Abstract
Introduction
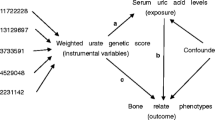
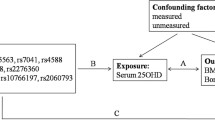
The mechanistic target of rapamycin (mTOR) regulates bone homeostasis, a crucial factor in osteoporosis (OP) development. However, most research is based on observational studies, and the causality remains uncertain. Therefore, we analyzed two samples of mendelian randomization (MR) to determine whether there is a causal relationship between mTOR-dependent circulating proteins and OP.
Methods
Mendelian weighting (weighted median [WM], inverse variance weighting [IVW], and MR-Egger regression) were applied to analyze the causality between bone phenotypes (bone mineral density [BMD] in forearm, femoral neck, lumbar spine, and heel) and mTOR-dependent circulating proteins (RP-S6K, 4EBP, EIF-4E, EIF-4A, and EIF-4G). Horizontal pleiotropy and heterogeneities were detected using Cochran’s Q test, MR-Pleiotropy RE-Sidual Sum and Outlier (MR-PRESSO), and “leave-one-out” analysis. The proteomics-GWAS INTERVAL study was used to select the instrumental variables (IVs) for mTOR proteins.
Results
As phenotypes for OP, estimations of BMD were taken in four different sites: forearm (FA) (n = 8143), femoral neck (FN) (n = 32,735), lumbar spine (LS) (n = 28,498), and heel (eBMD) (n = 426,824). Based on IVW analysis, EIF4E is causally related to FA-BMD (OR = 0.938, 95% CI 0.887, 0.991, p = 0.024) but not to BMD elsewhere.
Conclusion
MR analysis revealed a causal relationship between EIF-4E and FA-BMD, which may provide new insights into the underlying pathogenesis of OP and a new therapeutic target for OP.




Similar content being viewed by others
Data Availability
These data were derived from the following resources available in the public websites: the exposure data for mTOR-dependent circulating proteins (https://www.phpc.cam.ac.uk/ceu/proteins/), and the outcome data for BMD (https://gwas.mrcieu.ac.uk/datasets/).
References
Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet (London, England). 2019;393(10169):364–76.
Ensrud KE, Crandall CJ. Osteoporosis. Ann Intern Med. 2017;167(3):17–32.
Holroyd C, Cooper C, Dennison E. Epidemiology of osteoporosis. Best Pract Res Clin Endocrinol Metab. 2008;22(5):671–85.
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–75.
Kanis JA, Borgstrom F, De Laet C, Johansson H, Johnell O, Jonsson B, Oden A, Zethraeus N, Pfleger B, Khaltaev N. Assessment of fracture risk. Osteoporos Int. 2005;16(6):581–9.
Jeyabalan J, Shah M, Viollet B, Chenu C. AMP-activated protein kinase pathway and bone metabolism. J Endocrinol. 2012;212(3):277–90.
Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168(6):960–76.
Liu DM, Zhao L, Liu TT, Jiao PL, Zhao DD, Shih MS, Tao B, Sun LH, Zhao HY, Liu JM. Rictor/mTORC2 loss in osteoblasts impairs bone mass and strength. Bone. 2016;90:50–8.
Zhan JK, Wang YJ, Wang Y, Wang S, Tan P, Huang W, Liu YS. The mammalian target of rapamycin signalling pathway is involved in osteoblastic differentiation of vascular smooth muscle cells. Can J Cardiol. 2014;30(5):568–75.
Roman B, Montei C, Zhang L, Biswas P, Donofrio R, Xu S, Zhang Y, Liu B, Li K, Huang B, Yan B, Zhang Z, Liang K, Jia C, Lin J, Zeng C, Cai D, Jin D, Jiang Y, Bai X. Activation of mTORC1 in B lymphocytes promotes osteoclast formation via regulation of β-catenin and RANKL/OPG. J AOAC Int. 2016;31(7):1320–33.
Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35.
