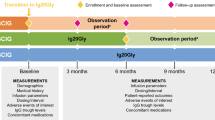
Abstract
Introduction
The CORE study aimed to provide a detailed understanding of real-world immune globulin subcutaneous (human) 20% solution (Ig20Gly) utilization in patients with primary immunodeficiency diseases (PIDs) in Germany and Switzerland.
Methods
Patients with PIDs receiving a stable dose of any subcutaneous immunoglobulin for ≥ 3 months before enrollment were eligible for this multicenter (n = 5), phase 4, non-interventional, prospective, longitudinal cohort study. Besides baseline demographics and clinical characteristics, Ig20Gly utilization and safety data, and patient-reported outcomes (Life Quality Index/Treatment Satisfaction Questionnaire for Medication) were collected at baseline, 6 and 12 months. Statistical analysis was descriptive.
Results
Overall, 36 patients provided data at baseline [69.4% female; mean age: 41.6 years (7–78 years)]. Totals of 23 and 26 patients attended 6- and 12-month visits, respectively; 16 attended all three visits. One patient withdrew consent before 6-month follow-up. Median maximum infusion rates of Ig20Gly at baseline, 6 months, and 12 months were 26.7, 24.5, and 40.0 mL/h, respectively (10–60 mL/h). Infusion and dosing parameters remained consistent across time points: patients used a median of two infusion sites, primarily the abdomen, and all patients used an infusion pump; all but one infused at home and most self-administered Ig20Gly (80.8–83.3%) at once-weekly intervals (69.2–73.9%). During follow-up, 10 adverse events were reported: none were rated serious, while 2 were considered probably related to Ig20Gly. Total patient-reported outcome scores remained high throughout the study.
Conclusion
The CORE study provides real-world evidence of the flexibility, feasibility, safety, and tolerability of Ig20Gly infusions, at mostly weekly intervals, over 1 year in patients with PIDs.
Trial Registration
German Clinical Trials Register, DRKS00014562. Registered April 9, 2018, https://drks.de/search/en/trial/DRKS00014562
Plain Language Summary
Primary immunodeficiency diseases are rare diseases that make patients more likely to develop infections than the general population. Many patients with primary immunodeficiency diseases do not produce enough antibodies, which are an important part of the immune system that fight infection. Replacing antibodies is the main way to treat primary immunodeficiency diseases and reduce the risk of infection. Ig20Gly is a type of medication used to replace antibodies and treat primary immunodeficiency diseases. Patients receive Ig20Gly through a needle inserted under the skin and can learn to do this themselves at home. Ig20Gly can be delivered more quickly than other antibody treatments that are less concentrated. CORE was a study of 36 patients (children and adults) taking Ig20Gly for primary immunodeficiency diseases for 1 year in Germany and Switzerland. The aim of the study was to understand how patients use and experience Ig20Gly as part of their normal treatment. In this study, nearly all patients received Ig20Gly treatment at home, and most patients gave Ig20Gly to themselves once a week. A few patients developed serious bacterial infections while being treated with Ig20Gly, and patients were generally satisfied with the treatment. Overall, the CORE study describes how patients with primary immunodeficiency diseases use Ig20Gly in their daily lives, and shows that Ig20Gly treatment can be tailored to suit each patient’s needs. Information from this study will help doctors to support patients in making decisions about their treatment.
AbstractSection Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Patients with primary immunodeficiency diseases (PIDs), also referred to as inborn errors of immunity, often require lifelong immunoglobulin replacement therapy. |
Real-world insights into the use of immune globulin subcutaneous (human) 20% solution (Ig20Gly) are needed to understand the patient experience, how the treatment is used, and to supplement clinical trial data. |
The primary objective of the CORE study was to collect data on real-world patterns of Ig20Gly administration in a broad sample of patients with PIDs in Germany and Switzerland. |
Almost all patients received their infusion at home, enabling independent infusions and providing a high level of treatment satisfaction and quality of life to patients receiving Ig20Gly. |
The study results provide real-world evidence of the flexibility, feasibility, safety, and tolerability of Ig20Gly infusions, at mostly weekly intervals, over 1 year in patients with PIDs, and supplement our understanding of how patients use this treatment to match their clinical and daily-life needs. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.23932341.
