Abstract
Introduction
Patients with hypertension and additional cardiovascular risk factors pose a challenge by requiring more intensive blood pressure (BP) control. Single-pill combination (SPC) therapy can benefit these patients by improving medication adherence.
Methods
This prospective, multicenter observational study assessed the real-world safety and effectiveness of an SPC containing olmesartan, amlodipine, and hydrochlorothiazide (O/A/H) in South Korean patients with hypertension and cardiovascular risk factors. BP control rates, defined as the percentage of patients achieving systolic BP (SBP) < 130 mmHg and diastolic BP (DBP) < 80 mmHg for intensive BP control, and < 140 mmHg and < 90 mmHg, respectively, for standard BP control, were investigated across various cardiovascular risk groups, along with changes in SBP and DBP from baseline to week 24.
Results
The most prevalent cardiovascular risk factor was age (≥ 45 years in men, ≥ 55 years in women, 86.1%), followed by cardiovascular diseases (64.4%), dyslipidemia (53.7%), body mass index ≥ 25 kg/m2 (53.5%), and diabetes mellitus (DM) (46.3%). Switching to O/A/H showed significant BP reduction, with a mean change of − 17.8 mmHg/− 9.3 mmHg in SBP/DBP within 4 weeks. The intensive BP control rate was 41.4% (95% confidence interval [CI] 39.5, 43.4), and the standard BP control rate was 73.3% (95% CI 71.5, 75.1), with better control rates in the risk age group (43.1% and 74.1%, respectively) and cardiovascular disease group (42.0% and 73.8%, respectively). The DM group had relatively lower control rates (37.5% for intensive control and 69.4% for standard control). Common adverse drug reactions included dizziness (2.91%), hypotension (1.51%), and headaches (0.70%).
Conclusion
The SPC therapy of O/A/H caused a rapid and sustained reduction in SBP/DBP in patients’ hypertension and additional cardiovascular risk factors. The therapy was safe and well tolerated.
Study Registration Number
KCT0003401 (https://cris.nih.go.kr/cris/search/detailSearch.do/20795).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Blood pressure (BP) control remains a challenge in some patients with hypertension, particularly in those with cardiovascular risk factors, who require more intensive BP control. |
Single-pill combination (SPC) therapy has been proposed as a solution to improve poor medication adherence, which contributes to inadequate BP control, especially in patients taking multiple antihypertensive drugs. |
This prospective, multicenter observational study assessed the real-world safety and effectiveness of an SPC containing olmesartan, amlodipine, and hydrochlorothiazide (O/A/H), in South Korean patients with hypertension and cardiovascular risk factors. |
What was learned from the study? |
After switching to O/A/H, a mean reduction of − 17.8 mmHg/− 9.3 mmHg in systolic BP/diastolic BP was observed at 24 weeks (P < 0.05). The intensive BP control rate was 41.4% and the standard BP control rate was 73.3%. Intensive and standard BP control rates were relatively higher in the risk age group (43.1% and 74.1%, respectively) and cardiovascular disease group (42.0% and 73.8%, respectively) and relatively lower in the diabetes mellitus group (37.5% and 69.4%, respectively). |
Patients with hypertension and additional cardiovascular risk factors benefit from O/A/H treatment. More intensive BP control is needed in patients with hypertension and diabetes mellitus, in line with the new treatment guidelines. |
Among patients without prior calcium channel blockers (CCB) use (535 patients), the incidence of edema with O/A/H was 1.1%. The lower edema incidence in the present study may be attributed to excluding patients with a history of edema while using CCB before switching to O/A/H. Additionally, the concurrent administration of hydrochlorothiazide, which has diuretic properties, might have contributed to the lower edema incidence observed in this study. |
Introduction
Hypertension is a significant global health burden, affecting approximately 1.4 billion individuals worldwide and approximately 11 million cases in Korea alone [1, 2]. Although the prevalence of elevated blood pressure (BP) has been decreasing in recent decades, BP control among patients with hypertension remains poor, with fewer than 50% of them achieving target BP level [3, 4]. Poor medication adherence, especially in patients prescribed multiple antihypertensive drugs, is one of the reasons for the lack of adequate BP control [2, 3, 5].
Single-pill combination (SPC) therapy has been proposed as a solution to improve medication adherence by simplifying multidrug regimens. Numerous studies have demonstrated that SPC therapy improves medication adherence, leading to improved clinical outcomes in terms of BP control. Several meta-analyses and observational studies have shown the association between SPC therapy and a significant reduction in both systolic BP (SBP) and diastolic BP (DBP), with a higher percentage of patients achieving their target BP by improving adherence and persistence [6,7,8,9]. Based on these findings, recent guidelines recommend using SPC therapy as a first-line treatment in high-risk patients to achieve immediate BP response, improve tolerability, and increase medication adherence [5, 10, 11].
One such SPC therapy available worldwide is the triple combination of olmesartan, amlodipine, and hydrochlorothiazide (O/A/H), whose safety and efficacy have been demonstrated in numerous studies [12,13,14,15]. In the pivotal trial (TRINITY), O/A/H showed a greater least-square mean reduction in seated BP compared with the dual combination SPC in European patients with uncontrolled hypertension (SBP − 37.1 mmHg [O/A/H] vs. − 30.0 to − 27.5 mmHg [dual combination SPC], P < 0.01; DBP − 21.8 mmHg vs. − 18.0 to − 15.1 mmHg, P < 0.01) [12]. Recently, the RESOLVE and RESOLVE-PRO studies have provided real-world evidence of the safety and effectiveness of O/A/H in Asian patients with hypertension, demonstrating significant reductions in mean BP compared to baseline [16, 17].
