Abstract
Introduction
Data describing real-world treatment patterns in patients with metastatic urothelial carcinoma (mUC) in Central-Eastern Europe are scarce, and data from Hungary have not been published. This retrospective, nationwide, real-world study investigated patient characteristics, treatment patterns, comorbidities, and clinical outcomes in patients with mUC in Hungary.
Methods
Adults diagnosed with mUC from January 2016 through June 2021 were identified using the National Health Insurance Fund Administration database. Overall survival (OS) was estimated using the Kaplan–Meier method.
Results
In total, 2523 patients with mUC were identified. Median follow-up was 7.1 months. Overall, 50% of patients received an identified systemic anticancer treatment; within this subgroup, first-line treatment was platinum-based chemotherapy (PBC) in 86%, non-PBC in 8%, and immune checkpoint inhibitor (ICI) in 6%. The proportion of patients receiving treatment increased from 41% in 2016 to 59% in 2020, driven by increased use of first-line PBC or first-line ICI treatment. Comorbidities were more common in patients receiving first-line ICI treatment vs PBC or non-PBC and in patients receiving carboplatin + gemcitabine vs cisplatin + gemcitabine. Overall, only 24% received a second-line treatment. Unadjusted median OS from the start of first-line treatment in the PBC, non-PBC, and ICI subgroups was 12.8, 7.5, and 6.3 months, respectively. Median OS from date of diagnosis in untreated patients was 7.8 months. OS comparisons adjusted for differences in baseline characteristics between subgroups could not be performed.
Conclusion
To our knowledge, this is the first study to assess treatment patterns in patients with mUC in clinical practice in Hungary, using the National Health Insurance Fund Administration. Rates of first- and second-line treatment were consistent with those observed in other countries. Avelumab first-line maintenance treatment became available for reimbursement in Hungary in late 2022, after the study period. Given the evolving landscape of reimbursed treatments in Hungary, further analyses are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Data describing real-world treatment patterns for metastatic urothelial carcinoma in Central-Eastern Europe are scarce. |
This study retrospectively analyzed patient claims from the Hungarian National Health Insurance Fund Administration database to obtain data about patient characteristics, treatment patterns, comorbidities, and clinical outcomes in patients with metastatic urothelial carcinoma in Hungary. |
What was learned from the study? |
Approximately 50% of patients received a systemic anticancer treatment and 24% received second-line treatment; these rates are similar to those observed in studies from other countries. |
The proportion of patients receiving first-line treatment increased between 2016 and 2020, with more patients receiving platinum-based chemotherapy and immune checkpoint inhibitors. |
Given the evolving treatment landscape in Hungary, this study can serve as a benchmark for future analyses of treatment patterns and clinical outcomes in patients with metastatic urothelial carcinoma in Central-Eastern Europe. |
Introduction
Urothelial carcinoma (UC) is the most common malignancy involving the urinary system and the sixth most common tumor type in men worldwide [1]. More than 90% of UC tumors originate in the bladder [2]. UC is approximately four times more common in men than in women, with worldwide incidence rates of 9.6 per 100,000 men and 2.4 per 100,000 women [3]. In Hungary in 2020, the estimated age-standardized incidence and mortality rates for all ages and sexes were 15.2 per 100,000 and 3.8 per 100,000, respectively [4]. However, no standardized local data for patients with metastatic UC (mUC) have been published, and data describing treatment patterns and associated clinical outcomes for mUC in routine clinical practice in Central-Eastern Europe are scarce.
