Abstract
Introduction
Immunoglobulin A nephropathy (IgAN) is a kidney disorder that can lead to progressive kidney disease. Currently, there lacks a comprehensive overview of the symptoms and impacts experienced by those living with IgAN that would help inform the selection or development of fit-for-purpose clinical outcome assessments (COA) to be used in clinical trials. The aim of this study was to develop a conceptual model of the adult and pediatric patient experience of IgAN, including disease signs and symptoms, treatment side effects, and impact on functioning and well-being.
Methods
This study comprised a systematic review and thematic analysis of qualitative studies with adults and children diagnosed with IgAN. Data sources were identified through an electronic database search of journal articles (MEDLINE, Embase, PsycINFO; June 2021), hand-searching of conference proceedings, patient advocacy group websites, and gray literature. Non-English articles were excluded. Identified data (patient/caregiver quotes, author summaries, and interpretations of patient experiences) were extracted from articles. Extracted data were qualitatively analyzed, aided by ATLAS.ti v7. Codes were applied to data; concepts (i.e., symptoms) were identified, named, and refined. A conceptual model was developed by grouping related concepts into domains.
Results
In total, five sources were identified for analysis: two journal articles, two online anthologies of patient stories, and one patient organization-sponsored “Voice of the Patient” meeting report. Conceptual model symptom domains included swelling/puffiness (edema), pain/aches/discomfort, fatigue, weight gain, sleep problems, urinary problems, and gastrointestinal problems. Impact domains included emotional/psychological well-being, physical functioning/activities of daily living, social functioning, work/school, and relationships.
Conclusions
Secondary analysis of published qualitative literature permitted development of a novel conceptual model depicting the patient experience of IgAN; however, its depth is limited by a lack of available literature. Further qualitative research is recommended to refine and/or confirm the concepts and domains, determine any relationships between them, and explore the outcomes that are most meaningful to patients. The refined model will provide a useful tool to inform the selection, development, and/or amendment of COAs for use in future IgAN clinical trials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A novel conceptual model depicting the patient experience of immunoglobulin A nephropathy (IgAN) was developed from a secondary analysis of published qualitative literature. |
The model provides a useful tool to inform the selection and development of clinical outcome assessments for use in future IgAN clinical trials. |
Symptom domains included swelling/puffiness (edema), pain/aches/discomfort, fatigue, weight gain, sleep problems, urinary problems, and gastrointestinal problems. |
Impact domains included emotional/psychological well-being, physical functioning/activities of daily living, social functioning, work/school, and relationships. |
Further qualitative research is recommended to refine the concepts and domains, determine relationships between them, and explore the outcomes that are most meaningful to patients. |
Introduction
Immunoglobulin A nephropathy (IgAN) is a progressive disease characterized by IgA deposition in the renal mesangium. IgAN is the most common form of primary glomerulonephritis [1] and progresses to end stage kidney disease (ESKD) in 25–30% patients within 20–25 years of presentation [1]. The cause of IgAN is unknown and the precise pathogenetic mechanisms have not been fully described. IgAN can occur at any age and exhibits high heterogeneity in epidemiology, clinical presentation, progression, and treatment response across different ethnic populations and different geographical locations [2, 3]. The clinical presentation can range from being asymptomatic to having gross hematuria, with minimal to nephrotic range proteinuria, and can be associated with progressive CKD [4]. IgAN is most predominant in people of Asian and European backgrounds, but is relatively rare in African populations [3]. A recent meta-analysis of incidence rates in the USA calculated the annual incidence of IgAN of 1.29/100,000 persons [5].
IgAN can have a considerable impact on health-related quality of life (HRQoL) including the mental, social, and physical well-being of adult and pediatric patients [5,6,7]. The HRQoL impact on patients is caused by the disease pathology and by treatment regimens that may eventually require long-term dialysis or kidney transplantation.