Although the safety and efficacy (effectiveness) of O/A/H in the general population of patients with hypertension have been established, little is known about its effects on patients with hypertension and cardiovascular risk factors. Over 80% of patients with hypertension have at least one additional cardiovascular risk factor, with approximately half of them having more than two factors [18]. Notably, such patients require more intensive BP control than the general population of patients with hypertension to reduce the risk of cardiovascular disease (CVD) [19]. Confirming the safety and efficacy (effectiveness) of O/A/H in patients with hypertension and additional cardiovascular risk factors would allow these patients to benefit from SPC therapy. Therefore, we conducted a prospective, multicenter observational study to observe the safety and effectiveness of O/A/H in South Korean patients with hypertension and additional cardiovascular risk factors.
Methods
Study Population
The study included adult individuals (≥ 19 years old) diagnosed with hypertension, for whom O/A/H was indicated under routine clinical practice, and who had at least one of the following cardiovascular risk factors defined by the 2013 Korean Society of Hypertension (KSH) guidelines (REF): age (≥ 45 years in men, ≥ 55 years in women), smoking, diabetes mellitus (DM), dyslipidemia, chronic kidney disease (CKD), or concurrent or history of CVD. Conversely, patients were excluded from the study if they had participated in other intervention studies, as this could potentially introduce confounding bias due to restrictions on medication use and the more intensive care setting compared to routine care. Furthermore, in order to mitigate potential bias arising from carryover effects stemming from previous use of O/A/H, we excluded patients who had previously taken O/A/H.
Study Design and Procedures
This non-interventional, observational, multicenter, prospective cohort study was conducted between August 2018 and November 2020. A total of 63 investigators from 47 hospitals in Korea recruited patients and collected study-related information. Voluntary informed consent was obtained from O/A/H-naïve eligible patients. Patient demographics, medical histories, O/A/H prescription information, prior and other concurrent medications, and treatment-emergent adverse events (TEAEs) were collected from enrolled patients. Pre-specified cardiovascular risk factors were identified at baseline and subsequent follow-up visits. Baseline characteristics were assessed on the day of enrollment, and patients were followed up until week 24. Dose of O/A/H (20/5/12.5 mg, 40/5/12.5 mg, and 40/10/12.5 mg are approved doses in Korea) and the frequency of follow-up visits was not defined in the study protocol but was determined according to the routine clinical practice at each institution and the clinical judgement of the investigator. All data were de-identified to protect the privacy of participating patients and were captured in an electronic case report form. The study protocol and informed consent forms were approved by the institutional review board (IRB) of each participating institution. A list of IRB approval numbers is provided in Table S1. The study was conducted in accordance with the principles of the Declaration of Helsinki 1964 and its later amendments as well as the rules of each IRB.
Effectiveness Endpoints
The primary endpoint was the intensive BP control rate, which was based on the 2017 American College of Cardiology/American Heart Association guidelines [11] and defined as the proportion of patients with SBP < 130 mmHg and DBP < 80 mmHg at week 24. The secondary endpoint was the standard BP control rate at week 24, which was defined as the proportion of patients with SBP < 140 mmHg and DBP < 90 mmHg based on the 2013 Korean Society of Hypertension (KSH) guidelines [20] and up-to-date local guidelines at the time of preparing the study protocol. Other secondary endpoints included changes in BP over time and changes in BP at week 24 from baseline.
Safety Endpoints
TEAEs were collected and assessed to identify the safety and tolerability of O/A/Hs. TEAEs were assessed for seriousness, relationship with O/A/Hs, and expectedness. TEAEs with causalities assessed as certain, probable/likely, possible, conditional/unclassified, and unassessable/unclassifiable were considered as adverse drug reactions (ADRs). If a TEAE was not a known side effect of O/A/H, the event was classified as an unexpected TEAE, regardless of its relationship with O/A/H. Additionally, if a known adverse event listed in the product leaflet worsened in severity or recurred during the study period, the event was classified as an unexpected TEAE.
Statistical Methods
For the sample size calculation, the intensive BP control rate was assumed to be 50%, which was based on a previous study in which 55–66% of patients achieved the intensive BP target (SBP/DBP < 140/90 mmHg) at any time of the study with two different-dose combinations of the O/A/H triple regimen [21]. A sample size of 2401 patients was calculated to observe 50% of patients achieving the intensive BP target with a precision of 4% at 95% confidence interval (CI). Assuming a dropout rate of 20%, we targeted 3002 patients for registration.
Patient demographics and baseline characteristics were summarized using descriptive statistics. Continuous variables were presented as means, standard deviations (SD), medians, and ranges (minimum and maximum), whereas categorical variables were presented as frequencies and percentages.
Regarding the endpoints of intensive or standard BP control rate, the percentages of patients achieving the target BP at week 24 were calculated, along with their 95% CI, using the exact binomial method. Both intensive and standard BP control rates were presented for the following cardiovascular risk subgroups as well: risk age (≥ 45 years in men, ≥ 55 years in women), smoking, BMI ≥ 25 kg/m2, DM, dyslipidemia, CVD, and CKD.