Platinum-based chemotherapy (PBC) has been the standard first-line (1L) treatment for patients with mUC for approximately two decades [2, 5,6,7]. In addition, immune checkpoint inhibitors (ICIs) were added to international guidelines for mUC [2, 5,6,7], which are followed in Hungary, based on the results of several phase 2 trials [8,9,10,11]. ICIs were approved by the European Commission (EC) from 2017 onward for 1L treatment of patients who were cisplatin-ineligible with locally advanced or metastatic UC with PD-L1+ tumors (atezolizumab and pembrolizumab), and for second-line (2L) treatment in patients who have received prior platinum-containing chemotherapy (atezolizumab, pembrolizumab, and nivolumab) [12,13,14]. In Hungary, 1L and 2L ICI treatment for these indications was accepted into the Named-Patient Based Reimbursement (NPBR) program [15] in 2018 and 2019, respectively. Following the results of the JAVELIN Bladder 100 phase 3 trial, avelumab was approved by the EC in 2021 for 1L maintenance treatment of patients who are progression free following PBC [16]. Avelumab 1L maintenance has since been incorporated into international treatment guidelines based on level 1 evidence, including those developed by the European Society for Medical Oncology and European Association of Urology, as a standard of care [2, 5,6,7]. Avelumab was accepted into the NPBR program in Hungary in November 2022. Additionally, enfortumab vedotin was approved by the EC in 2022 for the treatment of patients with mUC who have received prior PBC and ICI treatment [17], and nivolumab was also approved by the EC in 2022 for the adjuvant treatment of patients with high-risk, muscle-invasive, PD-L1+ UC after radical resection [13]; however, in Hungary, reimbursement decisions for these drugs in these indications are awaited at the time of writing.
The aim of this study was to describe real-world patient characteristics, treatment patterns, and clinical outcomes in patients diagnosed with mUC in Hungary.
Methods
Study Design and Patients
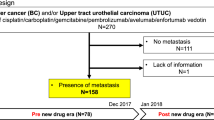
This study was a retrospective analysis of anonymized patient claims data from the National Health Insurance Fund Administration (NHIFA) database, the only health insurance fund database in Hungary, which covers all Hungarian residents (approximately 10 million people) [18]. This comprehensive database captures all insurance claims and deaths. The study period was from January 1, 2016 through June 30, 2021, with a 1-year censoring period starting from January 1, 2015 (Fig. 1a). Eligible patients were aged 18 years or older and had a diagnosis of mUC based on at least two outpatient claims or at least one inpatient claim (e.g., surgery, chemoradiotherapy, or diagnostics) for malignant neoplasm of the urinary tract (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision [ICD-10] codes C65–C68) with evidence of metastatic disease (ICD-10 code C77–C79) or a relevant 1L ICI treatment for mUC. Patients who had only one reported ICD-10 code and had no relevant systemic treatment (chemotherapy or ICI) were excluded.
Study design (a) and patient attrition (b). Study inclusion criteria: aged ≥ 18 years, diagnosis of mUC based on ≥ 2 outpatient claims or ≥ 1 inpatient claim for malignant neoplasm of the urinary tract (ICD-10 codes C65–C68), and evidence of metastatic disease (ICD-10 code C77–C79) or received relevant 1L ICI treatment. Study exclusion criteria: only 1 reported ICD-10 code, and no relevant systemic treatment. 1L first line, ICD-10 International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, ICI immune checkpoint inhibitor, mUC metastatic urothelial carcinoma, NHIFA National Health Insurance Fund Administration, PBC platinum-based chemotherapy, UC urothelial carcinoma. aFirst reported UC ICD-10 code. b1L treatment subgroup: start date of 1L systemic treatment; untreated subgroup: date of mUC diagnosis
Patients were categorized into two subgroups: untreated and treated (Fig. 1b). The untreated subgroup comprised patients with mUC identified as described above who had not received a relevant 1L systemic anticancer treatment. The treated subgroup was subdivided by the type of 1L systemic treatment received (defined as the first identified treatment for mUC received) within the following protocol-specified categories: (1) PBC, (2) non-PBC, or (3) ICI monotherapy. The 1L PBC subgroup was further subdivided between patients who initially received cisplatin + gemcitabine or carboplatin + gemcitabine. Subgroups receiving different treatment sequences (any 2L treatment received after a specific 1L treatment [PBC or ICI]) were also analyzed. To analyze proportions of patients who received different 1L treatments over time, incidence per year was based on the date when treatment was first observed following the censoring period, and prevalence per year was based on all patients who received the specified treatment (or who were untreated) each year.