The current mainstay of treatment for IgAN is optimized supportive therapy generally focused on a non-immunosuppressive-based strategy that includes management of hypertension and proteinuria with renin–angiotensin system (RAS) blockade, and lifestyle and dietary changes [1]. Immunosuppressants such as corticosteroids may also be administered, particularly in patients at high risk of disease progression; however, their efficacy and safety are widely debated [8]. Consequently, there remains an unmet need for well-tolerated disease-modifying therapies [3]. Besides the recent approval of an oral targeted release formulation of the corticosteroid budesonide by US Food and Drug Administration (FDA) in adults with primary IgAN, there are multiple ongoing clinical trials evaluating new therapies with disease-modifying mechanisms that address the unmet need for targeted treatments for this heterogeneous disease [8]. In the future it may become possible to treat patients with IgAN with precision medicine. The change in the treatment paradigm towards a personalized therapy could reduce the morbidity and mortality rates for patients in need.
To achieve patient-focused drug development, it is important to identify the symptoms and impacts of IgAN that are most important from the patient and caregiver perspective for a treatment to target, and the side effects of existing treatments that are most problematic. Identifying the important outcomes for measurement can then inform the selection of fit-for-purpose clinical outcome assessments (COAs) for inclusion in clinical trials [9]. Meanwhile, the Standardised Outcomes in Nephrology-Glomerular Disease (SONG-GD) initiative aims to identify core outcomes domains for patients with glomerular diseases including, but not limited to, IgAN. To date, the initiative has identified important outcomes for the broader population of patients with glomerular diseases [10].
The importance of selecting fit-for-purpose COAs to measure the outcomes that matter to patients is detailed in the US FDA Patient-Reported Outcome (PRO) Guidance for Industry [11] and the Patient-Focused Drug Development final and draft documents [12,13,14]. Appropriate COAs for IgAN may include patient-reported outcome measures to assess key symptoms and impacts, clinician-reported outcome measures to assess disease defining signs, and observer-reported outcome measures to assess observable concepts in children too young to self-report through PRO. The first step in identifying important outcomes for measurement is the development of a conceptual model. However, the current literature does not include a comprehensive overview or illustrative conceptual model that reflects patient-reported symptoms and impacts of IgAN in both adult and pediatric populations.
The objective of this study was to conduct a qualitative literature review to understand the lived patient experience of IgAN and develop a conceptual model of the disease that can inform the selection of COAs to measure important outcomes for patients and caregivers in future clinical trials that evaluate potential treatments for IgAN.
Methods
Selection Criteria
Qualitative studies, interviews, and blog posts that described the patient experience of IgAN were eligible. Blog posts and other gray literature were included because of the anticipated paucity of traditional published qualitative research in IgAN. Titles and abstracts of publications from the electronic searches and hand-searching were screened using the eligibility criteria outlined in Table 1.
Data Sources and Searches
A detailed search strategy protocol was developed and included several data sources. The electronic databases MEDLINE (1946–present), Embase (1980–present), and PsycINFO (1806–present) were run via OVID in June 2021 to identify relevant publications from the published literature. The search strategies are provided in Tables S1–S3 in the electronic supplementary material. Hand-searching was also conducted and included the FDA and European Medicines Agency (EMA) websites, the websites of patient advocacy organizations, the IgA Nephropathy Foundation and NephCure Kidney International, three kidney conference proceedings (2019–2021), and the reference lists of any included studies and relevant reviews from the database search. Two of the authors (TaZ and MT) screened the search results on the basis of titles and abstracts (first pass). Non-relevant studies were excluded, and potentially relevant studies were retrieved for full publication review (second pass).
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Quality Assessment
All identified qualitative studies were independently assessed for quality by two authors (TaZ and MT) using the Critical Appraisal Skills Programme (CASP) checklist [15] to ensure all included studies met an appropriate threshold of reliability. Any discrepancies were resolved by discussion.
Data Extraction and Analysis
Extracted quotes from patient and caregivers, and author descriptions and interpretations underwent secondary analysis using inductive, semantic analysis techniques. Quotes were coded using ATLAS.ti version 7, computer-assisted qualitative data analysis software which facilitates the coding and organization of data. Author MT led coding and identified concepts relevant to the patient experience, and together with authors NVJA, TaZ, and HK grouped concepts into themes and sub-themes. MT led development of the conceptual model, a pictorial representation of the patient experience of IgA comprising the identified concepts, and was refined through discussions with all other authors, including clinical experts SST and LZ.