Descriptive statistics were calculated for SBP and DBP values every 4 weeks from baseline until week 24. The statistical significance of the change in BP values from baseline to week 24 was tested using the paired t test or Wilcoxon signed-rank test. Changes in BP at week 24 were further assessed in subgroups according to the use of previous antihypertensive drugs in terms of the number of drugs and combinations of drug classes. We also conducted additional analysis to ascertain the impact of enhanced medication adherence on BP reduction. Specifically, patients who employed an identical combination of antihypertensive medications as O/A/H were categorized on the basis of the quantity of pills (2-pill or 3-pill) consumed. Differences in BP changes at week 24 between the subgroups were compared using a two-sample t test or Wilcoxon rank-sum test.
As a post hoc analysis, BP change at week 24 from baseline was summarized in the subgroups of elderly (≥ 65 years), very elderly (≥ 80 years), high-risk hypertension, CVD, high-risk CVD, high-risk DM, stroke, CKD with or without albuminuria, and CKD with DM. Two sets of BP control rates were also calculated on the basis of the intensive BP target used in the primary endpoint and the targets recommended in the 2022 KSH guidelines [22] for these subgroups. The definition and classification of the subgroups used for post hoc analysis were based on the 2022 KSH guidelines [22].
Adverse events were coded using MedDRA version 21.0. The rate of occurrence of TEAEs was calculated and presented with the number of events. TEAEs were further analyzed according to their seriousness, relationship with O/A/H, and expectedness. Effectiveness outcomes and baseline data were analyzed using an effectiveness analysis set, which comprised patients who received at least one dose of O/A/H and underwent BP measurement after week 20. Safety outcomes were analyzed using a safety analysis set, which comprised patients who received at least one dose of O/A/H and were assessed for safety at least once. All hypothesis testing was two-sided, and a P value less than 0.5 was considered statistically significant. All statistical analyses were conducted using the Statistical Analysis Software (SAS) version 9.4 (SAS Institute Inc, Cary, North Carolina, USA).
Results
Patient Characteristics
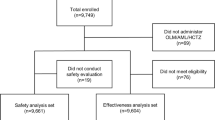
Among 3145 patients enrolled in the study, 2401 patients (76.3%) completed the 24-week follow-up period (Fig. 1). After 41 patients with missing measurements of SBP and/or DBP at 24 weeks were excluded, a total of 2360 patients were included in the effectiveness analysis set assessing the effectiveness endpoints. The demographic and clinical characteristics of the effectiveness analysis set are presented in Table 1. The mean age was 63.3 years, and 86.1% of the patients were included in the risk age group (≥ 45 years in men, ≥ 55 years in women). Concurrent or history of CVDs was the second most prevalent cardiovascular risk factor (64.4%) in this study population, and when only the high-risk CVDs only were considered, more than half of the patients (51.5%) fell into this subgroup. Other cardiovascular risk factors (percentage of relevant patients) included, in the order of frequency, dyslipidemia (53.7%), obesity (BMI ≥ 25 kg/m2) (53.5%), DM (46.3%), smoking (14.8%), and CKD (3.8%). As for prior antihypertensive treatment, double-combination treatment was the most common strategy (45.8%).
Treatment Exposure and Compliance
In the effectiveness population, the mean treatment duration of O/A/H was 197 days (median 185 days). The most frequently prescribed dose-strength combination of O/A/H was 20/5/12.5 mg, given to 1558 patients (66.0%); meanwhile, 626 (26.5%) and 309 (13.1%) patients were prescribed 40/5/12.5 mg and 40/10/12.5 mg, respectively. Based on the total treatment duration and cumulative dose of O/A/H, the number of O/A/H tablets taken per day was approximately 1, as instructed in the approved label (Table 2).
Effectiveness Outcomes
At week 24, the intensive BP control rate was 41.4% (95% CI 39.5%, 43.4%), while the standard BP control rate was 73.3% (95% CI 71.5%, 75.1%). The mean baseline BP (SBP/DBP) was 146.9/83.9 mmHg, and both SBP and DBP values showed a decreasing trend from week 4, with the level of BP reduction maintained until week 24 (Fig. S1 in the supplementary material). Reductions in both SBP and DBP at week 24 were statistically significant (P < 0.0001), with a mean change of − 17.8 mmHg and − 9.3 mmHg for SBP and DBP, respectively (Table 3).
In the subgroups with different cardiovascular risks, the intensive BP control rate ranged from 36.8% to 43.1%. The risk age group had the highest intensive BP control rate at 43.1%, followed by the CVD group (42.0%) (Fig. 2). Regarding the standard BP control rates among the cardiovascular risk subgroups, the risk age group had the highest standard BP control rate at 74.1%, followed by the CVD group (73.8%) (Fig. 2). The standard BP control rate was less than 70% in the DM (69.4%) and CKD subgroups (68.5%).