Statistical Analyses
Descriptive statistical analyses of the study population were conducted to show baseline demographics, treatment patterns, and clinical outcomes. Treatment patterns and comorbidities were analyzed for all claims. Comorbidities were described using the Charlson Comorbidity Index, based on reported ICD-10 codes starting 12 months before the index date. The index date was defined differently for the untreated and treated subgroups. The index date was the start date of 1L systemic treatment for the 1L treated subgroups, the start date of 2L systemic treatment for the 2L treated subgroups, and the date of mUC diagnosis for the untreated subgroup. Unadjusted median overall survival (OS) was calculated from the index date and estimated by the Kaplan–Meier method (R 4.0.4 survival package). Statistical comparisons of median OS between treatment subgroups were analyzed at a significance level of p < 0.05. Comparisons for OS, adjusted for differences in baseline characteristics between subgroups, could not be performed.
Ethics Approval
Ethics approval, as required by Ministerial Decree No. 23/2002 (V.9) for non-interventional studies, was provided by the Hungarian Medical Research Council (No. IV/7775-4/2021/EKU) [19]. The NHIFA database was accessed under license and is not publicly available. Patient claims data received from the NHIFA database were anonymized.
Results
Patient Characteristics and Treatment Patterns
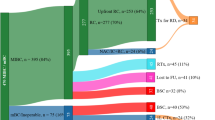
In total, 2523 patients with mUC were identified, of whom 1822 (72.2%) were men. Median age was 67 years (interquartile range [IQR], 63–73 years) (Table 1). Approximately half of the patients (n = 1256 [49.8%]) had received an identified systemic anticancer treatment. The type of 1L treatment was PBC in 1082 patients (86.1% of treated patients; 42.9% overall), non-PBC in 97 (7.7% of treated patients; 3.8% overall), and ICI in 77 (6.1% of treated patients; 3.1% overall). The 1L PBC regimen was cisplatin + gemcitabine in 841 patients (77.7% of those who received any 1L PBC; 33.3% overall) and carboplatin + gemcitabine in 241 (22.3% of those who received any 1L PBC; 9.6% overall).
The proportion of treated patients in the study population increased during the study period when full-year data were available (Fig. 2), with the prevalence of treated patients per year increasing from 41% in 2016 to 59% in 2020. This was driven by an increase in patients receiving 1L PBC (2016, 37%; 2020, 52%) and 1L ICI treatment (2016, 0%; 2020, 4%).
Incidence (a) and prevalence (b) of different types of treatment over the study period (2016–2021). To analyze proportions of patients receiving different 1L treatments over time, incidence per year was based on the date when treatment was first observed following the censoring period, and prevalence per year was based on all patients who received the specified treatment (or who were untreated) each year. Actual patient numbers are reported in the figures. 1L first line, H1 first half of year, ICI immune checkpoint inhibitor, PBC platinum-based chemotherapy
Among all patients, 11.8% received 2L treatment (n = 298 [23.7% of patients who received 1L treatment]). Among these patients, most received 2L ICI treatment after 1L chemotherapy (n = 287 [96.3%]) and other patients received 2L chemotherapy after 1L ICI treatment (n = 11 [3.7%]). Patient characteristics among subgroups defined by 2L treatment sequence are presented in Supplementary Table S1. A summary of treatment sequencing in the overall population is shown in Fig. 3.
