Abstract
Type 2 diabetes mellitus (T2DM) and hypertension are leading risk factors for death and disability in the Middle East. Both conditions are highly prevalent, underdiagnosed and poorly controlled, highlighting an urgent need for a roadmap to overcome the barriers to optimal glycaemic and blood pressure management in this region. This review provides a summary of the Evidence in Diabetes and Hypertension Summit (EVIDENT) held in September 2022, which discussed current treatment guidelines, unmet clinical needs and strategies to improve treatment outcomes for patients with T2DM and hypertension in the Middle East. Current clinical guidelines recommend strict glycaemic and blood pressure targets, presenting several treatment options to achieve and maintain these targets and prevent complications. However, treatment targets are infrequently met in the Middle East, largely due to high clinical inertia among physicians and low medication adherence among patients. To address these challenges, clinical guidelines now provide individualised therapy recommendations based on drug profiles, patient preferences and management priorities. Efforts to improve the early detection of prediabetes, T2DM screening and intensive, early glucose control will minimise long-term complications. Physicians can use the T2DM Oral Agents Fact Checking programme to help navigate the wide range of treatment options and guide clinical decision-making. Sulfonylurea agents have been used successfully to manage T2DM; a newer agent, gliclazide MR (modified release formulation), has the advantages of a lower incidence of hypoglycaemia with no risk of cardiovascular events, weight neutrality and proven renal benefits. For patients with hypertension, single-pill combinations have been developed to improve efficacy and reduce treatment burden. In conjunction with pragmatic treatment algorithms and personalised therapies, greater investments in disease prevention, public awareness, training of healthcare providers, patient education, government policies and research are needed to improve the quality of care of patients with T2DM and/or hypertension in the Middle East.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Type 2 diabetes mellitus (T2DM) and hypertension are highly prevalent, underdiagnosed and poorly controlled in the Middle East, mainly due to high clinical inertia among physicians and low medication adherence among patients. |
Early glycaemic control with oral antihyperglycaemic medications is recommended for the management of patients with T2DM; of these, gliclazide MR (modified release formulation) has the advantage of providing excellent efficacy with a lower incidence of hypoglycaemia, weight neutrality and higher cardiovascular safety compared with older sulfonylureas. |
Clinical guidelines for T2DM advocate a patient-centred treatment approach, and the T2DM Oral Agents Fact Checking programme represents an evidence-based resource to guide individualised clinical decisions. |
Single-pill combination therapies are now recommended as the first-line treatment option for most patients with hypertension. |
Greater physician adherence to clinical guidelines, increased patient education and support, and further investment in disease prevention and management are needed to improve long-term outcomes for patients with T2DM and/or hypertension in the Middle East. |
Introduction
Cardiometabolic syndrome refers to a group of interrelated risk factors associated with cardiovascular disease (CVD), the leading cause of death worldwide [1]. Two major cardiometabolic disorders are type 2 diabetes mellitus (T2DM) and hypertension, both of which are highly prevalent in the Middle East as a result of rapid urbanisation in the region and high rates of obesity, physical inactivity and smoking [2,3,4,5]. Despite increasing prevalence, rates of T2DM and hypertension diagnosis in the Middle East are relatively low and guideline-recommended glycaemic and blood pressure (BP) targets are infrequently met [2, 4, 6], for reasons including high clinical inertia and poor medication adherence. To improve the outcomes for patients with T2DM or hypertension in the Middle East, there is an urgent need for strategies that improve disease awareness and prevention, promote prompt diagnosis and intervention, reduce treatment burden, achieve treatment targets and improve long-term cardiovascular outcomes.