Reductions in both SBP and DBP at week 24 were statistically significant (P < 0.05), regardless of the number of prior antihypertensive agents or the combinations of classes of prior antihypertensive agents. The degree of BP reduction was inversely proportional to the number of prior antihypertensive agents used (Fig. 3). Among the subgroups that used double-combination treatment before the study, the ARB + DU group (− 18.9/− 10.8 mmHg) had significantly (P < 0.05) greater mean BP reduction (SBP/DBP) than the ARB + CCB group (− 14.6/− 7.7 mmHg). Among the subgroups that used triple-combination treatment before the study, the ARB + CCB + DU group showed the lowest BP reduction (− 5.2/− 2.6 mmHg, P < 0.05), although there was a significant change from baseline. Compared with the ARB + CCB + DU group, the ARB + CCB + BB group (− 12.3/− 5.0 mmHg) or ARB + DU + BB group (− 7.2/− 4.1 mmHg) had significantly (P < 0.05) greater mean BP reduction.
Subgroup analysis for mean change in BP at week 24 from baseline. a Subgroups are based on the number of classes of previous antihypertensive agents. b Subgroups are based on the combinations of classes of antihypertensive agents taken prior to the study. c Mean BP change in the subgroup of patients using the triple combination of ARB, CCB, and DU before the study is presented. *ARB, CCB refers to the subgroup of patients using ARB, CCB combination treatment before the study. †ARB, DU refers to the subgroup of patients using ARB, DU combination treatment before the study. ‡ARB, CCB, BB refers to the subgroup of patients using ARB, CCB, BB combination treatment before the study. §ARB, CCB, DU refers to the subgroup of patients using ARB, CCB, DU combination treatment before the study. ‖ARB, DU, BB refers to the subgroup of patients using ARB, DU, BB combination treatment before the study. ¶Three-pill for ARB, CCB, DU refers to the subgroup of patients using three individual pills for ARB, CCB, and DU combination treatment before the study. ◊Two-pill for ARB, CCB, DU refers to the subgroup of patients using two individual pills for ARB, CCB, and DU combination treatment before the study. ↓A single-asterisk sign (*) indicates P < 0.05 versus the baseline and a double-asterisk sign (**) indicates P < 0.05 versus the comparator group. ARB angiotensin receptor blocker, BB beta-blocker, BP blood pressure, CCB calcium channel blocker, DU diuretic, DBP diastolic blood pressure, SBP systolic blood pressure
When the 2017 ACC/ACA guidelines were applied, the BP control rates in the subgroups with the following risk factors were relatively lower than the intensive BP control rate in the overall effectiveness population (41.4%): CKD with DM (38.6%), high-risk hypertension (37.7%), high-risk DM (37.4%), and CKD with albuminuria (28.1%) (Table 4). In the subgroups where the target SBP/DBP was < 140/90 mmHg, according to the 2022 KSH guidelines, the very elderly subgroup (≥ 80 years old) had a relatively lower BP control rate (67.1%) than the standard BP control rate in the overall effectiveness population (73.3%). The CKD without albuminuria subgroup had the highest BP control rate at 77.2%.
Safety Outcomes
The safety outcomes are summarized in Table 5. During the 24-week follow-up period, 23.3% of patients included in the safety set (n = 2849) experienced 934 TEAEs, of which 109 events observed in 3.83% of the patients were reported as serious adverse events (SAEs). Common TEAEs (occurrence rate > 1%) included dizziness (4.32%), hypotension (1.54%), and headaches (1.44%), and the most common SAE was cerebral infarction (0.18%); none of these were considered as ADRs.
A total of 223 ADRs were observed in 7.8% of overall patients. The most common ADR was dizziness (2.91%), followed by hypotension (1.51%) and headache (0.70%). The occurrence rate of serious ADRs was 0.28%, with eight events, including two events of hyponatremia and the single event of headache, seizure, vestibular neuronitis, rib fracture, intervertebral disc protrusion, and death.
There were four deaths. Three deaths, namely due to cardiac arrest, cerebral hemorrhage, and hepatocellular carcinoma, were assessed as unlikely to be related to O/A/H. As for the one death classified as an ADR, the exact cause and date of death were unavailable, and its relationship to O/A/H was unassessable.
Discussion
This observational study identified the effectiveness and safety of O/A/H in South Korean patients with hypertension and at least one additional cardiovascular risk factor. The administration of O/A/H significantly reduced mean SBP/DBP at week 24 from baseline, with a mean change of − 17.8 mmHg and − 9.3 mmHg, respectively. The reduction in BP was consistently observed across all the risk groups. Moreover, patients with hypertension and additional cardiovascular risk factors who received O/A/H to control BP sustained a mean SBP/DBP below 130/80 mmHg, the target level recommended in the current ESC/ESH guidelines, throughout the study period [10]. Notably, a significant BP reduction was observed within 4 weeks after switching to O/A/H. This relatively rapid onset of O/A/H effectiveness is worth emphasizing, given that early and fast BP management is associated with a more effective and sustained BP control, and hence with greater long-term clinical benefits [23, 24].
At the end of the study period, the standard BP control rate for the study population was 73.3% (95% CI 71.5%, 75.1%), which is consistent with the results of previous real-world studies of O/A/H in the general population of patients with hypertension (BP control rates 70.6–82.6%). This also corresponds well with the published data on the percentage of South Korean patients with hypertension and uncontrolled BP (29%) [3, 16, 17]. Our study results highlighted the importance of O/A/H in reducing and sustaining BP in patients with hypertension and additional cardiovascular risk factors, a group in which BP control is considered challenging. However, the data on the O/A/H effectiveness presented herein should be interpreted with caution. The target BP for patients with additional cardiovascular risk factors was set lower than that for those without additional risk factors; hence, the beneficial effects of O/A/H in the present study might have been overestimated. Furthermore, patients with additional cardiovascular risk factors are subjected to more intensive care and close monitoring, which may lead to improved BP control.