Introduction
Primary immunodeficiency diseases (PIDs), also referred to as inborn errors of immunity [1], are a group of diseases that arise from germline variants and affect the functioning of the immune system [2]. Diseases involving antibody disorders made up more than 57% of PIDs in a German national registry study (2012–2017) [3] and 62% of PIDs in a Swiss national registry study (2008–2014) [4]; these patients can be treated with immunoglobulin replacement therapy (IgRT) to reduce their susceptibility to infection. Patients with PIDs often require lifelong IgRT, and therefore administration setting, infusion parameters, and tolerability are important considerations. Compared with intravenous immunoglobulin (IVIG), subcutaneous immunoglobulin (SCIG) gives patients the flexibility to self-administer their IgRT at home [5]. However, as subcutaneous tissues are unable to absorb the large volumes of SCIG required, treatment can be burdensome as patients require frequent infusions of smaller volumes at multiple sites [5].
Immune globulin subcutaneous (human) 20% solution [Ig20Gly (Cuvitru; Baxalta US, a member of the Takeda group of companies, Lexington, MA, USA)], indicated for the treatment of PIDs in adults and children (aged 2 years and older in the USA, and of any age in the EU) is a concentrated immunoglobulin G (IgG) formulation that allows for smaller infusion volumes and higher infusion rates compared with less concentrated SCIG products [6,7,8,9]. Ig20Gly offers patients multiple administration options, so that they can customize their immunoglobulin treatment to fit their needs; these include varying the volume per site, dosing frequency, and infusion rate [6, 7]. Two pivotal phase 2/3 clinical trials in adult and pediatric immunoglobulin-experienced patients with PIDs in Europe and North America (US and Canada) (NCT01412385, NCT01218438) demonstrated the safety and efficacy of Ig20Gly, with median maximum infusion rates of up to 60 mL/h/site [8, 9]. Real-world evidence studies from North America (HelloCuvitru [10], CANCUN [11], REToUCh [12]) also tended to show similar average infusion times (≤ 1 h) and average number of sites per infusion (two) to the pivotal clinical trials.
To continue building on the real-world evidence base for Ig20Gly use, the primary objective of the CORE study was to collect data on real-world patterns of Ig20Gly administration in patients with PIDs. This study also aimed to assess patterns of use in patients switching from other SCIG therapies to Ig20Gly and the frequency of adverse events, patient experience and satisfaction, use of healthcare resources, work productivity, and activity impairment with Ig20Gly.
Methods
Patients and Study Design
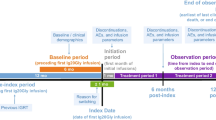
The CORE study was a phase 4, non-interventional, prospective, longitudinal cohort study (German Clinical Trials Register: DRKS00014562). It was conducted at five sites with substantial experience in IgG treatment (two in Germany and three in Switzerland) between November 27, 2018 and November 30, 2021.
Patients of any age (including those aged < 1 year) were eligible for the study if they had received a diagnosis of a primary immunodeficiency that involved a defect in antibody formation and required IgRT. All patients received a stable dose of any SCIG for at least 3 months prior to enrollment. Patients received Ig20Gly in line with the product specification at the time of first documentation and were expected to continue the therapy in future. Adult patients were recruited and treated at Swiss sites, while German sites recruited and treated both adult and pediatric patients. Patients aged < 18 years were treated by pediatric immunologists. Patients, or their legally authorized representative, provided informed written consent and were likely and willing to be available for follow-up. Patients were ineligible for the study if the dose of SCIG they were receiving changed in the 3 months prior to enrollment or if they were participating in another clinical study during the observation period.
In a deviation from the protocol-defined inclusion criteria, patients who received IVIG treatment or no SCIG therapy prior to enrollment were included in the overall study population (all patients in the study) to improve the sample size for analysis. Data were also evaluated for the ‘per protocol’ population, which included only patients who received a SCIG product other than Ig20Gly prior to enrollment; patients who received Ig20Gly, IVIG treatment, or no SCIG therapy prior to enrollment were excluded from this population.
Assessments
Data were collected at baseline (the first study visit) and 6 and 12 months after the first visit. Data for individual patients were extracted from their medical records by the investigator or study nurse. Patients documented the details of their infusions, concomitant medications, and any infections they experienced. If they consented, patients or their legally authorized representative received the Life Quality Index (LQI) questionnaire and the 9-item Treatment Satisfaction Questionnaire for Medication (TSQM-9) to complete at each visit. All data were extracted into an online data collection form at regular intervals, as determined by the documenting physician, from the patients’ medical records and questionnaires.