Results
Data Selection Summary
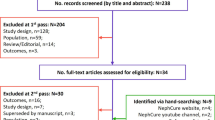
A total of five sources were identified for review, namely a journal article reporting a social media review [16], a conference abstract reporting a qualitative interview study [17], a transcript from a “Voice of the Patient” meeting led by the National Kidney Foundation and IgA Nephropathy Foundation of America [18], and two anthologies of patient testimonials collated by NephCure Kidney International and the IgA Nephropathy Foundation [19, 20]. Of these sources, two included an adult population [17, 20], two included both pediatric and adult populations [18, 20] and one did not report the age of the population included [16]. Four studies were set in the USA, with two including participants from Europe. The process of study selection is documented in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [21] flow diagram in Fig. 1. A summary of the included studies is found in Table 2.
Conceptual Model of the Patient Experience of IgAN
A conceptual model of the patient experience of IgAN is presented in Fig. 2. The model is divided into two main sections: “Signs, Symptoms, and Treatment side effects” and “Impacts”, with the former hypothesized to cause the latter. Signs, symptoms, and treatment side effects were grouped because it was not always possible to discriminate whether the experience reported was a disease symptom or a treatment side effect. Related experiences were grouped into hypothesized domains and were confirmed by clinical experts. Concepts are categorized in order of saliency, according to how frequently they were reported throughout the literature, and how bothersome patients described them to be. Much of this interpretation was informed by the “Voice of the Patient” meeting [18] where the audience was polled on their experiences.
Many of the key concepts such as fatigue, urinary problems, sleep problems, reduced heat and sun tolerance, and weight gain were reported by patients of all ages, while others were reported by adult or pediatric patients only—although it is unclear to what extent this is due to age differences in the manifestation of the disease compared with an artifact of the small number of included studies. Signs, symptoms, and treatment side effects of IgAN reported exclusively by adults included swelling and puffiness (edema), dizziness, general malaise, dental problems, furry face, and skin problems; while acne, chills/sweats, dizziness, and nail growth issues came from pediatric reports only. Included impact concepts were broadly relevant to both adults and children, although a greater number of emotional and relationship concerns were reported by adults, and understandably more “adult” concerns such as chores, driving, and finances were not raised by children. Stigma was only reported by children, in the context of being teased or bullied at school.
Signs, Symptoms, and Treatment Side Effects
A number of signs, symptoms, and treatment side effects were reported in the literature. Participants noted how frequently these experiences would “flare,” leading to “good days” and “bad days,” and distinct “episodes” of the disease [18, 20]. Patients reported that IgAN was a very unpredictable disease making it difficult to plan activities and causing stress and worry [18].
Fatigue
Fatigue was reported frequently by patients in the literature [16,17,18,19,20], and was the symptom most frequently experienced by polled audience members at the “Voice of the Patient” meeting [18]. Patients described fatigue as both a physical and mental experience, using words or descriptions such as “tired” [18, 20], “lack of energy” [16, 17], “constant weariness” [18], “exhaustion” [18], being “mentally and/or physically drained” [18], being “filled with lead” [18], or feeling like you have been “hit by a ton of bricks”. Patients described difficulties with maintaining focus on daily tasks, not being “mentally sharp”, and taking significantly longer periods of time to carry out tasks that require concentration. Fatigue was also named during the “Voice of the Patient” meeting to be the symptom that most negatively impacts daily life [18], and reported to interfere with basic activities of daily living (ADLs) [18]. Fatigue limited the ability to exercise [16, 18], impacted patients’ ability to work [18, 19], and increased the need for sleep—both at night and during the day [18]. While fatigue was reported as a general symptom of IgAN, some patients also attributed fatigue to corticosteroid and/or blood pressure treatment [18].