Recognising the unmet clinical needs in this region, the virtual Evidence in Diabetes and Hypertension Summit (EVIDENT) was held in September 2021 to discuss the current state of T2DM and hypertension management in the Middle East [7]. Following the success of this symposium, EVIDENT 2022 was held on September 9, 2022, in Jeddah, Kingdom of Saudi Arabia. EVIDENT 2022 was endorsed by the Gulf Association of Endocrinology and Diabetes (GAED), the Gulf Heart Association (GHA), the Canadian Heart Research Centre (CHRC) and the European Society of Hypertension (ESH), and was accredited for continuing medical education by the Dubai Health Authority (DHA) and the Saudi Commission for Health Specialties (SCHS). This review presents a roadmap that stemmed from the presentations made at EVIDENT 2022, which discussed the prevalence and burden of T2DM and hypertension in the Middle East, current treatment guidelines for these conditions, barriers to optimal glycaemic and BP control, and recommendations to improve clinical outcomes for patients in this region. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
T2DM in the Middle East
Prevalence, Diagnosis and Control
The Middle East and North Africa (MENA) region currently leads the world in T2DM prevalence, and is expected to extend its lead over the next 20 years [2]. The number of adults (aged 20–79 years) with diabetes mellitus (DM) worldwide was estimated at 537 million people in 2021 (global prevalence, 10.5%), and this is projected to rise by 46% in 2045 (783 million people; global prevalence, 12.2%). In comparison, the 73 million people in MENA with DM in 2021 (region prevalence, 16.2%) is expected to rise by 87% in 2045 (136 million people; region prevalence, 19.3%) [2]. T2DM accounts for approximately 90% of DM cases worldwide [2], and current prevalence estimates for T2DM in the Arab world range between 4% and 32% (Fig. 1) [3]. Higher rates of T2DM were observed in Gulf Cooperation Council (GCC) countries, in part due to increased energy consumption, obesity, physical inactivity, healthcare expenditure and life expectancy in this region [3, 8].
Prevalence of type 2 diabetes mellitus across the Middle East and North Africa [3]
In 2021, almost half of all adults with DM worldwide (240 million people; 44.7%) were unaware of their diagnosis [2]. While the prevalence of people with undiagnosed DM in MENA was lower than the global estimate (27 million people; 37.6%), Egypt had the fifth-highest number of people with undiagnosed DM worldwide (6.8 million people, representing 62% of all DM cases in Egypt) [2]. Among individuals diagnosed with DM, rates of good glycaemic control are achieved in only 11–41% of the population across GCC countries [6].
On the basis of data from the global DISCOVER study programme, patients with T2DM in the Middle East typically present as follows: aged 47–61 years (63% of patients are aged 41–60 years); secondary (38%) or higher (37%) education level; hypertension (40% of patients); mean systolic BP (SBP) of 133 mmHg; hyperlipidaemia (42% of patients); mean low-density lipoprotein cholesterol level of 114 mg/dL; and mean body mass index (BMI) of 31.1 kg/m2 (BMI > 30 kg/m2 is considered obese; Table 1) [9]. Notably, patients with T2DM in the Middle East have a mean glycated haemoglobin (HbA1c) of 8.7% and approximately two-thirds present with poor glycaemic control (66% of patients with HbA1c ≥ 8.0%) [9].
Compared with patients with T2DM in other regions, patients in the Middle East are typically younger and have a higher BMI, but with a similar or lower incidence of microvascular or macrovascular complications (Table 1) [9]. Therefore, early diagnosis, prompt treatment and long-term glycaemic control are particularly important to reduce the burden of T2DM in this region. Furthermore, efforts to improve prediabetes and T2DM screening, as well as intensive glucose control, will substantially minimise the long-term complications of T2DM [10].
Clinical Guidelines for the Management of T2DM
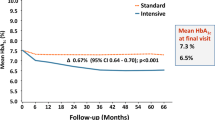
It is widely established that lowering HbA1c is critical to reduce the risks of complications and mortality associated with T2DM [11], and that these long-term benefits are maximised with early and intensive glycaemic control (also known as metabolic memory or the glycaemic legacy effect) [10, 12]. The mechanisms underlying this phenomenon are not fully understood; however, hyperglycaemia may cause lasting epigenetic modifications, endothelial dysfunction, oxidative stress and inflammation that contribute to future micro- and macrovascular complications of T2DM [13]. These lasting effects of hyperglycaemia may be minimised with early and intensive glycaemic control, but are difficult to reverse with delayed intervention.
Regardless of when treatment is initiated, international guidelines for the management of T2DM recommend that treatment should aim to reduce HbA1c levels to ≤ 7.0% in most patients [14,15,16,17,18]. However, glycaemic control rates suggest this glycaemic target has been difficult to achieve in the Middle East.