Zheng HF, Forgetta V, Hsu YH, Estrada K, Rosello-Diez A, Leo PJ, Dahia CL, Park-Min KH, Tobias JH, Kooperberg C, Kleinman A, Styrkarsdottir U, Liu CT, Uggla C, Evans DS, Nielson CM, Walter K, Pettersson-Kymmer U, McCarthy S, Eriksson J, Kwan T, Jhamai M, Trajanoska K, Memari Y, Min J, Huang J, Danecek P, Wilmot B, Li R, Chou WC, Mokry LE, Moayyeri A, Claussnitzer M, Cheng CH, Cheung W, Medina-Gómez C, Ge B, Chen SH, Choi K, Oei L, Fraser J, Kraaij R, Hibbs MA, Gregson CL, Paquette D, Hofman A, Wibom C, Tranah GJ, Marshall M, Gardiner BB, Cremin K, Auer P, Hsu L, Ring S, Tung JY, Thorleifsson G, Enneman AW, van Schoor NM, de Groot LC, van der Velde N, Melin B, Kemp JP, Christiansen C, Sayers A, Zhou Y, Calderari S, van Rooij J, Carlson C, Peters U, Berlivet S, Dostie J, Uitterlinden AG, Williams SR, Farber C, Grinberg D, LaCroix AZ, Haessler J, Chasman DI, Giulianini F, Rose LM, Ridker PM, Eisman JA, Nguyen TV, Center JR, Nogues X, Garcia-Giralt N, Launer LL, Gudnason V, Mellström D, Vandenput L, Amin N, van Duijn CM, Karlsson MK, Ljunggren Ö, Svensson O, Hallmans G, Rousseau F, Giroux S, Bussière J, Arp PP, Koromani F, Prince RL, Lewis JR, Langdahl BL, Hermann AP, Jensen JE, Kaptoge S, Khaw KT, Reeve J, Formosa MM, Xuereb-Anastasi A, Åkesson K, McGuigan FE, Garg G, Olmos JM, Zarrabeitia MT, Riancho JA, Ralston SH, Alonso N, Jiang X, Goltzman D, Pastinen T, Grundberg E, Gauguier D, Orwoll ES, Karasik D, Davey-Smith G, Smith AV, Siggeirsdottir K, Harris TB, Zillikens MC, van Meurs JB, Thorsteinsdottir U, Maurano MT, Timpson NJ, Soranzo N, Durbin R, Wilson SG, Ntzani EE, Brown MA, Stefansson K, Hinds DA, Spector T, Cupples LA, Ohlsson C, Greenwood CM, Jackson RD, Rowe DW, Loomis CA, Evans DM, Ackert-Bicknell CL, Joyner AL, Duncan EL, Kiel DP, Rivadeneira F, Richards JB. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015;526(7571):112–7.
Morris JA, Kemp JP. An atlas of genetic influences on osteoporosis in humans and mice. Nat Genet. 2019;51(2):258–66.
Tang Y. Absence of causal association between Vitamin D and bone mineral density across the lifespan: a Mendelian randomization study. Nat Genet. 2022;12(1):10408.
Sun BB, Maranville JC, Peters JE, Stacey D, Staley JR, Blackshaw J, Burgess S, Jiang T, Paige E, Surendran P, Oliver-Williams C, Kamat MA, Prins BP, Wilcox SK, Zimmerman ES, Chi A, Bansal N, Spain SL, Wood AM, Morrell NW, Bradley JR, Janjic N, Roberts DJ, Ouwehand WH, Todd JA, Soranzo N, Suhre K, Paul DS, Fox CS, Plenge RM, Danesh J, Runz H, Butterworth AS. Genomic atlas of the human plasma proteome. Sci Rep. 2018;558(7708):73–9.
Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23(R1):R89-98.
Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res. 2007;16(4):309–30.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65.
Holmes MV, Ala-Korpela M, Smith GD. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat Rev Cardiol. 2017;14(10):577–90.
Hemani G, Zheng J. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7: e34408.
Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89.
Hocking LJ, Whitehouse C, Helfrich MH. Autophagy: a new player in skeletal maintenance? J Immunol Res. 2012;27(7):1439–47.
Zhang L, Guo YF, Liu YZ, Liu YJ, Xiong DH, Liu XG, Wang L, Yang TL, Lei SF, Guo Y, Yan H, Pei YF, Zhang F, Papasian CJ, Recker RR, Deng HW. Pathway-based genome-wide association analysis identified the importance of regulation-of-autophagy pathway for ultradistal radius BMD. J Bone Miner Res. 2010;25(7):1572–80.
Sugatani T, Hruska KA. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J Biol Chem. 2005;280(5):3583–9.
Darcy A, Meltzer M, Miller J, Lee S, Chappell S, Ver Donck K, Montano M. A novel library screen identifies immunosuppressors that promote osteoblast differentiation. Bone. 2012;50(6):1294–303.
Manolagas SC, Parfitt AM. What old means to bone. Trends Endocrinol Metab. 2010;21(6):369–74.
Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol CB. 1997;7(4):261–9.
Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37(Pt 1):217–22.
Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169(3):381–405.
Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13(2):132–41.
Wen HY, Wang J. Low-dose sirolimus immunoregulation therapy in patients with active rheumatoid arthritis: a 24-week follow-up of the randomized, open-label, parallel-controlled trial. J Immunol Res. 2019;2019:7684352.
Beck N. Validation of modifications to the Soleris® E. coli method for detection and threshold determination of Escherichia coli in select foods: level 3 modification to AOAC performance tested method SM 101101. Nat Commun. 2022;105(2):483–91.
Tobeiha M, Moghadasian MH, Amin N, Jafarnejad S. RANKL/RANK/OPG pathway: a mechanism involved in exercise-induced bone remodeling. J Immunol Res. 2020;2020:6910312.
Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009;5(12):667–76.