At baseline, patient characteristics, concomitant diseases, concomitant medications, and SCIG treatment history were recorded. At baseline, 6-month, and 12-month follow-up visits, data on Ig20Gly dosing, administration and infusion characteristics, IgG levels, adverse events (up to 3 weeks after the last infusion), tolerability, infections (including acute serious bacterial infections defined as sepsis, bacterial pneumonia, bacterial meningitis, osteomyelitis/arthritis, and visceral abscess caused by a recognized bacterial pathogen), patient-reported outcomes (PROs; as measured using the LQI and TSQM-9), and healthcare resource utilization were collected. The LQI [13, 14] consists of four domains, each scored out of 100 (higher scores indicate better quality of life): treatment interference, therapy-related problems, therapy setting, and treatment costs. The TSQM-9 [15, 16] consists of three domains, each scored out of 100 (higher scores indicate better satisfaction): effectiveness, convenience, and global satisfaction.
The primary outcome measure was the maximum infusion rate of Ig20Gly; all other assessments were secondary outcome measures.
Statistical Analysis
No formal sample size calculation was performed, but the initial plan was to enroll 150 patients from up to 30 sites in Germany and Switzerland. Owing to recruitment difficulties, partly associated with the COVID-19 pandemic, enrollment was stopped at 36 patients. Statistical evaluation was descriptive and performed on the total patient cohort; no statistical hypothesis was tested. The number of missing values was recorded for each parameter. No missing values were imputed, except for incomplete calendar dates (i.e., if day and/or month were missing) that were required to calculate durations.
Ethics and Study Conduct
This study was conducted in accordance with the International Council for Harmonization Good Clinical Practice, the Declaration of Helsinki 1964, and national and local laws and regulations. Ethics approval was given by the ethics committee of Saxony Physician Chamber and Swissethics (EK-BR-12/18-1). The study is registered in the German Clinical Trials Register under the identifier DRKS00014562. All patients or their legally authorized representative provided written informed consent to participate and consent for publication prior to study enrollment.
Results
Patients
Patient disposition data are presented in Fig. 1. In the overall study population, 36 patients were enrolled and provided data at baseline, 23 patients were present at a 6-month follow-up visit, 26 patients attended a 12-month follow-up visit, and 16 patients attended all three visits. One patient withdrew consent before the 6-month follow-up visit. No patients discontinued owing to adverse events, death, pregnancy, physician decision, or loss to follow-up. The median (range) observation period was 12.9 (7.3–13.5) months. In the ‘per protocol’ population, 18, 16, and 12 patients provided data at baseline, 6 months, and 12 months, respectively.
Patient demographics, clinical characteristics, and SCIG treatment history are presented in Table 1. In the overall study population, mean (range) patient age was 41.6 (7–78) years, seven patients (19.4%) were aged < 18 years, and most patients were female (69.4%). Twelve women (33.3% of all patients) were of childbearing age (15–49 years). Patients had a diagnosis of PIDs for a mean [standard deviation (SD)] of 8.9 (7.3) years. Common variable immunodeficiency (CVID) represented the most common type of PID in this patient population (58.3%). Patients most commonly received immune globulin subcutaneous (human) 16% solution (SCIG 16% [Subcuvia; Baxalta Innovations, Vienna, Austria]) prior to starting Ig20Gly (44.4%). The median (range) maximum infusion rate of previous SCIG therapy was 20 (10–60) mL/h. Reasons for discontinuing any prior SCIG therapy included patient request (16.7%) and tolerability issues (5.6%). Patient demographics, clinical characteristics, and SCIG treatment history presented by country are shown in Supplementary Material Table S1. Baseline characteristics were broadly similar in the ‘per protocol’ population (n = 18); in this population, the mean age (range) was 40.8 (9.0–78.0) years and most patients were female (66.7%). CVID was the most common PID diagnosis (55.6%) and most patients had received SCIG 16% prior to enrollment (83.3%).