Brain Fog
Patients described experiencing “brain/mental fog”, which appeared to be closely related to fatigue, although limited descriptions in the literature made it difficult to determine if it was a component of fatigue, such as feeling tired, or lacking energy, or a separate experience.
Urinary Problems
Dark-colored urine (hematuria) and foamy urine (proteinuria) were commonly reported by patients as a symptom, and were often the initial trigger for seeking diagnosis [18, 20]. Some patients likened their urine color to the color of Coca-Cola [18, 20] or “dark tea” [18, 20]. These urinary problems could be a constant reminder of the condition, causing depressive feelings [18]. Other concerns reported included frequent urination (both as a symptom of IgAN and also a side effect of diuretic medications to treat fluid-retention) [18], painful urination [18], and a feeling that the bladder was never fully empty [18], although these last two concepts were not widely reported and may be individual experiences not associated with the broader patient population. Urinary problems were not an option during audience polling at the “Voice of the Patient” meeting to determine the bothersome symptoms of IgAN, but they were named by one meeting participant as particularly bothersome [18], and were amongst the symptoms most frequently reported throughout the literature.
Sleep Problems
Patients reported a spectrum of sleep problems caused by IgAN including excessive sleeping [18], restless/low-quality sleep [16, 18, 20], difficulty sleeping, and insomnia [18]. Restless sleep and insomnia seemed to be also attributed to treatment [18, 20]. Some patients also reported they had received a clinical diagnosis of sleep apnea in relation to their IgAN or IgAN treatments [18]. Sleep problems were not an option during audience polling at the “Voice of the Patient” meeting to determine the bothersome symptoms of IgAN, but were named by individuals in the literature as particularly bothersome [18, 20], and were amongst the symptoms most frequently reported throughout the literature.
Swelling/Puffiness (Edema)
Swelling or “puffiness” in the face, legs, feet, and ankles was commonly reported by patients, both as a symptom of IgAN and a side effect of corticosteroid treatment [16, 18]. This concept was ranked fourth of the symptoms that most negatively impact daily life during audience polling at the Voice of the Patient meeting, with participants describing how it affects self-esteem and causes mobility difficulties [18].
Reduced Heat/Sun Tolerance
“Heat or cold intolerance or sensitivity” was reported as the fourth most experienced concept and the sixth most bothersome concept at the “Voice of the Patient” meeting, although only reduced heat or sun tolerance was discussed directly by patients during the presented testimonials [18]. Patients described how this intolerance made it difficult to go outside and take part in outdoors activities, which could affect their social life. Many patients attributed this concept to immunosuppressive or corticosteroid treatment.
Gastrointestinal Problems
Patients reported a range of gastrointestinal problems, including diarrhea, cramping, vomiting, and nausea [18]. Patients described vomiting as particularly bothersome with some reporting unpredictable occurrences while others experienced it daily. These problems were associated with IgAN and various treatments [18]. During the “Voice of the Patient” meeting gastrointestinal symptoms were named in the top five symptoms that most negatively impacted patients’ daily lives [18].
Weight Gain
Patients reported experiencing weight gain that was attributed to either fluid retention or corticosteroid treatment [16, 18,19,20]. As with swelling, this caused patients difficulties with self-esteem and limited physical function [18].
Pain/Aches/Discomfort
Patients reported experiencing discomfort in several locations including, joint pain [18, 20], muscle pain/cramping [18], body aches [18], and abdominal (or “kidney”) pain [16, 18, 19]. Pain could also be secondary to skin problems [18], urinary problems [18], or bone/joint problems [16, 18, 20]. Some instances of pain were attributed as side effects of treatment [18, 20], while others were attributed to symptoms of IgAN [16, 18].
Pain had a significant impact on ADLs, limiting patients’ mobility [19] and making it difficult to do grocery shopping, carry out household chores, or perform their jobs [18]. Some participants reported that pain was so severe they could not leave their bed [18]. Pain would also limit patients’ abilities to perform their favorite activities, such as running or dancing [18]. Emotionally, the pain could cause patients to be irritable [18].
