Abstract
Introduction
Sugemalimab is the first China-developed programmed death-ligand 1 inhibitor that has proved to be effective as a first-line treatment for both metastatic squamous and non-squamous non-small cell lung cancer (NSCLC) when used in combination with chemotherapy. This study compared the cost-effectiveness of sugemalimab plus chemotherapy (sugema + chemo) with placebo plus chemotherapy (placebo + chemo) among metastatic squamous and nonsquamous NSCLC, respectively.
Methods
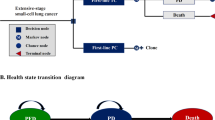
Separate Markov models were constructed to generate the cumulative healthcare costs and quality-adjusted life-years (QALYs) associated with two treatment strategies over a 20-year time horizon. Transition probabilities were estimated using survival data reported in the GEMSTONE-302 trial. Health state utilities and costs were derived from published literature, national databases, and local general hospitals. Sensitivity analyses were performed to test the robustness of our conclusions.
Results
Compared with first-line placebo + chem, sugema + chemo achieved an incremental cost-effectiveness ratio (ICER) of $57,842/QALY for patients with metastatic squamous NSCLC and achieved an ICER of $78,249/QALY for patients with metastatic non-squamous NSCLC. In our sensitivity analyses of a willingness-to-pay (WTP) threshold of $35,663 per QALY, the first-line sugema + chemo was only cost-effective for patient groups when the price of sugemalimab decreased.
Conclusion
Sugema + chemo was not cost-effective as a first-line treatment for either metastatic squamous or metastatic nonsquamous NSCLC in Chinese patients compared with placebo + chemo. However, we found that sugema + chemo would be cost-effective in patients with metastatic squamous and non-squamous NSCLC when sugemalimab’s price was decreased by > 39.0% and 64.8%, respectively.


Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134(7):783–91.
Gridelli C, Rossi A, Carbone DP, et al. Non-small-cell lung cancer. Nat Rev Dis Primers. 2015;21(1):15009.
Dafni U, Tsourti Z, Vervita K, Peters S. Immune checkpoint inhibitors, alone or in combination with chemotherapy, as first-line treatment for advanced non-small cell lung cancer. A systematic review and network meta-analysis. Lung Cancer. 2019;134:127–40.
Socinski MA, Obasaju C, Gandara D, et al. Current and emergent therapy options for advanced squamous cell lung cancer. J Thorac Oncol. 2018;13(2):165–83.
Guidelines Working Committee of Chinese society of Clinical Oncology. Guidelines of Chinese society of clinical oncology (CSCO) for non-small cell lung cancer [M] 2021 edition. Beijing: People's Medical Publishing House, 2021. p. 201.
Wu YL, Zhang L, Fan Y, et al. Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD-L1-positive locally advanced or metastatic non-small-cell lung cancer: KEYNOTE-042 China Study. Int J Cancer. 2021;148(9):2313–20.
Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–92.
Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for first-line treatment of PD-L1-selected patients with NSCLC. N Engl J Med. 2020;383(14):1328–39.
Yang Y, Wang Z, Fang J, et al. Efficacy and safety of sintilimab plus pemetrexed and platinum as first-line treatment for locally advanced or metastatic nonsquamous NSCLC: a randomized, double-blind, phase 3 study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol. 2020;15(10):1636–46.
Zhou C, Wu L, Fan Y, et al. Sintilimab plus platinum and gemcitabine as first-line treatment for advanced or metastatic squamous NSCLC: Results From a Randomized, Double-Blind, Phase 3 Trial (ORIENT-12). J Thorac Oncol. 2021;16(9):1501–11.
Wang J, Lu S, Yu X, et al. Tislelizumab plus chemotherapy vs chemotherapy alone as first-line treatment for advanced squamous non-small-cell lung cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(5):709–17.
Lu S, Wang J, Yu Y, et al. Tislelizumab plus chemotherapy as first-line treatment for locally advanced or metastatic nonsquamous NSCLC (RATIONALE 304): a randomized phase 3 trial. J Thorac Oncol. 2021;16(9):1512–22.
Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med. 2021;9(3):305–14.
Shi JF, Huang HY, Guo LW, et al. Quality-of-life and health utility scores for common cancers in China: a multicentre cross-sectional survey. Lancet. 2016;388:S29.
Zhou C, Wang Z, Sun Y, et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. 2022;23(2):220–33.
National Medical Products Administration. The State Drug Administration approved the listing of sugemalimab injection. https://www.nmpa.gov.cn/yaopin/ypjgdt/20211221091119147.html. Accessed 21 Dec 2021.
Chinese Pharmaceutical Association. Chinese Guidelines for Pharmacoeconomic Evaluations (2020) https://www.cpa.org.cn/cpadmn/attached/file/20201203/1606977380634185.pdf. Accessed 21 Dec 2021.