Barriers to Optimal Glycaemic Control
Recent real-world studies have identified that clinical inertia (defined as the delay to initiate or modify treatment to achieve recommended targets) is a key reason for suboptimal glycaemic control in the Middle East. For example, the DISCOVER study programme examined global treatment patterns in almost 16,000 patients receiving second-line therapy for T2DM across 38 countries worldwide [9, 19]. At the time of initiating second-line treatment, patients from the MENA region had a higher mean HbA1c than patients in all other regions (8.7% vs 8.3% overall), 34% of these patients had HbA1c ≥ 9.0% and the mean time from diagnosis to second-line treatment was 5.8 years [9, 19]. Similarly, the VISION study (Verifying Insulin Strategy and Initial Health Outcome aNalysis) explored patterns of insulin treatment among almost 1200 patients in the MENA region, and found that mean HbA1c at insulin initiation was 9.9%, 68% of patients were receiving two or more oral antidiabetic drugs and the mean time from diagnosis to insulin initiation was 8.9 years [20]. Together, these data demonstrate the significant delay in early intensification of treatment to achieve treatment targets, as well as in adjusting treatment despite poor glycaemic control, thus highlighting the need for initiatives that promote timely intervention, reduce clinical inertia and improve long-term outcomes for patients in the Middle East.
Oral Glucose-Lowering Agents for T2DM
Treatment options for T2DM have expanded beyond metformin, sulfonylureas and insulin in recent decades to include thiazolidinediones, sodium-glucose cotransporter 2 (SGLT2) inhibitors, glucagon-like peptide 1 (GLP-1) receptor agonists and dipeptidyl peptidase 4 (DPP4) inhibitors. These agents vary in terms of glucose-lowering efficacy, cardiorenal effects, weight change, cost, hypoglycaemia risk and adverse effects, and subsequently provide physicians and patients with greater opportunity to individualise treatment and optimise clinical outcomes. Current clinical guidelines recommend a patient-centred approach to T2DM management through pragmatic treatment algorithms (Fig. 2) [17, 18, 21].
Guideline-recommended treatment algorithm for type 2 diabetes mellitus (T2DM) [17, 18]. Adapted from reference [17] Davies MJ, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65:1925–66 © Springer Nature, with permission, and reference [18] Cosentino F, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323, by permission of © Oxford University Press. ASCVD atherosclerotic cardiovascular disease, CV cardiovascular, DPP-4i dipeptidyl peptidase 4 inhibitor, GLP-1 RA glucagon-like peptide 1 receptor agonist, SGLT2i sodium-glucose cotransporter 2 inhibitor, SU sulfonylurea, TZD thiazolidinedione
International societies, such as the American Diabetes Association, European Association for the Study of Diabetes and European Society of Cardiology (ESC), advocate a cardiocentric treatment approach to prevent macrovascular complications in at-risk patients and a glucocentric approach to prevent microvascular complications in patients with lower cardiovascular risk [17, 18, 21, 22]. For example, recent guidelines and consensus reports recommend initiating treatment with an SGLT2 inhibitor or GLP-1 receptor agonist (with or without metformin, based on glycaemic needs) for patients with established atherosclerotic CVD (ASCVD), heart failure and/or chronic kidney disease (CKD), or those at high risk for ASCVD (Fig. 2) [17, 18, 21]. These recommendations are based on large cardiovascular outcome trials, which found that these agents significantly improved cardiorenal outcomes in patients with T2DM, ASCVD, heart failure or CKD [23,24,25,26].
A meta-analysis of cardiovascular outcome trials found that the cardioprotective benefits of SGLT2 inhibitors and GLP-1 receptor agonists were only observed in patients with pre-existing CVD at baseline; in these patients, intensive glycaemic control significantly reduced the risk of major cardiovascular events independent of the treatment regimen used [23]. Hence, clinical guidelines recommend first-line metformin and comprehensive lifestyle modifications for most other patients, with additional agent(s) incorporated as needed on the basis of comorbidities and patient-centred treatment needs [18, 21, 22]. To achieve and maintain glycaemic targets (HbA1c < 7%) in the absence of other factors, oral agents with comparatively greater glucose-lowering efficacy, such as GLP-1 receptor agonists, sulfonylureas and thiazolidinediones, are usually prescribed [26, 27]. In patients with a compelling need to minimise hypoglycaemia, preferred agents include DPP4 inhibitors, GLP-1 receptor agonists, SGLT2 inhibitors and/or thiazolidinediones [21].