Patients also reported experiencing headaches and migraines, typically attributed as both a symptom of IgAN and as blood pressure medication side effects, which could be so severe that they vomited and completely limited their activities [16, 18].
Mood Swings/Irritability
Mood swings or feelings of irritability were mentioned primarily as a common side effect of corticosteroid treatment [18], but also as a result of pain [18]. Patients described feeling “moody” and “mean”, with one pediatric patient reporting they would “yell at everyone” and “refuse to do what my parents ask” [18].
Skin Problems
Patients reported that they experienced skin issues, including itchy skin, and rashes, which could be painful. Some patients reported these were an indication that their kidney function had declined [16, 18].
Other Symptoms
Patients reported an array of additional symptoms and treatment side effects which could not be easily categorized into a domain or theme, and for which limited detail or description was available in the literature. These included concepts such as appetite increase and decrease [18, 20], chills and sweats [18], dizziness [16, 18], dental issues [18], irregular periods [18], acne [18], a “furry face” [16, 18] and hair loss [18], and ingrown toenails [18]. It is important to note that many of these concepts were attributed as side effects of treatment.
Clinical Signs/Complications
There were several clinical signs and complications that patients could not directly report, but were able to describe as a result of insight provided by their clinicians and through clinical tests. Some of these signs and complications were possibly related to corticosteroid treatment. Moreover, the complications are not disease-defining symptoms of IgAN and could not be included in a patient-reported outcome measure; however, they are important to consider when conceptualizing the patient experience because they contribute to the experience of those living with IgAN. Commonly reported in the literature and noted as particularly bothersome at the “Voice of the Patient” meeting complications [18] were high blood pressure [16, 18, 20], a compromised immune system [18, 20], gout [18], and kidney failure or ESKD [18,19,20].
Other complications identified in the literature included vitamin or mineral deficiency [18], bone-mineral disorder [18], high cholesterol [18], cardiac abnormalities [18, 20], diabetes [16, 18], anemia [18], dehydration [18], and sleep apnea [18]. Patients also described experiencing collapsed veins [18], glaucoma [18], and hormonal imbalance [18], but these were reported only by one patient each and may be individual experiences not generalizable to the broader population.
Impacts
Emotional/Psychological
Patients reported substantial emotional and psychological impacts of living with IgAN [17, 18, 20]. Patients described anxiety and fear, specifically feeling fearful of symptom flare-ups and disease progression [17, 18]. Some fears were related to uncertainty about kidney transplant waiting lists, transplant rejections, and the requirement of frequent dialysis [17, 18, 20]. Patients also described experiencing depression and hopelessness and, in some cases, patients reported these feelings as the most bothersome impact of their disease [18]. Many patients also reported frustration and irritability due to the lack of control they had over the trajectory of their disease or due to symptoms such as constant pain [18]. Feelings of helplessness and frustration were also combined with the guilt of feeling like a burden on others [18]. Some patients reported feeling overwhelmed and stressed by their IgAN diagnosis or the process required for receiving a kidney transplant [17, 18, 20]. Additionally, some patients felt that feelings of stress contributed to the flare-up of their IgAN symptoms [18]. Feelings of low self-esteem due to weight gain and joint pain attributed to corticosteroid treatment were also reported [20].
Some emotional impacts were also specific to the experience of diagnosis; some patients experienced disbelief or felt in denial [17].
Difficulty Adhering to Dietary Restrictions
Patients reported difficulty in adhering to the dietary restrictions needed to manage their symptoms [16, 18, 20] and this was described as bothersome to patients. The most difficult dietary restriction was removing protein and sodium from their diet [18].
Social Functioning
Patients reported feeling isolated and having a reduced social life [17, 18], often due to self-isolation as a result of symptoms or corticosteroid side effects that impacted their appearance, e.g., swelling, weight gain, and acne [18]. Children and adolescents in particular reported experiencing stigma and bullying due to their appearance [18]. Some patients reported feelings of isolation and social anxiety which they attributed to the nature of IgAN being an invisible disease and a lack of public awareness about the disease symptoms [18]. Fatigue and corticosteroid-induced photosensitivity also reduced patients’ ability to participate in social activities [18].