National Bureau Of Statistics Of China. China statistical yearbook 2021. http://www.stats.gov.cn/english/Statisticaldata/AnnualData/. Accessed 14 Nov 2021.
Liu Q, Zhou Z, Luo X, et al. First-line ICI monotherapies for advanced non-small-cell lung cancer patients with PD-L1 of at least 50%: a cost-effectiveness analysis. Front Pharmacol. 2021;12: 788569.
Liu Q, Zhou Z, Zeng X, Tan C. Cost-effectiveness of domestic PD-1 inhibitor camrelizumab combined with chemotherapy in the first-line treatment of advanced nonsquamous non-small-cell lung cancer in China. Front Pharmacol. 2021;12: 728440.
Shen Y, Wu B, Wang X, Zhu J. Health state utilities in patients with advanced non-small-cell lung cancer in China. J Comp Eff Res. 2018;7(5):443–52.
Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non-small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2017;13(5):e195–203.
China's health industry data platform. Bid winning information of drugs. https://www.yaozh.com/. Accessed 14 Feb 2022.
Briggs AH, Weinstein MC, Fenwick EAL, Karnon J, Sculpher MJ, Paltiel AD, ISPOR-SMDM Modeling Good Research Practices Task Force. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med Decis Making. 2012;32(5):722–732.
Miao Y, Yuan X, Gu J, et al. Constructing a value-based healthcare system for hypertensive patients through changing payment mode: evidence from a comparative study in rural China. J Med Econ. 2019;22(3):245–51.
Liang X, Chen X, Li H, Liu X, Li Y. Sugemalimab plus chemotherapy vs. chemotherapy for metastatic non-small-cell lung cancer: a cost-effectiveness analysis. Front Public Health. 2023;11:1054405.
Li W, Wan L. Cost-effectiveness analysis of sugemalimab vs. placebo, in combination with chemotherapy, for treatment of first-line metastatic NSCLC in China. Front Public Health. 2022;10:1015702.
Zheng Z, Zhu H, Fang L, Cai H. Cost-effectiveness analysis of sugemalimab vs. chemotherapy as first-line treatment of metastatic nonsquamous non-small cell lung cancer. Front Pharmacol. 2022;13:996914.
Chen P, Li Y, Jing X, Chen J, Chen S, Yang Q. Cost-effectiveness analysis of sugemalimab in combination with chemotherapy as first-line treatment in Chinese patients with metastatic NSCLC. Lung Cancer. 2022;174:157–64.
Central People's Government of the People's Republic of China. Catalogue of drugs for national basic medical insurance, industrial injury insurance and maternity insurance (2020). http://www.gov.cn/zhengce/zhengceku/2020-12/28/content_5574062.htm. Accessed 14 Feb 2022.
Cai H, Zhang L, Li N, et al. Cost-effectiveness of osimertinib as first-line treatment and sequential therapy for EGFR mutation-positive non-small cell lung cancer in China. Clin Ther. 2019;41(2):280–90.
Rothwell B, Kiff C, Ling C, Brodtkorb TH, et al. Cost effectiveness of nivolumab in patients with advanced, previously treated squamous and non-squamous non-small-cell lung cancer in England. Pharmacoecon Open. 2021;5(2):251–60.
Aziz MIA, Foo WYX, Toh CK, Lim WT, Ng K. Cost-effectiveness analysis of osimertinib for first-line treatment of locally advanced or metastatic EGFR mutation positive non-small cell lung cancer in Singapore. J Med Econ. 2020;23(11):1330–9.
Zhang L, Li N, Liu M, Zheng B, Wu Z, Cai H. Cost-effectiveness analysis of dacomitinib versus gefitinib in the first-line treatment of EGFR-positive advanced or metastatic non-small cell lung cancer. Cancer Manag Res. 2021;13:4263–70
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Rihua Cheng and Qiao Liu developed the economic model, performed the analyses, interpreted the results and drafted the article. Rihua Cheng and Zhen Zhou collected and reviewed the data. Qiao Liu contributed to the conception and design of the primary model. All authors read and approved the final article.
Disclosures
Rihua Cheng, Zhen Zhou and Qiao Liu declare they have nothing to disclose.
Compliance with Ethics Guidelines
The model used in this analysis was based on previously conducted studies and other economic models; no studies with human participants or animals were performed by any of the authors. Since no individual patient-level data were involved, this study was exempted from the review by the Chinese ethics review committee.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cheng, R., Zhou, Z. & Liu, Q. The Cost-Effectiveness of Sugemalimab Plus Chemotherapy as First-Line Treatment for Metastatic Squamous and Non-squamous NSCLC in China. Adv Ther 40, 4298–4309 (2023). https://doi.org/10.1007/s12325-023-02594-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02594-y