It is important to recognise high intra-class variation between sulfonylurea agents. For example, a recent meta-analysis including 229 randomised trials and almost 122,000 patients showed that the relative risk of hypoglycaemia was greatest with sulfonylureas, but was considerably lower with gliclazide compared with glimepiride, glipizide and glyburide [27]. Similarly, randomised studies of Muslim patients during Ramadan found that the incidence of hypoglycaemia with gliclazide was lower than with other sulfonylureas (namely glimepiride and glyburide) and was similar to that observed with sitagliptin [28, 29]. In a real-world study, patients with poor glycaemic control with metformin as a single agent received second-line gliclazide modified release (MR) tablets or sitagliptin [30]. Overall, patients in the gliclazide MR group were 35% more likely to achieve HbA1c < 7% than patients in the sitagliptin group, with low rates of hypoglycaemic events (4.7 and 2.6 events per 1000 patient years with gliclazide MR and sitagliptin, respectively). A further distinction between sulfonylurea treatments can be made on the basis of their cardiovascular risk profile: in a network meta-analysis of 24 controlled studies, gliclazide was associated with a lower risk of all-cause and cardiovascular-related mortality compared with glibenclamide, while glimepiride was associated with a lower risk of all-cause, but not cardiovascular-related, mortality than glibenclamide [31]. In addition to excellent glycaemic efficacy and reduced cardiovascular risk, the randomised ADVANCE trial also showed that intensive gliclazide MR-based therapy for T2DM significantly reduced the risk of end-stage renal disease by 65%, new-onset microalbuminuria by 9% and macroalbuminuria by 30% over 5 years, when compared with standard glucose control [32]. Taken together, these data suggest that gliclazide MR is a reasonable second-line treatment option for patients who have no known cardiovascular or renal disease [7, 33]
GLP-1 receptor agonists and SGLT2 inhibitors are recommended to minimise weight gain or promote weight loss, while DPP4 inhibitors may be recommended because they are weight neutral (Fig. 2) [21]. Although most sulfonylureas have been associated with modest weight gain overall, no major weight gain concerns have been observed with gliclazide MR use [34]. Data from the ADVANCE randomised trial similarly suggest that gliclazide MR may have a weight-neutral profile [35]. Additionally, the real-world ZODIAC-39 study found that adding a sulfonylurea (gliclazide, glibenclamide, glimepiride or tolbutamide) to metformin did not significantly change body weight over 5 years of follow-up [36].
For patients and healthcare systems where access is a factor, sulfonylureas and thiazolidinediones are advocated owing to the availability of generic agents and their comparatively lower cost [21]. Finally, international guidelines recommend that patients should be regularly assessed (every 3–6 months) to limit clinical inertia, and encourage physicians to modify treatment without delay in order to achieve and maintain glycaemic targets (Fig. 2) [17, 21].
To help navigate the wide range of oral therapies available for T2DM, the T2DM Oral Agents Fact Checking programme was developed by a neutral physician organisation (the International Centre for Professional Development in Health and Medicine [ICPDHM] in Canada) as an evidence-based resource to educate physicians and optimise patient outcomes [37]. The programme was circulated to healthcare professionals in the MENA region through webinars and face-to-face meetings, was developed in consultation with 17 clinical experts worldwide, and comprises four modules that describe and differentiate current oral therapies to guide individualised treatment decisions based on patient preferences and management priorities. The T2DM Oral Agents Fact Checking programme represents a one-stop resource that aims to support clinical decision-making, improve glycaemic control and alleviate the burden of T2DM on patients and healthcare systems worldwide.
Several patient-, physician-, culture- and government-related factors have been identified as challenges to optimal T2DM management in the Middle East. These factors are summarised in Table 2, alongside recommendations that can form part of the roadmap to improve T2DM management in the region.