The intensive BP control rate for the study population was 41.4% (95% CI 39.45%, 43.4%), with rates varying among different cardiovascular risk subgroups. A recent study on patients with resistant hypertension in Korea found that the average rate of achieving intensive BP control was approximately 50%, which is somewhat higher than the rate observed in our study [25]. This discrepancy can be explained by several factors. First, the target BP for patients with resistant hypertension may differ from that for patients with hypertension and cardiovascular risk factors. While the target BP for patients with resistant hypertension is generally SBP < 130 mmHg and DBP < 80 mmHg, the 2018 KSH recommends a target SBP/DBP of < 140/90 mmHg for patients with hypertension and cardiovascular risk factors, except for those with DM and concomitant cardiovascular risk factors, high-risk hypertension, CVD, and CKD with albuminuria [5]. The relatively high target BP of the patients enrolled in the present study may have contributed to the lower intensive BP control rate. Another reason may be that some physicians might have been doubtful whether the benefits of pursuing more intensive BP control outweighed the risks in patients with hypertension and cardiovascular risk factors [20, 26,27,28]. Such an approach could lead to therapeutic inertia, with a resultant failure in achieving intensive BP goals in some patients with inadequate BP control owing to the lack of timely adjustment of O/A/H treatment [17].
Subgroup analysis based on combinations of previous antihypertensive drug classes showed the efficacy of O/A/H in managing high baseline BP levels, effectively attaining target BP levels within 24 weeks in antihypertensive treatment-naïve patients (Fig. 3 and Table S3). Nevertheless, the utilization of the triple combination therapy in treatment-naïve patients continues to cause controversy. The study’s protocol permits the administration of O/A/H at the physician’s discretion, specifically reserved for patients necessitating a SBP reduction of 20 mmHg or more to achieve the target BP level.
The result of subgroup analysis based on combinations of previous antihypertensive drug classes also showed that patients who used a combination of one or two antihypertensive drugs before switching to O/A/H had a more significant BP reduction. These findings indicate that combining drugs from different and complementary classes provides a synergistic effect on BP reduction, which in turn leads to greater antihypertensive efficacy. Moreover, patients who switched from a combination of at least three antihypertensive agents to O/A/H showed a significant BP reduction. Interestingly, a significant BP reduction was also documented in a subgroup of patients in whom O/A/H was introduced instead of ARB + CCB + DU, the free combination of agents from the same groups as SPC components. This finding supports the use of SPC to improve medication adherence and BP control. However, any conclusion on this matter should be formulated carefully as the introduction of O/A/H may also be associated with dose escalation.
Both DM and metabolic syndrome are potent cardiovascular risk factors, and the 2022 KSH guidelines recommend intensive BP control (< 130/80 mmHg) for patients with high-risk DM, defined as DM accompanied by asymptomatic organ damage or a cardiovascular risk factor [22]. In the present study, intensive and standard BP control rates in patients with DM were lower than those in the overall population. Patients with hypertension and DM require multiple medications, including antihypertensive and antidiabetic drugs, and this polypharmacy may negatively affect their medication adherence compared with other cardiovascular risk factors. Poor medication adherence in patients with hypertension and DM can interfere with achievement of the target BP. Furthermore, most of the participants with DM included in this study (1087 out of 1092, 99.5%) were classified as having high-risk DM and thus might present with kidney-related complications; in these cases adequate BP control is generally more challenging. A relatively poor BP control rate in patients with DM and/or CKD was also observed in patients who received more than two antihypertensive drugs in a cross-sectional study [29]. Although SPC is beneficial for BP control by improving medication adherence, further studies are needed to investigate the risk factors that interfere with BP control in patients with hypertension and DM and/or CKD because SPC could be only one of the many prescribed medications in patients with polypharmacy. Therefore, physicians should care more closely for patients with hypertension and additional cardiovascular risk factors and monitor their BP to ensure adequate control.
Within this study, patients who exhibited SBP below 130 mmHg were included. Those were found to be previously utilizing a regimen consisting of three or more antihypertensive medications. In the case of these patients, the utilization of SPC therapy, such as the combination of O/A/H, could be considered as a means to enhance treatment adherence. In addition, the study included 26.9% of patients with controlled hypertension (Table 1). The classification of patients with uncontrolled hypertension was based on the criteria of SBP exceeding 140 mmHg and DBP exceeding 90 mmHg. However, it is important to note that this study focused on patients with hypertension and concurrent CV risk factors, including those with high-risk DM, high-risk CVD, and high-risk hypertension. KSH guidelines recommended a lower target BP of less than 130 mmHg for SBP and 80 mmHg for DBP in these patient groups. Given the lower target BP in these patients, switching to higher dose O/A/H SPC than the doses of the free combination could be considered when switching to O/A/H SPC.