Ig20Gly Infusion and Dosing Parameters
Infusion and dosing parameters of patients’ most recent Ig20Gly infusion were recorded at each time point and are presented in Table 2 (overall population). In the overall study population, median maximum infusion rates at baseline, 6 months, and 12 months were 26.7, 24.5, and 40.0 mL/h, respectively (10–60 mL/h at all time points). Other infusion and dosing parameters remained broadly similar at all time points. The dose of Ig20Gly at the most recent infusion was 7 g at baseline and 8 g at 6- and 12-month follow-up visits (2–49 g across all time points). The median monthly dose of Ig20Gly at the most recent infusion was 0.4 g/kg at baseline and 0.5 g/kg at 6- and 12-month follow-up visits (range: 0.1–1.4 g/kg). At all time points, patients used between one and three infusion sites (median of two sites), and most commonly infused into the abdomen. Median infusion duration was 60 min (20–150 min). All patients used an infusion pump. Infusion and dosing parameters were broadly similar in the ‘per protocol’ population, except for a lower median maximum infusion rate at 12 months [25.0 (10–60) mL/h] and lower median total volumes at baseline and 12 months (30 and 27.5 mL, respectively) compared with the full patient population (40 and 35 mL, respectively) (Table 3).
Ig20Gly Administration Characteristics
Administration characteristics of patients’ most recent Ig20Gly infusion were recorded at each time point and are presented in Fig. 2. In the overall study population, all but one patient (at baseline) infused at home. More than 80% of patients at each time point administered the infusion themselves. All patients whose infusion was administered by a parent or caregiver were aged < 18 years. The patients whose infusions were administered by a nurse were aged ≥ 18 years. At all time points, most patients (69–74%) infused once weekly.
Safety and Tolerability
No adverse drug reactions were recorded at baseline or at 6 months in the overall study population. At 12 months, four adverse reactions were recorded in three patients (11.5%) during or within 72 h of the infusion (local: hematoma and redness; systemic: tiredness and chills, but no fever during the infusion). There were 10 spontaneous adverse events in eight patients reported at or between visits. None were rated serious by the investigator. Two adverse events were considered by the investigator to be probably related (subcutaneous nodules), two possibly related (shivering and fatigue), and six not related to the study medication.
Infections
At baseline in the overall study population, 15 patients (41.7%) reported at least one bacterial infection (other than acute severe bacterial infections) in the past 12 months; at both 6- and 12-month visits, seven patients (30.4% and 26.9% of patients respectively) reported at least one bacterial infection since the last documented visit, of whom six (26.1%) and five (19.2%) patients, respectively, required antibiotic treatment. At 6 months, one patient was hospitalized for infection for 2 days; no patients were hospitalized for infection at 12 months. Acute severe bacterial infections were reported in three patients (8.3%) at baseline, and acute serious bacterial infection was reported in one patient (3.8%) at 12 months; no acute serious bacterial infections were reported at 6 months.
Healthcare Resource Utilization
Patients in the overall study population reported up to four training sessions at baseline to learn how to administer Ig20Gly (median: one session). A median of one patient visited the physician’s office at 6 months (0–3 visits) and 12 months (0–30 visits). At baseline, 14 patients required a nurse visit to their home (1–52 visits); there were no nurse visits at 6 months and one patient required one visit at 12 months. There was a median of 7 days missed in daycare, school, or work due to sickness at baseline (0–365 days), and a median of 0 sickness days at 6 months (0–10 days) and 12 months (0–100 days). Days of hospitalization for PIDs ranged from 0 to 4 (median: 0 days) at 6 months; no patients were hospitalized for PIDs at 12 months.
Patient-Reported Outcomes
In the overall study population, TSQM-9 and LQI total scores remained high throughout the study (Fig. 3). At baseline, 6 months, and 12 months, mean (SD) total LQI scores were 82.7 (8.5), 80.8 (8.8), and 84.1 (9.5), respectively. At baseline, 6 months, and 12 months, mean (SD) total TSQM-9 scores were 78.9 (12.1), 76.7 (12.1), and 83.0 (12.2), respectively. At all time points, the LQI component with the highest mean scores was therapy setting (91.8–94.3), and treatment-related costs was the component with the lowest mean scores (67.4–71.0). Global satisfaction was the TSQM-9 component with the highest mean scores at all time points (83.9–88.9); the lowest mean scores were for convenience at baseline (73.8) and for effectiveness at 6 and 12 months (70.0 and 79.7, respectively).
Discussion
The CORE study provides insight into the real-world use of Ig20Gly in a broad sample of patients with PIDs in Germany and Switzerland. Given the often lifelong requirement for IgG therapy in patients with PIDs, real-world insights into Ig20Gly use are needed to understand the patient experience, how the treatment is used, and to supplement clinical trial data. The primary outcome measure was the median maximum infusion rate, which increased from 26.7 and 24.5 mL/h at baseline and 6 months, respectively, to 40.0 mL/h at 12 months in the overall study population.