Hypertension in the Middle East
Prevalence, Diagnosis and Control
High BP is the leading risk factor for death and disability in the Middle East and worldwide [38]. In a recent analysis that included 1201 studies, 104 million participants and 184 countries, the global prevalence of hypertension was found to have stagnated at approximately 32% between 1990 and 2019, while the number of adults with hypertension doubled from 648 million to 1.28 billion during the same period [4]. These global estimates were largely driven by an increased prevalence of hypertension among people in low- and middle-income countries, despite reduced prevalence in high-income countries over the last three decades [4]. This disparity is particularly evident in the MENA region, where the estimated prevalence of hypertension in 2019 ranged between 26% and 48% across countries (Fig. 3) [4] and an overall prevalence of 43% was observed [39]. High rates of smoking, obesity and physical inactivity have been identified as some of the key modifiable factors that contribute to the prevalence and burden of hypertension in the Middle East [5], where it remains a leading cause of death and disability. Additionally, hypertension awareness, treatment and BP control are suboptimal in the Middle East, where a study of 10,516 people in the United Arab Emirates, Saudi Arabia, Iran and Occupied Palestinian Territory found that only 49% of those with hypertension were aware of their diagnosis, and only 19% had their BP levels effectively controlled [40].
Rates of prevalence, diagnosis, treatment and control of hypertension across the Middle East and North Africa [4]
Despite improving prevalence rates in some countries, the underdiagnosis, undertreatment and inadequate control of high BP remains a global public health issue. Among all women with hypertension worldwide, 59% were estimated to be diagnosed, 47% were treated and 23% achieved BP control [4]. In other words, 41% of women with hypertension were undiagnosed, 12% were diagnosed but not treated and 24% were treated but not controlled. Similarly, global estimates of diagnosis, treatment and control among all men with hypertension were 49%, 38% and 18%, respectively [4]. At a country level, rates of diagnosis, treatment and control varied across the MENA region, where it was estimated that 34–73% of men and women with hypertension were diagnosed, 20–67% were treated and 6–39% achieved BP control (Fig. 3) [4]. Low rates of BP control worldwide stand in contrast to landmark studies showing that effective BP management is achievable in approximately 50–80% of patients across different grades of hypertension and cardiovascular risk profiles [41,42,43,44]. Together, these data highlight the urgent need to identify and close the gaps that currently exist between hypertension prevalence and diagnosis, diagnosis and treatment, and treatment and optimal BP control.
Clinical Guidelines for the Management of Hypertension
The Saudi Heart Association and National Heart Center, American College of Cardiology and American Heart Association (ACC/AHA), ESC and ESH, International Society of Hypertension (ISH) and World Health Organization provide evidence-based recommendations for the diagnosis and management of patients with hypertension [45,46,47,48,49]. To improve rates of BP control in clinical practice, these guidelines strongly recommend treating to target BP and advocate strategies that achieve rapid and sustained BP control, as well as cardiovascular event risk reduction.
Treat to Target BP
Current international guidelines recommend that antihypertensive medication should be considered for patients with BP ≥ 130/80 mmHg depending on comorbidities and other cardiovascular risk factors, and/or advocate treating to a target BP of < 130/80 mmHg in most cases [45,46,47,48,49]. These recommendations represent a lowering of the BP thresholds and targets used in previous guidelines and are informed by increasing evidence that even small improvements in BP are associated with significant improvements in CVD risk and mortality [50, 51]. The Systolic Blood Pressure Intervention Trial (SPRINT) investigated which SBP target (< 120 mmHg [intensive treatment] or < 140 mmHg [standard treatment]) was associated with a lower rate of clinical events in non-diabetic individuals at an increased risk for CVD [52]. The study found that individuals treated to a < 120 mmHg SBP target had a significantly lower rate of major adverse cardiovascular events (1.65% per year) compared with those treated to a < 140 mmHg SBP target (2.19% per year; hazard ratio [HR] 0.75). The all-cause mortality rate for patients on intensive treatment was also lower than for patients on standard treatment (1.03% vs 1.40%, respectively; HR 0.73) [52]. A meta-analysis by the Blood Pressure Lowering Treatment Trialists’ Collaboration found that a 5-mmHg reduction in SBP lowered the risk of major cardiovascular events (i.e. stroke, myocardial infarction, ischaemic heart disease or heart failure causing death or hospitalisation) by approximately 10%, even in patients with normal or high-normal BP [53]. The authors concluded that decisions to initiate antihypertensive therapy should be based on CVD risk rather than BP thresholds and that treatment should be viewed as an effective tool to prevent CVD in at-risk patients, regardless of their BP [53].