The majority of patients with hypertension previously using the combinations of ARB + CCB + BB and ARB + DU + BB had received O/A/H as an additive to BB, with some entirely switching to O/A/H therapy (Table 1). For those patients who underwent the switching to O/A/H, it is postulated that such changes were based on clinical judgment, likely due to the absence of relevant medical histories warranting the continued use of BB, such as a history of myocardial infarction or heart failure. Furthermore, it is contemplated that in cases where there exists a potential risk of edema associated with the usage of ARB + CCB + BB combinations, the substitution of diuretics instead of BB may be deemed an appropriate therapeutic consideration.
This study was conducted in a group of patients with hypertension and additional cardiovascular risk factors. As previously mentioned, the target BP values in such patients are set lower than in those without concomitant risks. Although the more aggressive approach to antihypertensive treatment in patients with additional cardiovascular risk factors might theoretically lead to higher frequency of side effects associated with hypotension, the hypotension rate in the present study (1.51%) was within the range reported in previous studies on patients with similar characteristics (0.6–2.4%) [12,13,14, 16, 17, 30]. An extension study of the TRINITY trial also showed that ADRs occurring in patients with additional cardiovascular risk were consistent with the established safety profile of O/A/H [15]. This implies that safety concerns should not constitute an argument against pursuing more aggressive BP targets in patients with additional cardiovascular risk.
During the observation period, the incidence of edema associated with O/A/H use was approximately 0.3% among patients included in the effective dataset. Among the subgroup of 385 patients taking 40/10/12.5 mg of O/A/H containing 10 mg of amlodipine, six patients (1.5%) experienced edema due to O/A/H administration. In comparison, a previous study by Guthrie reported a significantly higher edema incidence of 10.8% in a subset using an SPC of telmisartan and amlodipine 10 mg [30]. The lower edema incidence observed in the present study is likely due to the exclusion of patients who had previously experienced edema while using CCB before switching to O/A/H. Notably, among patients without prior CCB use (535 patients), the incidence of edema with O/A/H was 1.1%, higher than the overall incidence of 0.3%. These findings suggest that physicians may have opted to prescribe O/A/H to patients, considering the potential risk of edema associated with CCB usage. Furthermore, the low incidence of edema observed in this study may be attributed to the concurrent administration of hydrochlorothiazide, which possesses diuretic properties.
During this study, a dropout rate of 22% (701 subjects) was observed among the enrolled patients, attributed to various factors. One primary reason was the loss to follow-up, possibly caused by enrolled patients’ non-compliance with scheduled medical visits at tertiary general hospitals, reflecting the influence of the Korean healthcare delivery system’s discouragement of frequent hospital visits solely for obtaining antihypertensive medications. Additionally, some cases resulted in discontinuation of O/A/H therapy, with reasons ranging from patient reluctance to adhere to the treatment regimen to the occurrence of adverse events related to O/A/H use. Notably, the incidence of serious adverse events during the study was relatively low, indicating that drug intolerance may not have been the primary cause of O/A/H discontinuation (Table 5).
Some potential limitations of the present study should be considered. First, this was a non-interventional, prospective observational study without a comparator group, rather than a randomized controlled study. Underreporting is unavoidable in such a study design, which may lead to a potential bias. Also, the present study is not able to establish causality, as it cannot control for all potential confounding variables, and it is unable to manipulate exposure or intervention. Therefore, our findings must be interpreted with caution and in conjunction with other studies. Additionally, variables related to routine care (such as the use of concomitant medications) were left to the discretion of the individual physicians in charge. All these factors, which could not be controlled under a real-world study design, should be considered as potential confounders influencing the results. Second, the treatment period was relatively short. If the treatment period were longer, the effect of O/A/H on BP would probably be even more pronounced. Despite these potential limitations, the results of the present study provide real-world evidence for the beneficial effects of O/A/H in a large sample of patients with hypertension and additional cardiovascular risk factors, in whom BP control is generally considered a challenge.
Conclusion
The use of O/A/H, a triple SPC therapy, resulted in a rapid and sustained reduction in SBP/DBP in South Korean patients with hypertension and additional cardiovascular risk factors. O/A/H was safe and well tolerated by the study population. However, it is imperative to acknowledge that the study design employed was that of a prospective observational nature, lacking a comparator group. Therefore, prudent consideration and cautious interpretation of the clinical implications of the findings are warranted. It is also important to recognize that further investigations may be necessary to corroborate and validate the results obtained from this study.
Data Availability
Data used for this study were based on the KCT000340. The data underlying this article will be shared on reasonable request to the corresponding author and after permission of the relevant medical ethical committees.
References
Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–50. https://doi.org/10.1161/CIRCULATIONAHA.115.018912.
Kim HC, Ihm S-H, Kim G-H, et al. 2018 Korean Society of Hypertension guidelines for the management of hypertension: part I-epidemiology of hypertension. Clin Hypertens. 2019;25:16. https://doi.org/10.1186/s40885-019-0121-0.
Korean Society Hypertension, Hypertension Epidemiology Research Working Group, Kim HC, Cho M-C. Korea hypertension fact sheet 2018. Clin Hypertens. 2018;24:13. https://doi.org/10.1186/s40885-018-0098-0.
Edwards EW, Saari HD, DiPette DJ. Inadequate hypertension control rates: a global concern for countries of all income levels. J Clin Hypertens (Greenwich). 2022;24:362–4. https://doi.org/10.1111/jch.14444.