The median age was similar in untreated and treated patients, but the level of comorbidity was higher in untreated patients (median Charlson Comorbidity Index score, 2.1 vs 1.7) (Table 1). In an analysis of individual comorbidities using ICD-10 codes, slightly different proportions of patients in the untreated vs treated subgroups (respectively) had diseases of the digestive system (82.3% vs 76.4%), respiratory system (76.8% vs 72.1%), and nervous system (32.1% vs 24.8%). Among treated patients, those who received 1L ICI treatment were older than those who received 1L PBC or 1L non-PBC (median age, 70, 67, and 66 years, respectively). In addition, patients who received 1L ICI treatment had more comorbidities than those who received 1L PBC or 1L non-PBC, as indicated by a higher mean Charlson Comorbidity Index score (2.3, 1.6, and 2.0, respectively) and a greater proportion of patients with several types of comorbidities (Table 2). Among patients who received any 1L PBC, median age was similar in those who received 1L cisplatin + gemcitabine or 1L carboplatin + gemcitabine; however, those who received 1L cisplatin + gemcitabine had a lower mean Charlson Comorbidity Index score (1.5 vs 2.0, respectively).
Clinical Outcomes
Median follow-up in the overall population was 7.1 months (IQR 2.2–17.0 months). Median follow-up was shorter in untreated patients (5.3 months [IQR 1–16.5 months]) and in patients who received 1L ICI treatment (2.1 months [IQR 1–8 months]) than in those who received 1L PBC or 1L non-PBC (8.9 months [IQR 4.4–18.3 months] or 6.4 months [IQR 2.8–13.5 months], respectively).
In the overall population, the unadjusted median OS (measured from the date of mUC diagnosis in untreated patients and from the start of 1L systemic treatment in treated patients) was 9.9 months (95% CI 9.2–10.7 months). Median OS from the index date was 7.8 months (95% CI 6.7–8.8 months; Fig. 4a) in untreated patients vs 11.7 months (95% CI 10.5–12.9 months) in treated patients (p < 0.0001). Unadjusted median OS from the start of 1L treatment was 12.8 months (95% CI 11.5–14.1 months) with any 1L PBC, 7.5 months (95% CI 5.8–10.1 months) with 1L non-PBC, and 6.3 months (95% CI 2.9–9.0 months) with 1L ICI treatment (Fig. 4b). In patients who received 1L cisplatin + gemcitabine vs 1L carboplatin + gemcitabine, median OS from the start of 1L treatment was 14.6 months (95% CI 12.8–16.0 months) vs 8.4 months (95% CI 7.4–10.6 months; p < 0.0001; Fig. 4c).
Unadjusted analysis of OS in subgroups. a OS from index date in treated patients (measured from start of 1L treatment) and untreated patients (measured from date of mUC diagnosis). b OS in patients receiving different types of 1L treatment (measured from start of 1L treatment). c OS in patients receiving different types of PBC (measured from start of 1L treatment). d OS in patients receiving different sequences of 1L and 2L treatment (measured from start of 2L treatment). 1L first line, 2L second line, ICI immune checkpoint inhibitor, mUC metastatic urothelial carcinoma, OS overall survival, PBC platinum-based chemotherapy
Among patients with any 2L systemic treatment, unadjusted median OS from start of 2L treatment was 6.8 months (95% CI 5.6–8.2 months) (Fig. 4d). Median OS from start of 2L treatment was similar in patients who received 2L ICI treatment following 1L cisplatin + gemcitabine or 1L carboplatin + gemcitabine treatment (6.4 months [95% CI 4.6–8.3 months] vs 5.6 months [95% CI 3.4–6.9 months]; p = 0.6914).
Discussion
This nationwide, noninterventional, retrospective study is the first to assess real-world treatment patterns in patients with mUC in Hungary. Although nationwide studies in mUC have been carried out in other countries [20,21,22,23,24], to our knowledge, no similar study has been performed in Central-Eastern Europe. The population of patients with mUC identified in Hungary was mostly men and median age was 67 years, similar to other real-world studies [21,22,23, 25].