Rapid and Sustained BP Control
The most recent ACC/AHA, ESC/ESH and ISH guidelines recommend that antihypertensive therapy should aim to achieve BP control within 3 months of initiation [46,47,48]. Once achieved, these guidelines also recognise the importance of maintaining sustained BP control, given that uncontrolled BP over 24 h (e.g. masked hypertension) and increased BP variability have each been associated with elevated risks of CVD and death [54, 55]. One strategy recommended for rapid and sustained 24-h BP control is single-pill combination (SPC) therapy, which combines two or more antihypertensive agents to increase BP-lowering efficacy, reduce time to reach target BP and extend the duration of action to cover a 24-h period [56]. An extended duration of action over at least 24 h would ideally provide BP control to the very end of the dosing period, and thus limit natural diurnal variation in BP [57]. SPC therapies for the management of hypertension are discussed further below.
Barriers to Optimal BP Control
High clinical inertia among physicians and poor medication adherence among patients have been identified as two of the key reasons for suboptimal BP control in clinical practice [57]. Clinical inertia in hypertension was demonstrated in the Supporting Hypertension Awareness and Research Europe-wide (SHARE) survey, which found that physicians were willing to tolerate higher than recommended BP levels in their patients before taking action [58]. On average, physicians reported that a BP level of 132/82 mmHg was considered satisfactory, 149/92 mmHg was of concern and 168/100 mmHg would require immediate action [58]. More recently, a Dutch analysis of more than 66,000 patients with hypertension found that approximately 10% had uncontrolled BP despite treatment with one or two antihypertensive agents, and 87% of those did not have their treatment adjusted despite poor BP control (i.e. patients were clinically inert) [59]. The three most common reasons for inertia among surveyed physicians were (1) they were waiting for a repeat reading to confirm uncontrolled BP; (2) they recommended lifestyle modifications for BP control; and (3) they considered the reading to be non-representative because previous BP readings were < 140/90 mmHg [59]. Clinical inertia in hypertension is placing patients at greater cardiovascular risk; therefore, there is a need for greater physician awareness of clinical guidelines and the importance of prompt action to improve BP control.
Poor medication adherence (defined as the inability to initiate, comply and/or persist with prescribed treatment) represents a major barrier to optimal BP control among patients with hypertension [60]. Several sociodemographic, healthcare system-related, therapy-related, condition-related and/or patient-related factors have been associated with medication non-adherence in general [60]; however, increasingly complex treatment regimens may pose a particular challenge for patients with high BP. Hypertension is a multifactorial disease and most patients require multiple medications in order to achieve and maintain BP control [61, 62]; consequently, it is burdensome for patients to adhere to multiple drugs and dosing schedules, frequent treatment adjustments and often other medications for common comorbidities. A multicentre study of patients with hypertension in the UK (n = 676) and Czech Republic (n = 672) found that with each additional antihypertensive medication prescribed, there was a corresponding 85% and 77% increase in the odds of non-adherence (both P < 0.001), respectively [63]. Strategies to improve medication adherence include raising patient awareness of the risks of hypertension and benefits of treatment, encouraging shared decision-making between physicians and patients, and utilising newer treatment options that streamline and simplify BP-lowering therapy [60].
Antihypertensive Agents for BP Control
In an effort to improve patient adherence, reduce clinical inertia and facilitate rapid and sustained BP control, local and international guidelines now recommend SPC therapy as the first-line treatment option for most patients with hypertension [45, 47, 48]. Compared with the previous stepped-care approach, first-line SPC therapy addresses the need to target multiple pathways in hypertension and is associated with faster BP control, reduced risk of cardiovascular events, improved safety and tolerability, and greater convenience for patients and physicians [56]. A wide range of SPCs have been developed that vary in the number, class and dosage of drugs in combination, which in turn enables individualised therapy based on patient profiles and the mechanism(s) driving their hypertension (Fig. 4).