The maximum infusion rates in the CORE study fall within the range of values reported in the literature: a pivotal European study reported a lower median rate (20 mL/h; [8]), and a pivotal US study and two real-world evidence studies [HelloCuvitru (retrospective, observational study of adult and pediatric patients) and CANCUN (non-interventional, prospective study of patients with a diagnosis of PIDs or secondary immunodeficiency diseases)], reported higher median rates (60, 47, and 40–51 mL/h, respectively [9,10,11]). The HelloCuvitru study also reported a similar median monthly dose to the current study: 0.55 g/kg [10] compared with 0.4–0.5 g/kg. The remaining infusion parameters reported in the CORE study were mostly consistent over time, with the majority of patients receiving infusions at two sites, primarily the abdomen. They were also generally similar to those seen in previous real-world studies in North America [10,11,12], including the number and location of sites and infusion duration.
Ig20Gly also allowed for variation in administration, with almost all patients receiving their infusion at home by self-administration or administered by a parent/caregiver, similar to the REToUCh study in which most patients infused at home [12]. All patients whose infusion was administered by a parent/caregiver were children. Patients were also most likely to infuse once weekly in the current study, similar to previous real-world evidence studies [10, 11].
Adverse drug reactions in the CORE study were generally minor and did not lead to discontinuation of treatment. The overall tolerability profile was consistent with that seen in the clinical trial setting [8, 9], and no serious adverse events were reported. Post hoc analysis from the North American clinical trial also found that higher infusion rates were not associated with increased incidence of adverse events [17].
Healthcare resources, including nurse and physician visits, were rarely used, and acute serious bacterial infections and hospitalizations due to infection were rare. This may reflect the study inclusion criteria given that most patients were stable on another SCIG product at baseline. The training sessions provided at baseline may also have limited the need for healthcare resource utilization at 6 and 12 months. The rarity of acute serious bacterial infections is similar to results seen in both the European and North American Ig20Gly clinical trials [8, 9].
TSQM-9 and LQI total scores remained high throughout the study, indicating maintenance of quality of life and treatment satisfaction with Ig20Gly, and the lowest mean component scores at each time point were still high (mean scores ≥ 67/100). These results are consistent with those in previous studies. The CANCUN study found that patients were satisfied with Ig20Gly, and that all patients were interested in continuing the treatment [11]. The European pivotal clinical trial found that more than 80% of patients wanted to continue using Ig20Gly and liked the ease of administration and the convenience [8]. It should be noted that treatment-related costs, the lowest-scoring component of the LQI at all time points, is unlikely to play an important role for patients in Germany and Switzerland where co-payment is usually low, and patients do not need to negotiate with their (statutory) insurance company.
Strengths of the CORE study include that it was prospective and had broad eligibility criteria, which ensures good applicability of the results. Our findings also complement the pivotal North American and European clinical trial data [8, 9], including the flexibility in dosing and administration. Almost all patients received their infusion at home, enabling independent infusions and providing a high level of treatment satisfaction and quality of life to patients receiving Ig20Gly.
One limitation of this study is that it took place during the COVID-19 pandemic; this created challenges for recruitment and meant that some patients did not fulfil the inclusion criteria for SCIG treatment history. However, it should be noted that baseline characteristics and infusion and dosing characteristics in the ‘per protocol’ population were broadly similar to those in the overall study population for most parameters. In addition to the impact of the COVID-19 pandemic, recruitment may have been challenging owing to a delayed start to the study, limited availability of some immunoglobulin medications, and the high documentation requirement for sites. Some patients may have been reluctant to switch to a new immunoglobulin product when they experienced good tolerability and efficacy with their current therapy. Recruitment may also have been affected by a reluctance to switch from IVIG to SCIG owing to the feeling of security from regular visits to healthcare services. In Switzerland, switching to SCIG administration requires additional cost authorization from the health insurance company for both the immunoglobulin product and for the pump and equipment needed for SCIG administration. In addition, Switzerland also has a relatively high density of hospitals and thus short travel distances, which can make it more convenient/appealing for patients to travel to hospital for an infusion once a month compared with administering the treatment themselves at home on a weekly basis. Another limitation was that there were some missing data in the study, but not at higher levels than expected. Missing data may have been related to the COVID-19 pandemic during which observational research was a lower priority. Furthermore, patients did not always complete documentation as intended, which may have been improved through the use of patient diaries. Generalization to the wider population is limited owing to the small number of patients, the number of observations over 12 months (including missing data for several parameters), and the restriction to five sites. There may also have been selection bias in the physicians who chose to take part and patients selected by those physicians for SCIG therapy.