Physical Functioning and Activities of Daily Living (ADLs)
Patients reported that IgAN significantly impacted their day-to-day life. Many symptoms, but particularly pain, aches, fatigue, swelling, weight gain, and nausea, impacted the ability to carry out simple functions such as walking [18, 19]. Patients were unable to participate in hobbies, had reduced capacity to travel, and reduced ability to carry out chores and exercise [16, 18,19,20].
Impact on Relationships
Patients reported feeling that IgAN negatively impacted their relationship with their friends and family. They perceived their loved ones to be experiencing stress and concern due to the burden of caring for someone with a chronic disease. Patients also reported difficultly in discussing their disease with their friends and family [18, 19].
Work/School
Patients reported reduced productivity at work due to mental and physical fatigue; some were forced to give up work altogether as a result of IgAN [18, 20]. Some patients felt that it was difficult to get support from employers who did not understand the impact of their IgAN diagnosis. Patients also worried that prospective employers may not understand how IgAN could affect their ability to work [16, 18].
School life was impacted because of feeling ill, fatigue, and being unable to concentrate which resulted in school absences and missing out on school events. Some patients stopped attending school as a result of a perceived lack of understanding from teachers and/or the severity of their symptoms and were home-schooled or deferred their college attendance instead [18].
Financial Impact
Adult patients reported struggling financially because of their condition. Financial issues stemmed mainly from an inability to work and costs of healthcare [18, 19].
Discussion
This qualitative literature review conceptualized the patient experience of IgAN and highlighted the impact of IgAN on patients’ daily lives and well-being. Symptoms such as swelling (edema), pain/aches, fatigue, and urinary, gastrointestinal, skin and sleep problems can significantly impact patients’ ADLs, exercise routine, hobbies, and social activities. Work and school life can be affected as a result of the symptoms of the disease. Some patients are either unable to work or attend school and may experience reduced productivity due to their symptoms. Emotional and psychological impacts appear to be substantial and bothersome to patients. Patients have reported fearing symptom flare-ups/reoccurrence as well as disease progression to ESKD because this is associated with more substantial medical support such as dialysis and kidney transplant. These interventions can cause additional patient anxiety due to concerns around transplant rejection and uncertain futures. Patients can experience depression, frustration, and hopelessness due to living with a chronic incurable disease and managing debilitating symptoms. A common theme identified was that patients could feel isolated and perceived a lack of public awareness of IgAN. Overall, patients experience considerable disease burden and reduced HRQoL even prior to progression to the late stages of ESKD [5]. The findings from this study are supported by the ongoing Standardised Outcomes in Nephrology-Glomerular Disease (SONG-GD) initiative to identify core outcomes domains for patients with glomerular diseases including but not limited IgAN. To date, the initiative has identified that the broader population of patients with glomerular diseases consider kidney function, mortality, need for dialysis or treatment, life participation, and fatigue to be the five most important outcomes for future treatments [10].
To our knowledge, this is the first study to develop a conceptual model that depicts the adult and pediatric experience of IgAN. The conceptual model can be considered an initial visualization of the patient experience of IgAN, based on the limited number of qualitative studies/sources identified in this review. Most data were sourced from extensive patient accounts during the “Voice of the Patient” externally led patient-focused drug development meeting on IgA Nepropathy held by the National Kidney Foundation and the IGA Foundation of America in 2019. Initiatives such as this indicate that efforts are ongoing to understand the patient experience in the current landscape where there is active clinical research to develop new targeted therapies for patients with IgAN. This meeting also included audience voting on the symptoms and impacts of IgAN that are most frequently experienced and bothersome to patients, but as votes were limited to a short pre-defined list there is a need for further in-depth qualitative research with adults and pediatric/adolescent patients to refine the conceptual model and confirm the most important concepts to patients. This initial conceptual model provides a useful tool to inform the selection, development, and amendment of clinical outcome assessments for use in future IgAN clinical trials [12].