Hsu H, Lacey DL, Dunstan CR, Solovyev I, Colombero A, Timms E, Tan HL, Elliott G, Kelley MJ, Sarosi I, Wang L, Xia XZ, Elliott R, Chiu L, Black T, Scully S, Capparelli C, Morony S, Shimamoto G, Bass MB, Boyle WJ. Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Biomed Res Int. 1999;96(7):3540–5.
Takayanagi H, Sato K, Takaoka A, Taniguchi T. Interplay between interferon and other cytokine systems in bone metabolism. Immunol Rev. 2005;208:181–93.
Ono T, Takayanagi H. Osteoimmunology in bone fracture healing. Curr Osteoporos Rep. 2017;15(4):367–75.
Okamoto K, Nakashima T, Shinohara M, Negishi-Koga T, Komatsu N, Terashima A, Sawa S, Nitta T, Takayanagi H. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol Rev. 2017;97(4):1295–349.
Zaiss MM, Axmann R, Zwerina J, Polzer K, Gückel E, Skapenko A, Schulze-Koops H, Horwood N, Cope A, Schett G. Treg cells suppress osteoclast formation: a new link between the immune system and bone. Arthritis Rheum. 2007;56(12):4104–12.
Runyan CE, Liu Z, Schnaper HW. Phosphatidylinositol 3-kinase and Rab5 GTPase inversely regulate the Smad anchor for receptor activation (SARA) protein independently of transforming growth factor-β1. J Biol Chem. 2012;287(43):35815–24.
Blagden SP, Willis AE. The biological and therapeutic relevance of mRNA translation in cancer. Nat Rev Clin Oncol. 2011;8(5):280–91.
Iezaki T, Horie T, Fukasawa K, Kitabatake M, Nakamura Y, Park G, Onishi Y, Ozaki K, Kanayama T, Hiraiwa M, Kitaguchi Y, Kaneda K, Manabe T, Ishigaki Y, Ohno M, Hinoi E. Translational control of Sox9 RNA by mTORC1 contributes to skeletogenesis. Stem Cell Rep. 2018;11(1):228–41.
Li S, Fu J, Lu C, Mapara MY, Raza S, Hengst U, Lentzsch S. Elevated translation initiation factor eIF4E is an attractive therapeutic target in multiple myeloma. J Exp Clin Cancer Res CR. 2016;15(4):711–9.
Zuo D, Shogren KL, Zang J, Jewison DE, Waletzki BE, Miller AL 2nd, Okuno SH, Cai Z, Yaszemski MJ, Maran A. Inhibition of STAT3 blocks protein synthesis and tumor metastasis in osteosarcoma cells. J Exp Clin Cancer Res. 2018;37(1):244.
Volta V, Pérez-Baos S. A DAP5/eIF3d alternate mRNA translation mechanism promotes differentiation and immune suppression by human regulatory T cells. Nat Commun. 2021;12(1):6979.
Cheng T, Zhang SX, Wang J, Qiao J, Chang MJ, Niu HQ, Liu GY, Li XF. Abnormalities of peripheral lymphocyte subsets in rheumatoid arthritis patients complicated with osteoporosis. Rheumatol Ther. 2022;9(4):1049–59.
Acknowledgements
We are grateful to the GEFOS for BMD GWAS summary statistics and the proteomics-GWAS INTERVAL study for releasing the mTOR-dependent circulating proteins summary statistics.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82001740), the Natural Science Foundation of Shanxi Province (No. 202203021221269), and the Graduate Education Innovation Program of Shanxi Province (2021Y357). The journal’s Rapid Service Fee was funded by the authors.
Author information
Authors and Affiliations
Contributions
Ting Cheng and Yao-Chen Zhang: Methodology, Software, Writing-Original Draft; Ke-Yi Fan: Investigation; Jing-Xi Hu and Qian Wang: Visualization; Qi Wang, Liu Liu, He-Yi Zhang, Yao-Pu Hou: Data Curation; Xiao-Feng Li: Conceptualization; Sheng-Xiao Zhang: Supervision, Writing- Review & Editing. This article is authored by individuals who meet the International Committee of Medical Journal Editors (ICMJE) criteria, take responsibility for the authenticity of the work, and have provided their consent for the publication of this version.
Corresponding author
Ethics declarations
Conflict of Interest
Ting Cheng, Yao-Chen Zhang, Ke-Yi Fan, Jing-Xi Hu, Qian Wang, Qi Wang, Liu Liu, He-Yi Zhang, Yao-Pu Hou, Xiao-Feng Li, Sheng-Xiao Zhang have no conflicts of interest to disclose.
Ethical Approval
The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Patient consent for this study was not necessary because the study was non-interventional and data were de-identified.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, T., Zhang, YC., Fan, KY. et al. Genetic Evidence Supporting a Causal Association Between mTOR-Dependent EIF-4E Circulating Protein Level and Osteoporosis. Adv Ther 40, 4987–4998 (2023). https://doi.org/10.1007/s12325-023-02676-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02676-x