Conclusions
These data provide real-world evidence concerning the flexibility, feasibility, and tolerability of Ig20Gly infused via pump, mostly in weekly intervals over 1 year in pediatric and adult patients with PIDs. These findings, including the low rates of acute serious bacterial infections, are generally consistent with other clinical and real-world evidence studies of Ig20Gly to date, and supplement our understanding of how patients use this treatment to match their clinical and daily-life needs.
Data Availability
The data sets, including the redacted study protocol, redacted statistical analysis plan, and individual participant data supporting the conclusions of this article will be made available within 3 months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
References
Yamashita M, Inoue K, Okano T, Morio T. Inborn errors of immunity-recent advances in research on the pathogenesis. Inflamm Regen. 2021;41(1):9.
Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, et al. Human inborn errors of immunity: 2022 update on the classification from the International Union of Immunological Societies expert committee. J Clin Immunol. 2022;42(7):1473–507.
El-Helou SM, Biegner AK, Bode S, Ehl SR, Heeg M, Maccari ME, et al. The German national registry of primary immunodeficiencies (2012–2017). Front Immunol. 2019;10:1272.
Marschall K, Hoernes M, Bitzenhofer-Gruber M, Jandus P, Duppenthaler A, Wuillemin WA, et al. The Swiss National Registry for Primary Immunodeficiencies: report on the first 6 years’ activity from 2008 to 2014. Clin Exp Immunol. 2015;182(1):45–50.
Misbah S, Sturzenegger MH, Borte M, Shapiro RS, Wasserman RL, Berger M, et al. Subcutaneous immunoglobulin: opportunities and outlook. Clin Exp Immunol. 2009;158(Suppl 1):51–9.
European Medicines Agency. Cuvitru 200 mg/ml solution for subcutaneous injection. Summary of product characteristics. 2022. Available from: https://www.medicines.org.uk/emc/product/9191/smpc. Accessed 6 Sept 2023.
United States Food and Drug Administration. Cuvitru, Immune globulin subcutaneous (human) 20% solution. Prescribing information. 2021. Available from: https://www.fda.gov/media/100531/download. Accessed 6 Sept 2023.
Borte M, Krivan G, Derfalvi B, Marodi L, Harrer T, Jolles S, et al. Efficacy, safety, tolerability and pharmacokinetics of a novel human immune globulin subcutaneous, 20%: a phase 2/3 study in Europe in patients with primary immunodeficiencies. Clin Exp Immunol. 2017;187(1):146–59.
Suez D, Stein M, Gupta S, Hussain I, Melamed I, Paris K, et al. Efficacy, safety, and pharmacokinetics of a novel human immune globulin subcutaneous, 20% in patients with primary immunodeficiency diseases in North America. J Clin Immunol. 2016;36(7):700–12.
Meckley LM, Wu Y, Tzivelekis S, Gandhi V, Gladiator A. Infusion parameters of 20% subcutaneous immunoglobulin for primary immunodeficiency diseases among patient support program participants. Ann Allergy Asthma Immunol. 2021;127(5):568–74e1.
Keith PK, Cowan J, Kanani A, Kim H, Lacuesta G, Lee JK, et al. Transitioning subcutaneous immunoglobulin 20% therapies in patients with primary and secondary immunodeficiencies: Canadian real-world study. Allergy Asthma Clin Immunol. 2022;18(1):70.
Rosenbach K, Park M, Sanchirico M, Nwose O, Paris K. Real-world evidence of tolerability of 20% subcutaneous immunoglobulin treatment. J Clin Immunol. 2023;43:912.
Daly PB, Evans JH, Kobayashi RH, Kobayashi AL, Ochs HD, Fischer SH, et al. Home-based immunoglobulin infusion therapy: quality of life and patient health perceptions. Ann Allergy. 1991;67(5):504–10.