Overall, study data showed that treatment of mUC in Hungary follows established European guidelines [5,6,7]. Only half of patients received any systemic treatment for mUC. This rate is similar or higher than rates observed in other countries across a similar period [25,26,27,28,29,30,31,32]. However, rates of 1L systemic treatment increased during the study period when full-year data were available (2016–2020); a similar increase in 1L systemic treatment rate was also seen in a real-world study of mUC in Germany between 2015 and 2019 [21,22,23, 25]. No conclusions could be drawn for the year 2021 because data were incomplete. Of patients who received treatment, most received 1L PBC, consistent with treatment guidelines [5,6,7]. However, 1L ICI use in Hungary may have been limited by availability in the NPBR program, which occurred only later in the study period (since 2018), with use of 1L ICIs increasing thereafter. In our study, only 24% of patients who were treated with 1L therapy received subsequent 2L therapy. This rate is slightly lower than rates seen in some recent real-world studies [21,22,23, 30, 33]. The limited proportion of patients with mUC who receive systemic treatment, and the high rate of attrition between 1L and 2L therapy, are well recognized and highlight the need to use the most effective available frontline agents to improve outcomes in this population [34].
Patients who received 1L ICI treatment were older and had more comorbidity than patients who received 1L PBC, similar to patients in a previous real-world study [29]. In addition, patients who received 1L carboplatin + gemcitabine had more comorbidities than patients who received 1L cisplatin + gemcitabine. These observations are consistent with 1L ICIs being approved by the EC only in patients who were cisplatin-ineligible with PD-L1+ tumors during the study period [12, 13] and the recommendation of carboplatin + gemcitabine as an alternative to cisplatin-based chemotherapy in patients who were cisplatin-ineligible in guidelines [2, 5,6,7]. Specifically, established criteria for cisplatin ineligibility include specific comorbidities (reduced performance status, renal impairment, hearing loss, neuropathy, or heart failure) [2, 5,6,7]; thus, patients who were cisplatin-ineligible would be expected to have more comorbidities and to be generally frailer than patients receiving cisplatin-based chemotherapy.
Unadjusted OS was longer in patients with mUC who received any 1L treatment than in those who did not receive a relevant systemic treatment. Of those who received 1L treatment, unadjusted OS was longer in patients who received any 1L PBC than in those who received 1L ICIs or 1L non-PBC (median, 12.8 vs 6.3–7.5 months). These outcomes are consistent with those in previously published real-world data from unadjusted analyses [23, 24, 28, 29]. OS was also longer in patients who received 1L cisplatin + gemcitabine than in patients who received 1L carboplatin + gemcitabine, which was reported in previous studies [20,21,22, 29]. However, because detailed patient demographic and disease-specific data were not available at baseline, we were unable to perform adjusted OS analyses. Potential predictors for OS include patient demographics (e.g., performance status), comorbidity, and site of metastasis at the start of 1L treatment [35,36,37]. Thus, comparisons of OS between treatment subgroups in our analyses should be interpreted with caution.
This study had some limitations. First, the NHIFA is a payer-specific database, and information on mUC disease-specific parameters, such as stage at diagnosis, treatment outcomes, or known mUC risk factors, including smoking history, is lacking. Because of the lack of disease-staging information in the database, mUC diagnosis was identified either by ICD-10 codes or receipt of 1L systemic anticancer treatment. No information was available on the potential reasons for nonreceipt of 1L systemic treatment. Furthermore, claims-based studies are also inherently associated with factors such as coding errors and missing information. Because detailed baseline characteristics could not be collected, descriptive statistical analyses were conducted for efficacy results and OS was unadjusted, as discussed earlier. Median follow-up in the overall population was short because of the short median OS in some subgroups (untreated patients and patients receiving 1L ICI or 1L non-PBC treatment), which reflects the poor prognosis of patients with mUC. Moreover, subgroups of fewer than 10 patients were not included in the analysis, in line with the data protection regulations of the NHIFA database. Sizes of some subgroups were relatively small, specifically those receiving 1L ICI and 1L non-PBC vs any 1L PBC, meaning that data may not be fully representative. Lastly, 1L ICIs were only accepted in the NPBR program later in the study period (2018) [38].