The main limitation of this study, and perhaps therefore the key finding, was the paucity of published qualitative data available to inform the conceptual model. For example, side effects of immunosuppressive drugs other than steroids are not described in our model because no discussion of these were found in the literature. There were often difficulties distinguishing between the signs and symptoms of IgAN, the side effects of treatment, or whether they were specific to adult or pediatric patients only. Certainly, it was not possible to determine the precise frequency and severity of each of the identified concepts within this patient population, although we have been able to suggest a general impression of saliency based upon the frequency of report in the literature, and insights from the National Kidney Foundation and IGA Foundation of America “Voice of the Patient” meeting. In addition, it should be noted that only one data source included participants enrolled via clinician referral [16]; the remainder included patients self-reporting a diagnosis of IgAN, with no clinical confirmation required for participation in the study. Furthermore, it is difficult to ascribe signs, symptoms, or impacts specifically to IgAN versus nephrotic syndrome and/or CKD more generally, and this may be further confounded by the degree of kidney function which was not reported for the patients included in these reports. Further primary research is needed to confirm the disease defining signs/symptoms that are most important for measurement, and to fully explore patient characteristics and experience of IgAN. This additional research can help identify the most salient patient-relevant outcomes to measure clinical research. Additionally, this study did not explore diagnostic delays and the impacts these can have on patient outcomes.
Conversely, a strength of this study was that it included non-traditional sources, e.g., published patient testimonials from patient advocacy groups such as NephCure Kidney International and the IgA Nephropathy foundation to supplement the peer-reviewed literature, in addition to obtaining a clinical expert review of the conceptual model.
The search focused on English-language publications and included studies were conducted in North America and Europe; however, the eligibility criteria (Table 1) stipulated that any highly relevant non-English publications would be translated and included in the review—none were included because none were identified. It has been reported that IgAN exhibits high heterogeneity in clinical presentation and progression across different ethnic populations and different geographical locations [2, 3]. Consequently, further research into the patient experience of IgAN is recommended in other countries and cultures.
Conclusion
Secondary analysis of published qualitative literature informed the development of a novel conceptual model describing the patient experience of IgAN. The conceptual model provides a useful tool to inform the selection and development of clinical outcome assessments for use in future IgAN clinical trials. However, further qualitative research with patients with IgAN is required in order to further develop the model and develop our understanding of the concepts considered by patients to be most important for measurement during the assessment of novel treatments.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. Clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100:S1–S276.
Yeo SC, Goh SM, Barratt J. Is immunoglobulin A nephropathy different in different ethnic populations? Nephrology. 2019;24(9):885–95.
Zhang H, Barratt J. Is IgA nephropathy the same disease in different parts of the world? Semin Immunopathol. 2021;43(5):707–15.
Rodrigues JC, Haas M, Reich HN. IgA nephropathy. Clin J Am Soc Nephrol. 2017;12(4):677–86.
Kwon CS, Daniele P, Forsythe A, Ngai C. A systematic literature review of the epidemiology, health-related quality of life impact, and economic burden of immunoglobulin a nephropathy. J Health Econ Outcomes Res. 2021;8(2):36–45.
Mizerska-Wasiak M, Adamczuk D, Cichoń-Kawa K, et al. Health-related quality of life in children with immunoglobulin A nephropathy—results of a multicentre national study. Arch Med Sci. 2020;17(1):84–91.
Zhao Y, Chen Y-P, Wu Y-Q, Bao B-Y, Fan H. Effect of physical activity on depression symptoms in patients with IgA nephropathy. J Int Med Res. 2020;48(1):3.
Huang X, Xu G. An update on targeted treatment of IgA nephropathy: an autoimmune perspective. Front Pharmacol. 2021;12:715253.
Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1—eliciting concepts for a new PRO instrument. Value Health. 2011;14(8):967–77.
Carter SA, Gutman T, Logeman C, et al. Identifying outcomes important to patients with glomerular disease and their caregivers. Clin J Am Soc Nephrol. 2020;15(5):673–84.
Food and Drug Administration (FDA). Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009. https://www.fda.gov/media/77832/download. Accessed 20 Dec 2022.
