Exploratory Endpoints
Exploratory endpoints were the proportion of patients requiring rescue medications (i.e., temporary additional doses of BZRAs when current treatment is inadequate), change from baseline in the Insomnia Severity Index (ISI) score at the end of the 2-week titration phase, at a 6-week evaluation, and the end of the 12-week maintenance phase overall and for each treatment cohort, factors influencing the transition from pretreatment to LEM, and changes from the titration phase in dose of BZRA in patients with dose reduction or withdrawal of BZRA during the maintenance phase. A LEM responder was defined as a patient who continued on LEM at the end of the titration phase.
Statistical Analysis
The sample size was set based on feasibility, as there are few similar previous studies, making it difficult to design the sample size using statistical methods. As this study was open label, it was not designed or powered for efficacy or safety statistical comparisons between the treatment arms.
Analytical populations were all registered cases, the full analysis set (i.e., all enrolled patients, excluding those found to be ineligible after enrollment and those not receiving treatment), and the safety analysis set (i.e., all enrolled patients for whom LEM was administered).
Regarding data handling, missing values were not imputed unless otherwise stated. No multiplicity adjustments were applied in this study.
For the primary endpoint, 95% CIs were calculated using the Clopper-Pearson method. Summary statistics for continuous variables included mean, standard deviation, median, interquartile range, and range. Summary statistics for categorical or ordinal variables included frequency and percentage (%). The analyses of PGI-I were conducted using Bowker’s symmetry tests, and analyses of ISI were conducted using paired t-tests. The factors influencing the transition to LEM were analyzed using Student’s t-test/Fisher’s exact tests. Tests were two-sided with a significance level of 5%, and 95% confidence intervals (CIs) were used. Adverse events were reported as system organ classes and preferred terms from the Medical Dictionary of Regulatory Activities (MedDRA version 25.0). The statistical software used for analysis was SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient Disposition and Characteristics
A flow diagram of patients is shown in Fig. 2. Overall, 97 patients were enrolled: 25 in the Z-drug monotherapy cohort, 27 in the SUV monotherapy cohort, 23 in the SUV combination cohort, and 22 in the RMT combination cohort.
During the titration phase, one patient each withdrew from the Z-drug monotherapy and SUV combination cohorts. From the end of the titration phase to the start of maintenance phase, one patient each discontinued for worsening of insomnia/adverse drug reaction (ADR) in the Z-drug monotherapy, for an inadequate response to LEM in the SUV monotherapy, and for patient withdrawal in the RMT combination cohorts.
During the maintenance phase, three patients in the Z-drug monotherapy cohort, two in the SUV monotherapy cohort, three in the SUV combination cohort, and three in the RMT combination cohort discontinued early. The reasons for discontinuation during this phase (Fig. 2) included TEAEs (one in the SUV combination and two in the RMT combination cohorts), worsening of insomnia/ADR in one patient in the RMT combination cohort, patient withdrawal, withdrawal of consent in the Z-drug monotherapy cohort, protocol deviation, and lack of attendance to the study visit.
Baseline patient characteristics are summarized in Table 1. Overall, 40.0% were male patients, the mean ± SD age was 51.8 ± 15.4 years, the mean BMI was 23.71 ± 4.59 kg/m2, and 90.0% had received treatment for insomnia 1 month prior to enrollment. There was a higher proportion of females in the RMT combination cohort. In the Z-drug monotherapy cohort, 18 patients were treated with ESZ, 6 with ZOL, and 1 with ZOP. In the combination cohorts, 14 patients in the SUV combination cohort were treated with ESZ, 5 with ZOL, and 2 with brotizolam. In the RMT combination cohort, 11 patients were treated with ESZ, 5 with ZOL, 2 with flunitrazepam, and 1 with alprazolam.
Primary Endpoint
The proportion of patients transitioning from pre-treatment to LEM during the titration phase was 95.6% (95% CI 89.0–98.8%) overall and 92.0% (74.0–99.0%), 96.0% (79.6–99.9%), 95.2% (76.2–99.9%), and 100.0% (82.4–100.0%) in the Z-drug monotherapy, SUV monotherapy, SUV combination, and RMT combination cohorts, respectively (Table 2).