Lee H-Y, Shin J, Kim G-H, et al. 2018 Korean Society of Hypertension Guidelines for the management of hypertension: part II-diagnosis and treatment of hypertension. Clin Hypertens. 2019;25:20. https://doi.org/10.1186/s40885-019-0124-x.
Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009;122:290–300. https://doi.org/10.1016/j.amjmed.2008.09.038.
Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120:713–9. https://doi.org/10.1016/j.amjmed.2006.08.033.
Neutel JM. The role of combination therapy in the management of hypertension. Nephrol Dial Transplant. 2006;21:1469–73. https://doi.org/10.1093/ndt/gfk064.
Levi M, Pasqua A, Cricelli I, et al. Patient adherence to olmesartan/amlodipine combinations: fixed versus extemporaneous combinations. J Manag Care Spec Pharm. 2016;22:255–62. https://doi.org/10.18553/jmcp.2016.22.3.255.
Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953–2041. https://doi.org/10.1097/HJH.0000000000001940.
Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127-248. https://doi.org/10.1016/j.jacc.2017.11.006.
Oparil S, Melino M, Lee J, Fernandez V, Heyrman R. Triple therapy with olmesartan medoxomil, amlodipine besylate, and hydrochlorothiazide in adult patients with hypertension: the TRINITY multicenter, randomized, double-blind, 12-week, parallel-group study. Clin Ther. 2010;32:1252–69. https://doi.org/10.1016/j.clinthera.2010.07.008.
Volpe M, Christian Rump L, Ammentorp B, Laeis P. Efficacy and safety of triple antihypertensive therapy with the olmesartan/amlodipine/hydrochlorothiazide combination. Clin Drug Investig. 2012;32:649–64. https://doi.org/10.1007/BF03261919.
Volpe M, de la Sierra A, Ammentorp B, Laeis P. Open-label study assessing the long-term efficacy and safety of triple olmesartan/amlodipine/hydrochlorothiazide combination therapy for hypertension. Adv Ther. 2014;31:561–74. https://doi.org/10.1007/s12325-014-0117-9.
Kereiakes DJ, Chrysant SG, Izzo JLJ, et al. Olmesartan/amlodipine/hydrochlorothiazide in participants with hypertension and diabetes, chronic kidney disease, or chronic cardiovascular disease: a subanalysis of the multicenter, randomized, double-blind, parallel-group TRINITY study. Cardiovasc Diabetol. 2012;11:134. https://doi.org/10.1186/1475-2840-11-134.
Sohn IS, Ihm S-H, Kim GH, et al. Real-world evidence on the strategy of olmesartan-based triple single-pill combination in Korean hypertensive patients: a prospective, multicenter, observational study (RESOLVE-PRO). Clin Hypertens. 2021;27:21. https://doi.org/10.1186/s40885-021-00177-z.
Park S-J, Rhee SJ. Real-world effectiveness and safety of a single-pill combination of olmesartan/amlodipine/hydrochlorothiazide in Korean Patients with Essential Hypertension (RESOLVE): a large, observational, retrospective, cohort study. Adv Ther. 2020;37:3500–14. https://doi.org/10.1007/s12325-020-01404-z.
Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens. 2000;13:3S–10S. https://doi.org/10.1016/s0895-7061(99)00252-6.
Volpe M, Gallo G, Tocci G. Is early and fast blood pressure control important in hypertension management? Int J Cardiol. 2018;254:328–32. https://doi.org/10.1016/j.ijcard.2017.12.026.
Shin J, Park JB, Kim K-I, et al. 2013 Korean Society of Hypertension guidelines for the management of hypertension. Part II-treatments of hypertension. Clin Hypertens. 2015;21:2. https://doi.org/10.1186/s40885-014-0013-2.
Ram CV, Sachson R, Littlejohn T, et al. Management of hypertension in patients with diabetes using an amlodipine-, olmesartan medoxomil-, and hydrochlorothiazide-based titration regimen. Am J Cardiol. 2011;107:1346–52. https://doi.org/10.1016/j.amjcard.2010.12.045.
Korean Society of Hypertension. 2022 Korean Society of Hypertension Guidelines 2022. https://www.koreanhypertension.org/news/notice?mode=read&idno=10008. Accessed 24 May 2023.
Lloyd-Jones DM, Evans JC, Larson MG, Levy D. Treatment and control of hypertension in the community: a prospective analysis. Hypertension. 2002;40:640–6. https://doi.org/10.1161/01.hyp.0000035855.44620.da.
Weycker D, Edelsberg J, Vincze G, Levy DG, Kartashov A, Oster G. Blood pressure control in patients initiating antihypertensive therapy. Ann Pharmacother. 2008;42:169–76. https://doi.org/10.1345/aph.1K506.
Joo HJ, Yum Y, Kim YH, et al. Gender difference of blood pressure control rate and clinical prognosis in patients with resistant hypertension: real-world observation study. J Korean Med Sci. 2023;24: e124. https://doi.org/10.3346/jkms.2023.38.e124.
Cooper-DeHoff RM, Gong Y, Handberg EM, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61–8. https://doi.org/10.1001/jama.2010.884.
Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–85. https://doi.org/10.1056/NEJMoa1001286.
Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370:829–40. https://doi.org/10.1016/S0140-6736(07)61303-8.
Kim KI, Kim Y, Kim HJ, et al. Current status and characteristics of hypertension treatment by primary physicians in Korea: data from Korean epidemiology study on hypertension (KEY study). Am J Hypertens. 2008;21:884–9. https://doi.org/10.1038/ajh.2008.191.