Conclusion
This first-of-its-kind study evaluated patient characteristics, treatment patterns, and clinical outcomes in patients with mUC in routine clinical practice in Hungary using a nationwide, comprehensive database. This research provides important insights into real-world treatment outcomes and is complementary with other recently published international real-world evidence analyses from other data sources. Specifically, our data show that only 50% of patients received 1L systemic treatment and that clinical outcomes in this study were in line with those in similar real-world studies. The results can serve as a benchmark for future real-world analyses of treatment patterns and clinical outcomes in Central-Eastern European countries. Since this study was conducted, additional treatments have become available in Europe, including avelumab 1L maintenance treatment for patients with mUC who are progression free following PBC [16], enfortumab vedotin for patients with mUC who have received prior PBC and ICI treatment, and nivolumab for adjuvant treatment of patients with high-risk, muscle-invasive, PD-L1+ UC following radical resection [14, 17]. Avelumab was accepted into the NPBR program in 2022; however, reimbursement decisions for enfortumab vedotin and nivolumab in Hungary for these specific indications are awaited at the time of writing. Further studies are needed to assess the impact of emerging treatment options for mUC in Central-Eastern Europe.
Data Availability
The data that support the findings of this study are available from the National Health Insurance Fund Administration of Hungary, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. However, the datasets analyzed during the current study are available from the corresponding author on reasonable request and with permission of the National Health Insurance Fund Administration of Hungary.
Change history
09 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12325-023-02740-6
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Bladder Cancer Version 3.2023 - May 25.2023.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Global Cancer Observatory: cancer today. Lyon: International Agency for Research on Cancer. https://gco.iarc.fr/today. Accessed Sep 6, 2023.
Cathomas R, Lorch A, Bruins HM, et al. The 2021 updated European Association of Urology guidelines on metastatic urothelial carcinoma. Eur Urol. 2021;81(1):95–103.
Rouprêt M, Seisen T, Birtle AJ, et al. European Association of Urology guidelines on upper urinary tract urothelial carcinoma: 2023 update. Eur Urol. 2023;84(1):49–64.
Powles T, Bellmunt J, Comperat E, et al. Bladder cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(3):244–58.
Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67–76.
Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18(11):1483–92.
Géczi L, Dienes T, Küronya Z, Maráz A, Nagyiványi K. Immunotherapy in advanced urothelial cancer. Magy Onkol. 2021;65(4):339–46.
Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312–22.
European Medicines Agency. Pembrolizumab summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_en.pdf. Accessed May 25, 2023.
European Medicines Agency. Atezolizumab summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/tecentriq-epar-product-information_en.pdf. Accessed May 25, 2023.
European Medicines Agency. Nivolumab summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/opdivo-epar-product-information_en.pdf. Accessed May 25, 2023.
Inotai A, Csanádi M, Harsányi A, Németh B. Drug policy in Hungary. Value Health Regional Issues. 2017;13:16–22.
European Medicines Agency. Assessment report: Bavencio. 2020. https://www.ema.europa.eu/en/documents/overview/bavencio-epar-medicine-overview_en.pdf. Accessed May 25, 2023.
European Medicines Agency. Padcev (enfortumab vedotin). Summary of product characteristics. https://www.ema.europa.eu/en/documents/product-information/padcev-epar-product-information_en.pdf. Accessed May 25, 2023.
National Health Insurance Fund of Hungary (NEAK). Tasks of the National Insurance Fund of Hungary. http://www.neak.gov.hu/felso_menu/rolunk/kozerdeku_adatok/tevekenysegre_mukodesre_vonatkozo_adatok/a_szerv_feladata_alaptevekenysege_es_hatarkore/en_a_szerv_alaptevekenyege_feladata_es_hatarkore. Accessed May 25, 2023.
Egészségügyi Tudományos Tanács - Tudományos és Kutatásetikai Bizottság (ETT-TUKEB). Scientific and Research Ethics Committee. https://ett.aeek.hu/tukeb/. Accessed May 25, 2023.