Secondary Endpoints
LEM showed a retention rate (proportion of patients who remained on LEM) of 97.8% (95% CI 92.2–99.7%) overall at the end of the titration phase and 82.2% (95% CI 72.7–89.5%) at the end of the maintenance phase (Table 2). By cohort, the retention rates ranged from 95.2% (SUV combination cohort) to 100.0% (SUV monotherapy cohort) at the end of the titration phase. Retention rates at the end of the maintenance phase ranged from 78.9% (RMT combination cohort) to 88.0% (SUV monotherapy cohort).
In all cohorts, for PGI-I items 1 to 3, the proportions of patients with positive responses were higher than those with negative responses on study medication effects, and for PGI-I item 4, the proportions of patients who reported that their study medication was “just right” was greater than those at baseline. ISI scores generally improved over time after 2, 6, and 14 weeks of LEM transition (Fig. 3; Table S2). The proportions of patients with dose increases from LEM 5 to 10 mg/day were 18.9% and 13.3% at the 2-week titration and the 12-week maintenance phases, respectively (Table S3).
Changes in PGI-I. Proportion of patients who chose the answer option. For items 1 to 3, choices are: blue = positive, gray = negative, white = neutral. For item 4, choices are: blue = just right, gray = too strong, and white = too weak. Percentages were calculated by dividing the number of patients who chose the response option by the number of patients who transitioned to the maintenance phase of treatment (given as “N = ” at the head of each figure). Thus, the percentages do not total 100. Week 2 indicates the end of the transition phase, and Week 14 indicates the end of the maintenance phase. PGI-I Patient Global Impression of Insomnia, RMT ramelteon, SUV suvorexant, Z-drug non-benzodiazepine sleeping pills
Safety Endpoints
The safety and tolerability of LEM during the 2-week titration and the 12-week maintenance phases are summarized in Table 3. The overall incidence of TEAEs was 47.8%, that of ADRs (TEAEs considered related to the treatment) was 15.6%, and no serious TEAEs were observed. Most TEAEs were mild or moderate in severity. Frequent TEAEs were somnolence (overall, 7.8%; Z-drug monotherapy cohort, 16.0%; SUV monotherapy cohort, 4.0%; SUV combination cohort, 0%; and RMT combination cohort, 10.5%) and fever (overall, 6.7%; Z-drug monotherapy cohort, 0%; SUV monotherapy cohort, 20.0%; SUV combination cohort, 0%; and RMT combination cohort, 5.3%).
Exploratory Endpoints
No patients required rescue medications during the study.
Overall and in each cohort, there was an improvement in ISI scores over time after 2, 6, and 14 weeks of LEM transition from baseline (all P < 0.05) (Fig. 4), with the proportions of patients scoring < 8 (the cut-off value for subthreshold insomnia on the ISI) ranging from 13.3% at baseline to 49.3% at the end of the study at Week 14 (Table S4).
No background factors associated with the transition to LEM were identified overall or in each cohort (Table S5).
Approximately 12.5% (4/32) of patients underwent BZRA dose reduction/withdrawal in the combination cohorts at the end of the maintenance phase (14 weeks) (Table S6). Although the study did not pre-specify a method for achieving BZRA dose reduction, and there were not many overall, a small number of patients (combination cohorts, n = 4 at Week 2 and n = 4 at Week 14) were able to undergo a BZRA dose reduction.
Discussion
This is the first prospective interventional study to examine a direct transition to LEM from other insomnia treatments across four cohorts: Z-drug (ZOL, ZOP, or ESZ) monotherapy, SUV monotherapy, SUV combination therapy, and RMT combination therapy. The transitions to LEM from other insomnia medications were successful, with almost all patients (95.6% [95% CI 89.0–98.8%]) transitioning at the end of the titration phase. Retention rates during the maintenance phase were also favorable at 82.2%. The positive responses in PGI-I were generally increased over time after 2 and 14 weeks of LEM transition compared with baseline. In addition, ISI scores improved over time after 2, 6, and 14 weeks of LEM transition, and the proportion of patients scoring < 8 (the cut-off value for suspected insomnia on the ISI) increased from 13.3% at baseline to 49.3% at the end of the study. Furthermore, during the 14 weeks of treatment, no serious TEAEs were observed. Thus, direct transition therapy for LEM may be a valid treatment option for patients with insomnia who are dissatisfied with the efficacy of their previous treatment or have concerns about tolerability.