Food and Drug Administration (FDA). Patient-Focused Drug Development: Collecting Comprehensive and Representative Input. 2020. https://www.fda.gov/media/139088/download. Accessed 7 Oct 2022.
Food and Drug Administration (FDA). Patient-Focused Drug Development: Selecting, Developing or Modifying Fit-for-Purpose Clinical Outcomes Assessments (Draft Guidance). 2022. https://www.fda.gov/media/159500/download. Accessed 7 Oct 2022.
Food and Drug Administration (FDA). Patient-Focused Drug Development: Methods to Identify What Is Important to Patients. 2022. https://www.fda.gov/media/131230/download. Accessed 7 Oct 2022.
Critical Appraisal Skills Programme (CASP). CASP Qualitative Checklist. https://casp-uk.net/images/checklist/documents/CASP-Qualitative-Studies-Checklist/CASP-Qualitative-Checklist-2018_fillable_form.pdf. Accessed 10 May 2022.
Vasilica C, Oates T, Clausner C, Ormandy P, Barratt J, Graham-Brown M. Identifying information needs of patients with iga nephropathy using an innovative social media-stepped analytical approach. Kidney Int Rep. 2021;6(5):1317–25.
Zaour N, Maylander M, Walda S, George AT. Patient journey, perceptions, and burden associated with immunoglobulin a nephropathy (IgAN): a qualitative study. J Am Soc Nephrol. 2020;31:834.
Feldman D, White E, Julian B, et al. Voice of the Patient. Report on the Externally Led Patient-Focused Drug Development Meeting on IgA Nephropathy. https://nkf.egnyte.com/dl/aHGCS6tPNM/. Accessed 15 Jun 2021.
NephCure Kidney International. Adult IgAN Nephropathy Patient Stories. https://nephcure.org/adult-iga-nephropathy-patient-stories/. Accessed 15 Sep 2021.
IgA Nephropathy Foundation. IgA Nephropathy Foundation: Faces of IgAN. https://igan.org/faces-of-igan/. Accessed 29 Jun 2021.
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Acknowledgements
Medical Writing, Editorial, and Other Assistance.
Preparation of this article was performed by the study authors. Clarivate received consulting fees for preparation of the manuscript.
Funding
This study was sponsored by Pfizer. Clarivate received consulting fees for conducting this study. Pfizer funded the journal’s Rapid Service and Open Access Fees for this article.
Author information
Authors and Affiliations
Contributions
Christine L Baker, Randall Winnette, Natalie VJ Aldhouse and Helen Kitchen conceptualized and designed this study. Christine L Baker, Randall Winnette, Sandi See Tai and Linda Zhu approved the final protocol. Natalie VJ Aldhouse, Helen Kitchen, Tamara Al-Zubeidi and Madeleine Thursfield conducted the literature review and analyzed the data. Sandi See Tai provided clinical expert review of the conceptual model. Natalie VJ Aldhouse, Helen Kitchen, Tamara Al-Zubeidi, Madeleine Thursfield, Randall Winnette, Sandi See Tai, Linda Zhu, Cecilia Freitas, Nicolas Garnier and Christine L Baker interpreted the data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Natalie VJ Aldhouse, Helen Kitchen, Tamara Al-Zubeidi and Madeleine Thursfield are employees and stockholders of Clarivate, a health economic and outcomes research consultancy that consults with various pharmaceutical companies, including Pfizer. Christine L Baker, Sandi See Tai, Randall Winnette, Linda Zhu, Cecilia Freitas and Nicolas Garnier are employees and shareholders at Pfizer.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Additional information
Prior Presentation: This work was previously presented as an abstract/poster at ISPOR 2022, May 15–18, 2022. Washington, DC, USA (PCR134).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Aldhouse, N.V.J., Kitchen, H., Al-Zubeidi, T. et al. Development of a Conceptual Model for the Patient Experience of Immunoglobulin A Nephropathy (IgAN): A Qualitative Literature Review. Adv Ther 41, 1325–1337 (2024). https://doi.org/10.1007/s12325-024-02793-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02793-1