Omland LH, Lindberg H, Carus A, et al. Real-world treatment patterns and overall survival in locally advanced and metastatic urothelial tract cancer patients treated with chemotherapy in Denmark in the preimmunotherapy era: a nationwide, population-based study. Eur Urol Open Sci. 2021;24:1–8.
Niegisch G, Gerullis H, Lin SW, et al. A real-world data study to evaluate treatment patterns, clinical characteristics and survival outcomes for first- and second-line treatment in locally advanced and metastatic urothelial cancer patients in Germany. J Cancer. 2018;9(8):1337–48.
Cheeseman S, Thompson M, Sopwith W, et al. Current treatment and outcomes benchmark for locally advanced or metastatic urothelial cancer from a large UK-based single centre. Front Oncol. 2020;10:167.
Simeone JC, Nordstrom BL, Patel K, Mann H, Klein AB, Horne L. Treatment patterns and overall survival in metastatic urothelial carcinoma in a real-world, US setting. Cancer Epidemiol. 2019;60:121–7.
Geynisman DM, Broughton E, Hao Y, Zhang Y, Le T, Huo S. Real-world treatment patterns and clinical outcomes among patients with advanced urothelial carcinoma in the United States. Urol Oncol. 2022;40(5):195e1–11.
Niegisch G, Grimm M-O, Hardtstock F, et al. Treatment patterns, indicators of receiving systemic treatment, and clinical outcomes in metastatic urothelial carcinoma: a retrospective analysis of real-world data in Germany. J Clin Oncol. 2023;41(6 suppl): Abstract 464.
Wilke T, Zhang L, Hubscher E, Musat MG, Harricharan S, Kearney M. Undertreatment rates, associated factors, and survival among patients with locally advanced or metastatic urothelial cancer (la/mUC): a systematic literature review. Ann Oncol. 2022;33(7 suppl):S1345 (Abstract 1765P).
Kearney M, Zhang L, Hubscher E, Musat M, Harricharan S, Wilke T. First-line treatment patterns among patients with locally advanced or metastatic urothelial cancer (la/mUC): a systematic literature review. Value Health. 2022;25(12 suppl):S414 (Abstract PCR124).
Dinan MA, Georgieva MV, Li Y, et al. Real-world systemic therapy utilization in Medicare patients with locally advanced or metastatic urothelial carcinoma diagnosed between 2008 and 2012. J Geriatr Oncol. 2021;12(2):298–304.
Bilen MA, Robinson SB, Schroeder A, et al. Clinical and economic outcomes in patients with metastatic urothelial carcinoma receiving first-line systemic treatment (the IMPACT UC I study). Oncologist. 2023;28(9):790–8.
Richters A, Mehra N, Meijer RP, et al. Utilization of systemic treatment for metastatic bladder cancer in everyday practice: results of a nation-wide population-based cohort study. Cancer Treat Res Commun. 2020;25: 100266.
Knott C, Kearney M, Mahmoudpour H, Verpillat P. Factors associated with the receipt of systemic treatment (tx) for metastatic urothelial carcinoma (mUC) in England. Ann Oncol. 2022;33:S1338 (Abstract 750P).
Kearney M, Zhang L, Hubscher E, Musat M, Harricharan S, Wilke T. Undertreatment in patients with advanced urothelial cancer: systematic literature review and meta-analysis. Future Oncol. 2023. Epub ahead of print: Aug 1.
Hepp Z, Shah SN, Smoyer K, Vadagam P. Epidemiology and treatment patterns for locally advanced or metastatic urothelial carcinoma: a systematic literature review and gap analysis. J Manag Care Spec Pharm. 2021;27(2):240–55.
Swami U, Grivas P, Pal SK, Agarwal N. Utilization of systemic therapy for treatment of advanced urothelial carcinoma: lessons from real world experience. Cancer Treat Res Commun. 2021;27: 100325.
Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17(10):3173–81.