To date, no previous studies have been published that examine the effects of switching to LEM. This study explores the direct switch to LEM without tapering the previous drug and also includes monotherapy and combination therapy in transition strategies, providing useful information for physicians in real-world clinical practice. The present study showed high successful transition rates to LEM from ZOL monotherapy, similar to those of a study in the US evaluating direct transition from ZOL monotherapy to LEM [21]. In that study, as in our study, most patients (81.1%) transitioned to LEM, and most chose to continue LEM treatment during the maintenance phase. One possible reason for this high successful transition rate of LEM may be dose adjustment. In both studies, enrolled patients were allowed to adjust the dose of LEM, and the starting dose was 5 mg per the package insert [25, 26]. The approved dose of SUV is 20 mg for adults and 15 mg for the elderly in Japan, but dose adjustment was not permitted [27]. During the titration phase and after 1 week of treatment, the LEM dose was increased to 10 mg in 32% of patients, which may also reflect the high proportion of patients transitioning to LEM. As another possible reason, this study also considered background factors but did not identify any significant differences in background factors between those who transitioned to LEM and those not transitioning to LEM. In addition to transition of ZOL monotherapy, successful transition rates to LEM from all four cohorts were also high, which is consistent with a recent study suggesting that long-term gamma-aminobutyric acid-BZRA users may be able to successfully switch to LEM [28].
Each drug has different characteristics; therefore, the balance between efficacy and safety is vital for sleep medications. In a recently published network meta-analysis [29], LEM had the most favorable safety and efficacy profile. In the present study, a high rate of successful transition to LEM may have contributed to improvements in the time to fall asleep and improved sleep maintenance in PGI-I. This study comprised four cohorts considering real-world clinical treatment patterns, including combinations, and observed favorable LEM transition and retention rates across all cohorts. This suggests that LEM has good potential as a transition therapy in real-world settings. Thus, all patients with insomnia who have dissatisfaction with previous treatment could be considered eligible for LEM transition therapy.
As reported via meta-analyses, LEM treatment ranked higher than SUV for sleep-onset latency, was rated at least as effective in improving sleep maintenance and total sleep time [23], and was recommended in most clinical situations in Japan [12]. In terms of pharmacodynamics, the selectivity of orexin receptor 2 and rapid binding and dissociation kinetics of LEM compared with SUV might have contributed to sufficient efficacy, especially in sleep-onset latency and sleep maintenance [30, 31]. However, these examples may not truly represent the clinical situation, and more research is needed to verify this hypothesis. Although few reports have examined changing from DORA to DORA, and no numerical results were reported in terms of retention rate or sleep index, the transition rate of > 90% with SUV monotherapy, or in combination, suggests that it is worth considering not only switching to sleep medications with different mechanisms of action, such as Z-drugs or combination (RMT combination), but also switching to other DORAs when the initial DORA is ineffective. In addition, in the network meta-analysis of LEM and SUV, LEM 10 mg/day was rated superior to other active treatments in terms of efficacy, but because of the risk of somnolence, it is recommended to start with the well-tolerated LEM 5 mg/day [32]. In a prospective study examining the benefit of a direct switch to SUV or SUV add-on after 1 week in patients with insomnia with comorbid psychiatric disorders, the retention rate was 77.2% [33]. Similarly, the retention rate for LEM was high (> 83%) [23]. Both studies examined direct switching from single agents, but did not include examining changing to LEM in a combination cohort. Another retrospective observational study examined LEM in combination with BZRA and tapering of concomitant BZRA, with a retention rate of 91.3% (63/69) for LEM [22]. A retrospective study reported that switching to SUV from BZRA had > 70% continuation rate at 1 month and higher oversedation compared with SUV add-on to BZRA [34]. Transition to LEM from SUV is suggested to alleviate difficulties in initiating sleep (onset) [35], and LEM may be a useful alternative to BZRA or SUV [28]. In the current study, during the 12-week maintenance phase, the protocol did not require a reduction or withdrawal of BZRA in the combination cohort, resulting in a low frequency of BZRA withdrawal and dose reduction. Nevertheless, the patient satisfaction with LEM monotherapy and combination therapy was high based on the improvement of PGI-I and ISI over time. This suggests that dose reduction/withdrawal of BZRA can be considered as an option in clinical practice.