Guthrie RM. Review: a single-pill combination of telmisartan plus amlodipine for the treatment of hypertension. Postgrad Med. 2011;123:58–65. https://doi.org/10.3810/pgm.2011.11.2495.
Acknowledgements
The authors greatly acknowledge the contribution of all the investigators and research coordinators at each participating institution. We would also like to extend our sincere gratitude to all the subjects who generously participated in this study. Your valuable contributions have been crucial to the success of our research, and we are deeply appreciative of your involvement.
List of Investigators: Byung Su Yoo, Jong Hwan Choi, Sangwon Park, Yong Guk Cho, Sunil Lee, Dong Hoon Shin, Kwang Su Cha, Jaemin Shim, Young-dae Kim, Kyung-il Park, Jae Hyung Park, Bong Gu Yoo, Won Gu Lee, Jae Seung Yoon, Chang Hun Kim, Young Yeop Koh, Dong Yul Ryu, SungWan Chun, Kwang Je Lee, Jeong-Eun Yi, Pum-Joon Kim, Sung-Won Jang, Yun Seok Choi, Chang Beom Lee, Sung Hoon Yu, Min Goo Lee, Young Won Yoon, Dong-Gu Shin, Soo Joo Lee, Jae Guk Kim, Sang-Jin Han, Shin-Jae Kim, Oh-Hyun Lee, Eui Im, Sang Wook Kang, Jeong Hwan Cho, Gyu Hwan Park, Jong Min Lee, Seung Jin Han, Seo Hye Sun, In Kyung Jeong, Kyu-Jeung Ahn, Ho Yeon Chung, Kiyoung Lee, Won-Chul Shin, Sang Won Han, Jong Sam Baik, Sung-Pil Joo, Ung Jeon, Yong Hwan Park, Ki Hong Lee, Yu Jeong Choi, Hancheol Lee, A-Hyun Cho, Chan Seok Park, Ji Woong Roh, Jaechun Hwang, Won Young Lee, Yong-Hyun Kim, Jae Myung Yu, Hye Soo Chung, Shinje Moon, Young Rak Cho, Jung-Hee Lee, Young-Hyo Lim, Yun Seong Kim, Jin-Bae Kim, Moo Hyun Kim and Moo Young Park.
Medical Writing/Editorial Assistance
Editorial assistance in the preparation of this article was provided by Dr. Simon Kim (SciencePro, Seoul, Korea) and Hye-Ryon Kim (Medical Writing, Seoul, Korea) and was funded by Daiichi Sankyo Korea Co., Ltd., Seoul, Korea. Also, we would like to thank Editage and (www.editage.co.kr) and Ji-Hwan Bae for English language editing.
Funding
The sponsorship for this study and publication and open access fee for this article were funded by Daiichi Sankyo Korea Co., Ltd., Seoul, Korea.
Author information
Authors and Affiliations
Consortia
Contributions
Jaewon Oh, Gee-Hee Kim, Hack-Lyoung Kim, Sang-Don Park, Wonho Kim, and Jinho Shin contributed to the conception, design, and interpretation of the data. Jaewon Oh wrote the first draft and revised the manuscript. Jin-Ho Shin contributed to the review and critical revision of the manuscript. All authors reviewed and approved the final manuscript draft submitted for publication and agree to be accountable for all aspects of the work, ensuring the accuracy and integrity of the publication. Jaewon Oh, Gee-Hee Kim, Hack-Lyoung Kim, Sang-Don Park, Wonho Kim, Kyung Wan Min, Dongkeun Hyun, Jun Hwa Hong, Soo Lim, and Jinho Shin participated in the enrollment and performed clinical follow-up and data acquisition.
Corresponding author
Ethics declarations
Conflict of Interest
Jaewon Oh, Wonho Kim, Gee-Hee Kim, Hack-Lyoung Kim, Sang-Don Park, Kyung Wan Min, Dongkeun Hyun, Jun Hwa Hong, and Soo Lim declare that they have no competing interests. Jinho Shin received honoraria from Daiichi Sankyo, Organon, Bristol Myers Squibb, Menarini, Sanofi, Hanmi, Boryung, Chong Kun Dang, and Daewoong and grants from Sanofi and Hanmi.
Ethical Approval
The study protocol and informed consent forms were approved by the institutional review board (IRB) of each participating institution. The study was registered with the Clinical Research Information Service (KCT0003401; https://cris.nih.go.kr/cris/search/detailSearch.do/20795). The study was conducted in accordance with the principles of the Declaration of Helsinki 1964 and its later amendments as well as the rules of each IRB. All patients voluntarily provided written informed consent before enrollment in the study. Data were de-identified to protect the privacy of the participating patients and to be fully compliant with locally applicable regulations.
Additional information
The members of the “RESOLVE-INT Investigators” author group are listed in the Acknowledgements section.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Oh, J., Kim, W., Kim, GH. et al. Real-World Effectiveness and Safety of a Single-Pill Combination of Olmesartan/Amlodipine/Hydrochlorothiazide in Korean Patients with Hypertension and Cardiovascular Risk Factors. Adv Ther 40, 4817–4835 (2023). https://doi.org/10.1007/s12325-023-02632-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02632-9