Necchi A, Sonpavde G, Lo Vullo S, et al. Nomogram-based prediction of overall survival in patients with metastatic urothelial carcinoma receiving first-line platinum-based chemotherapy: retrospective international study of invasive/advanced cancer of the urothelium (RISC). Eur Urol. 2017;71(2):281–9.
Richters A, Boormans JL, van der Heijden MS, et al. Overall survival of patients receiving cisplatin or carboplatin for primary metastatic urothelial carcinoma of the bladder: a contemporary Dutch nationwide cohort study. Eur Urol Focus. 2022;8(4):995–1002.
Maráz A, Varga L, Pósfai B, Géczi L, Küronya Z. New aspects of chemotherapy and indications for maintenance immunotherapy in urothelial cancers. Magy Onkol. 2021;65(4):329–37.
Medical Writing, Editorial, or Other Assistance.
Medical writing support was provided by Jamie Ratcliffe of Clinical Thinking. Medical writing support was funded by the healthcare business of Merck KGaA, Darmstadt, Germany, and Pfizer.
Funding
This research was sponsored by the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945), and was previously conducted under an alliance between the healthcare business of Merck KGaA, Darmstadt, Germany, and Pfizer. The journal’s Rapid Service and Open Access Fees were funded by the healthcare business of Merck KGaA, Darmstadt, Germany, and Pfizer.
Author information
Authors and Affiliations
Contributions
Anikó Maráz: conceptualization, data curation, supervision, validation, and writing—review and editing. Bence Nagy: methodology, visualization, and writing—review and editing. Tamara Macher: methodology, visualization, and writing—review and editing. József Jeskó: methodology, visualization, and writing—review and editing. Erika Tischler: data curation, and writing—review and editing. Csaba Csongvai: data curation, and writing—review and editing. Mairead Kearney: conceptualization, supervision, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of Interest
Anikó Maráz has served in consulting or advisory roles for Merck & Co., Kenilworth, NJ, and the healthcare business of Merck KGaA, Darmstadt, Germany, and has provided speaker services for Bristol Myers Squibb, Merck & Co., Kenilworth, NJ, Roche, and the healthcare business of Merck KGaA, Darmstadt, Germany. Bence Nagy, Tamara Macher, and József Jeskó have served in consulting or advisory roles for the healthcare business of Merck KGaA, Darmstadt, Germany, for their work on this study. Erika Tischler and Csaba Csongvai are employees of Merck Kft., Budapest, Hungary, an affiliate of Merck KGaA, Darmstadt, Germany. Mairead Kearney is an employee of the healthcare business of Merck KGaA, Darmstadt, Germany, and reports stock and other ownership interests in Merck KGaA, Darmstadt, Germany, Novartis, and UCB Biopharma SPRL.
Ethical Approval
Ethics approval, as required by Ministerial Decree No. 23/2002 (V.9) for non-interventional studies, was provided by the Hungarian Medical Research Council (No. IV/7775–4/2021/EKU) [19]. The NHIFA database was accessed under license and is not publicly available. Patient claims data received from the NHIFA database were anonymized.
Additional information
The original article was revised due to update in article text
Prior Presentation
Some data reported in this manuscript were presented at the ESMO Congress 2022, Paris, France, September 9–13, 2022: Maráz AC, Nagy B, Macher T, Jeskó J, Tischler E, Csongvai C, Kearney M. 1756P Real-world (RW) treatment (Tx) patterns and clinical outcomes in patients (pts) with metastatic urothelial carcinoma (mUC): results of a nationwide, longitudinal, retrospective study in Hungary. Ann Oncol. 2022; 33(Suppl 7):S1341. Abstract 1756P. https://doi.org/10.1016/j.annonc.2022.07.1834.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Maráz, A., Nagy, B., Macher, T. et al. Nationwide Study of Real-World Treatment Patterns and Clinical Outcomes in Patients with Metastatic Urothelial Carcinoma in Hungary. Adv Ther 40, 5475–5488 (2023). https://doi.org/10.1007/s12325-023-02694-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02694-9