Regarding safety, although somnolence occurred in 7.8% of patients, no serious TEAEs occurred in this study, and most were mild or moderate, indicating good tolerability. These findings are consistent with the previously reported safety profile of LEM [19, 20]. Regarding sleep quality, there were few cases of nightmares, and patients experienced general improvements in sleep quality according to the assessment per the PGI-I and ISI scores.
Limitations
This study had some limitations. Since this study was small and open label, it was not designed or powered for efficacy or safety statistical comparisons between the treatment arms. Instead, it assessed only a specified approach to the dosing transition separately for each treatment regimen. It should be noted that this study did not compare LEM to a placebo or active control regarding efficacy, with participants, serving as their own control for before and after treatment assessments, as would be the case in routine clinical practice. Because the study period was 14 weeks, the long-term effects of the transition to LEM therapy are unknown. As the protocol did not specify a BZRA dose reduction, a small number of patients underwent dose reduction, and 4/39 and 4/32 patients in the combination cohorts underwent dose reduction in the titration and maintenance phases, respectively. However, to our knowledge, this is the first trial to show a direct transition to LEM from various hypnotic treatments, including combination therapy, and it provides important information about the direct transition to LEM in clinical practice.
Conclusions
Our findings highlight a successful transition to LEM from other insomnia medications, with > 95% of patients transitioning at the end of the titration phase. Patients who switched had improved subjective patient satisfaction scores over time based on the PGI-I and ISI. Most TEAEs were mild and moderate, indicating good tolerability. The results suggest that direct transition to LEM is an effective treatment option for patients with insomnia who are dissatisfied with, or anxious about, their current treatment. LEM substitution improved patient treatment satisfaction and was considered beneficial for reducing the BZRA dose and possibly BZRA discontinuation.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ohayon MM, Reynolds CF 3rd. Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD). Sleep Med. 2009;10:952–60.
Itani O, Kaneita Y, Munezawa T, et al. Nationwide epidemiological study of insomnia in Japan. Sleep Med. 2016;25:130–8.
Dopheide JA. Insomnia overview: epidemiology, pathophysiology, diagnosis and monitoring, and nonpharmacologic therapy. Am J Manag Care. 2020;26:S76-84.
Sateia MJ. International Classification of Sleep Disorders—third edition: highlights and modifications. Chest. 2014;146:1387–94.
Sutton EL. Insomnia. Ann Intern Med. 2021;174:ITC33–48.
Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504.
Kishi T, Nishida M, Koebis M, et al. Evidence-based insomnia treatment strategy using novel orexin antagonists: a review. Neuropsychopharmacol Rep. 2021;41:450–8.
Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical Practice Guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2017;13:307–49.
Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331:1169.
Murakoshi A, Takaesu Y, Komada Y, Ishikawa J, Inoue Y. Prevalence and associated factors of hypnotics dependence among Japanese outpatients with psychiatric disorders. Psychiatry Res. 2015;230:958–63.
Uemura SI, Imanishi A, Terui Y, et al. Residual effects of low dose of suvorexant, zolpidem, and ramelteon in healthy elderly subjects: a randomized double-blind study. Neuropsychopharmacol Rep. 2022;42:288–98.
Takaesu Y, Sakurai H, Aoki Y, et al. Treatment strategy for insomnia disorder: Japanese expert consensus. Front Psychiatry. 2023;14:1168100.
Watson NF, Benca RM, Krystal AD, McCall WV, Neubauer DN. Alliance for Sleep Clinical Practice Guideline on switching or deprescribing hypnotic medications for insomnia. J Clin Med. 2023;12:2493.
Tannenbaum C, Farrell B, Shaw J, et al. An ecological approach to reducing potentially inappropriate medication use: Canadian Deprescribing Network. Can J Aging. 2017;36:97–107.
American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227–46.
Riemann D, Baglioni C, Bassetti C, et al. K. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675–700.