Nicolay U, Haag S, Eichmann F, Herget S, Spruck D, Gardulf A. Measuring treatment satisfaction in patients with primary immunodeficiency diseases receiving lifelong immunoglobulin replacement therapy. Qual Life Res. 2005;14(7):1683–91.
Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12.
Bharmal M, Payne K, Atkinson MJ, Desrosiers MP, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36.
Gupta S, Stein M, Hussain I, Paris K, Engl W, McCoy B, et al. Tolerability of Ig20Gly during onboarding in patients with primary immunodeficiency diseases. Ann Allergy Asthma Immunol. 2019;123(3):271–9e1.
European Medicines Agency. Hizentra 200 mg/ml solution for subcutaneous injection. Summary of product characteristics. 2022. Available from: https://www.medicines.org.uk/emc/product/4643/smpc. Accessed 6 Sept 2023.
United States Food and Drug Administration. Immune globulin subcutaneous (human) (IGSC), 20% liquid, Hizentra. Prescribing information. 2021. Available from: https://www.fda.gov/media/78466/download. Accessed 6 Sept 2023.
Acknowledgments
The authors thank the patients who participated in this study, their caregivers, study-site personnel, and the investigators. The authors thank Dr Thomas Hauser and collaborators at IZZ Immunologie-Zentrum Zürich, Zurich, Switzerland, for their valued contribution to the study.
Medical Writing/Editorial Assistance
Medical writing services were provided by Rebecca Prince, BMBS, MSc, of Oxford PharmaGenesis Ltd, Oxford, UK, and funded by Takeda Development Center Americas, Inc. and Takeda Pharmaceuticals International AG.
Funding
Baxalta Innovations GmbH, a Takeda company, funded this study. GWT-TUD GmbH, Dresden, was the legal sponsor of this study. Takeda Pharmaceuticals International AG funded the journal’s Rapid Service and Open Access Fees.
Author information
Authors and Affiliations
Contributions
Maria Fasshauer and Peter Jandus contributed to methodology, investigation, resources, writing—review and editing. Michael Borte and Michaela Bitzenhofer contributed to investigation, resources, writing: review and editing. Christine Pausch contributed to software, validation, formal analysis, data curation, writing—review and editing, project administration. David Pittrow contributed to conceptualization, methodology, software, validation, formal analysis, data curation, writing—review and editing, project administration. Michelle Park and André Gladiator contributed to conceptualization, methodology, writing—review and editing, supervision, funding acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
Maria Fasshauer has participated in advisory boards for Baxalta/Shire, has received honoraria for lectures from CSL Behring and Shire, and has received travel grants from Octapharma until 2018. Michael Borte’s institution has received research grant support from Baxalta, CSL Behring, and Octapharma, and he has participated in advisory boards for CSL Behring, Octapharma, and Shire. Michaela Bitzenhofer has received a grant from Takeda. Christine Pausch has nothing to disclose. David Pittrow reports personal fees from Amgen, Aspen, Bayer, Biogen, Boehringer Ingelheim, Daiichi Sankyo, Janssen, MSD, Sandoz, and Sanofi Genzyme outside the submitted work. He has acted as a consultant for Baxalta. Michelle Park is an employee of Takeda Development Center Americas, Inc. and a Takeda shareholder. André Gladiator is an employee of Takeda Pharmaceuticals International AG and a Takeda shareholder. Peter Jandus has participated in advisory boards for AstraZeneca, CSL Behring, GSK, Sanofi, and Shire, has received honoraria for lectures from CSL Behring and Shire, has received travel grants from Biotest, CSL Behring, Octapharma, and Shire, and has received research grants to his institution from AstraZeneca, CSL Behring, Novartis, and Shire.
Ethical Approval
This study was conducted in accordance with the International Council for Harmonization Good Clinical Practice, the Declaration of Helsinki 1964 and national and local laws and regulations. Ethics approval was given by the ethics committee of Saxony Physician Chamber and Swissethics (EK-BR-12/18-1). The study is registered in the German Clinical Trials Register under the identifier DRKS00014562. All patients or their legally authorized representative provided written informed consent to participate and consent for publication prior to study enrollment.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fasshauer, M., Borte, M., Bitzenhofer, M. et al. Real-World Use, Safety, and Patient Experience of 20% Subcutaneous Immunoglobulin for Primary Immunodeficiency Diseases. Adv Ther 40, 5168–5187 (2023). https://doi.org/10.1007/s12325-023-02649-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02649-0