ClinicalTrials.gov. Facilitation of zolpidem (≥10 mg) discontinuation through use of ramelteon in subjects with chronic insomnia. Last updated 2010-07-20. Available at https://clinicaltrials.gov/study/NCT00492232. Accessed 06 July 2023.
Muehlan C, Vaillant C, Zenklusen I, Kraehenbuehl S, Dingemanse J. Clinical pharmacology, efficacy, and safety of orexin receptor antagonists for the treatment of insomnia disorders. Expert Opin Drug Metab Toxicol. 2020;16:1063–78.
Kärppä M, Yardley J, Pinner K, et al. Long-term efficacy and tolerability of lemborexant compared with placebo in adults with insomnia disorder: results from the phase 3 randomized clinical trial SUNRISE 2. Sleep. 2020;43:zsaa123.
Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2:e1918254.
Ahmad M, Kelly J, Montano B, et al. Transitioning insomnia patients from zolpidem to lemborexant: a multicenter, open-label study evaluating a next-dose transition approach to insomnia pharmacotherapy. Sleep Med X. 2024;7:10098. https://doi.org/10.1016/j.sleepx.2023.100098.
Usui K, Fujii Y, Okada K, Suzuki E. Low-dose benzodiazepine receptor agonists may be completely replaced by lemborexant at about the same dose. Psychiatry Clin Neurosci Reports. 2022;1:e32.
Kishi T, Sakuma K, Okuya M, Iwata N. Lemborexant for insomnia in adults with psychiatric disorders: a 1-week, open-label study. Psychiatry Clin Neurosci Reports. 2022;1:e23.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington: American Psychiatric Association; 2013.
Eisai Co., Ltd. Japanese package insert of DAYVIGO (lemborexant). 2022. Available at https://www.info.pmda.go.jp/go/pdf/170033_1190027F1022_1_04
Eisai Inc. US prescribing information of DAYVIGO (lemborexant). 2023. Available at https://www.dayvigo.com/-/media/Files/DAYVIGO/PDF/prescribing-information.pdf
MSD K.K. Japanese package insert of Belsomra (suvorexant). 2023. Available at https://pins.japic.or.jp/pdf/newPINS/00066563.pdf
Okino K, Suzuki H, Tomioka H, et al. Efficacy and safety of lemborexant as an alternative drug for patients with insomnia taking gamma-aminobutyric acid-benzodiazepine receptor agonists or suvorexant. Hum Psychopharmacol. 2023;38:e2868.
De Crescenzo F, D’Alò GL, Ostinelli EG, et al. Comparative effects of pharmacological interventions for the acute and long-term management of insomnia disorder in adults: a systematic review and network meta-analysis. Lancet. 2022;400:170–84.
Greenblatt DJ, Legangneux E, Harmatz JS, et al. Dynamics and kinetics of a modified-release formulation of zolpidem: comparison with immediate-release standard zolpidem and placebo. J Clin Pharmacol. 2006;46:1469–80.
Weinling E, McDougall S, Andre F, Bianchetti G, Dubruc C. Pharmacokinetic profile of a new modified release formulation of zolpidem designed to improve sleep maintenance. Fundam Clin Pharmacol. 2006;20:397–403.
Kishi T, Nomura I, Matsuda Y, et al. Lemborexant vs suvorexant for insomnia: a systematic review and network meta-analysis. J Psychiatr Res. 2020;128:68–74.
Kishi T, Sakuma K, Okuya M, et al. Suvorexant for insomnia in patients with psychiatric disorder: a 1-week, open-label study. Neuropsychopharmacol Rep. 2019;39:252–5.
Hatano M, Kamei H, Inagaki R, et al. Assessment of switching to suvorexant versus the use of add-on suvorexant in combination with benzodiazepine receptor agonists in insomnia patients: a retrospective study. Clin Psychopharmacol Neurosci. 2018;16:184–9.
Okino K, Suzuki H, Kondo S, et al. Effectiveness of change from suvorexant to lemborexant drug in the treatment of sleep disorders. Psychogeriatrics. 2022;22:595–604.
Acknowledgements
We thank all participating patients and their families, as well as investigators, physicians, nurses, and clinical research coordinators who helped in this study. We wish to acknowledge Ms. Yoshida Mitsuyo and Ms. Tomoko Ozawa, (Eisai Co., Ltd,) for their help in the study operation and Dr. Michiko Sugawara (Eisai Co., Ltd.) for her help as a publication coordinator.
Medical Writing, Editorial, and Other Assistance.
Medical writing support for the preparation of this article was provided by Keyra Martinez Dunn, MD, of Edanz (www.edanz.com), which was funded by Eisai Co., Ltd., in accordance with Good Publication Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022).
Funding
This research and journal’s Rapid Service and Open Access fees were funded by Eisai Co., Ltd.
Author information
Authors and Affiliations
Contributions
Motohiro Ozone, Susumu Hirota, Yu Ariyoshi, Kenichi Hayashida, Azusa Ikegami, Mitsunari Habukawa, Hayato Ohshima, Daisuke Harada, Hiroshi Hiejima, Nozomu Kotorii, Kenta Murotani, Takehiro Taninaga, and Naohisa Uchimura authors have made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; been involved in drafting the manuscript or revising it critically for important intellectual content; given final approval of the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of Interest
Motohiro Ozone received grants from Mitsubishi Tanabe Pharma Corporation, Tsumura Co., Ltd., Shionogi & Co., Ltd., Mochida Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Kyowa Kirin Co., Ltd., and Teijin Pharma Ltd.; and honoraria from Eisai Co., Ltd., Tsumura Co., Ltd., MSD Co., Ltd., Meiji Seika Pharma Co., Ltd., Takeda Pharmaceutical Company Limited, Nobelpharma Co., Ltd., Yoshitomi Pharmaceutical Industries, Ltd., and Mitsubishi Tanabe Pharma Corporation. Susumu Hirota received consulting fees from Viatris Inc.; honoraria from Eisai Co., Ltd., Meiji Seika Pharma Co., Ltd., Sumitomo Pharma Co., Ltd., and Takeda Pharmaceutical Company Ltd.; and is involved in clinical trials with Nippon Boehringer Ingelheim Co., Ltd., Shiongi & Co., Ltd., Sumitomo Pharma Co., Ltd., and Viatris Inc. Yu Ariyoshi received honoraria from Eisai Co., Ltd. and MSD Co., Ltd. Kenichi Hayashida received lecture fees from Eisai Co., Ltd. and MSD Co., Ltd. Azusa Ikegami received honoraria from Eisai Co., Ltd. Mitsunari Habukawa received honoraria from Eisai Co., Ltd., Otsuka Pharmaceutical Co., Ltd., MSD Co., Ltd., and Takeda Pharma Co., Ltd., Nozomu Kotorii received consulting fees from Eisai Co., Ltd., Takeda Pharmaceutical Company Limited, MSD Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Sumitomo Pharma Co., Ltd., and Janssen Pharmaceutical K.K. Takehiro Taninaga is an employee of Eisai Co., Ltd. Naohisa Uchimura received honoraria from Eisai Co., Ltd., Meiji Seika Pharma Co., Ltd., MSD Co., Ltd., Nobelpharma Co., Ltd., and Takeda Pharmaceutical Company Limited. The remaining authors have no conflicts of interest to declare.
Ethical Approval
The study protocol was approved by the NPO Clinical Research Network Fukuoka Certified Review Board and was registered at ClinicalTrials.gov under the identifier NCT04742699. The study adhered to the Declaration of Helsinki of 1964 and its later amendments, the Clinical Trials Act (Japanese law), and local regulations. All patients provided informed consent to participate in this study.
Additional information
Prior Publication: The overall results of this study were presented at the 45th Annual Meeting of the Japanese Sleep Society (held in Yokohama, Japan; September 15–17, 2023), abstract number O18-004. An abstract based on this work has also been presented at World Sleep (held in Rio de Janeiro, Brazil; October 21–25, 2023).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ozone, M., Hirota, S., Ariyoshi, Y. et al. Efficacy and Safety of Transitioning to Lemborexant from Z-drug, Suvorexant, and Ramelteon in Japanese Insomnia Patients: An Open-label, Multicenter Study. Adv Ther 41, 1728–1745 (2024). https://doi.org/10.1007/s12325-024-02811-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02811-2