Clinical guidelines recommend a two-drug SPC for the first-line treatment of hypertension, with a renin–angiotensin–aldosterone system (RAAS) inhibitor forming the basis of treatment (Fig. 5) [45, 47, 48]. RAAS inhibition may be achieved with either an angiotensin-converting enzyme (ACE) inhibitor or angiotensin II receptor blocker (ARB); however, there is evidence that ACE inhibitors may provide additional cardioprotective benefits and reduce cardiovascular risk beyond ARBs [64,65,66,67,68,69]. This is thought to be attributable to the different mechanisms of action between ACE inhibitors and ARBs [64, 70]. ARBs prevent angiotensin II (Ang II) from binding to its receptors, Ang II receptor type 1 (AT1R) and type 2 (AT2R) [70]. Prolonged ARB treatment results in significant increases in Ang II levels, which may lead to overactivation of AT2R and putative adverse effects on the cardiovascular system [64]. ACE inhibitors, like perindopril, prevent Ang II from being synthesised from Ang I and block the metabolism of bradykinin to inactive metabolites, thereby potentiating bradykinin levels and restoring the balance between Ang II and bradykinin [70]. Increased bradykinin levels lead to increased nitric oxide, endothelium-derived hyperpolarizing factor and prostaglandin levels, which are thought to lead, in turn, to vasodilation and antifibrotic, anti-inflammatory and antithrombotic effects [70]. As reviewed in depth previously, the cardiovascular benefits of ACE inhibitors over ARBs have been attributed to their specific effect on bradykinin levels and their consequent downstream pleiotropic effects [64]. Among the ACE inhibitors, perindopril is one of the most widely studied across the CVD continuum and provides durable BP-lowering effects over 24 h [65, 71], thus making it a favourable RAAS inhibitor for antihypertensive SPC therapy because of its cardioprotective properties.

Adapted from reference [47] Williams B, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39:3021–104, by permission of © Oxford University Press. ACEi angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, CCB calcium channel blocker, DM diabetes mellitus, RAASi renin–angiotensin–aldosterone system inhibitor, SPC single-pill combination
The preferred first-line SPC therapy for hypertension is a RAAS inhibitor plus a calcium channel blocker (CCB) or a thiazide or thiazide-like diuretic; if BP is not controlled with a two-drug SPC, then a triple combination (RAAS inhibitor, CCB and diuretic) is recommended (Fig. 5) [45, 48]. These recommendations are based on evidence including the landmark Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT), which found that amlodipine plus as-needed perindopril effectively controlled BP in patients with hypertension and reduced cardiovascular and all-cause mortality risk beyond combination therapy with a β-blocker and diuretic [72, 73]. Following on from ASCOT, the real-world SafeTy and efficacy analysis of coveRsyl amlodipine in uncOntrolled and Newly diaGnosed hypertension (STRONG) study showed that a once-daily SPC of perindopril and amlodipine rapidly and significantly reduced BP, while demonstrating an acceptable safety profile and enabling high treatment adherence [74]. Similarly in the AVANGARD study, target BP (i.e. < 140/90 mmHg) was achieved by 93.5% of patients who switched to a perindopril–amlodipine SPC following suboptimal BP control with ARB-based combination therapy [75]. A pooled analysis (n = 16,763) of the FORTISSIMO, FORSAGE, ACES and PICASSO observational studies found that a SPC of perindopril and indapamide resulted in significant mean reductions in SBP (− 23 mmHg) after 1 month of treatment compared with baseline (P < 0.001); by 3 months of treatment, 70% of patients had achieved BP control (< 140/90 mmHg in patients without diabetes; < 140/85 mmHg in patients with diabetes) [76]. In a subgroup of patients receiving triple therapy (CCB + SPC; n = 4002), mean SBP had decreased by 28 ± 15 mmHg at 3 months of treatment and BP had normalised in 65% of patients [76]. In patients requiring escalation to triple combination therapy, the Perindopril–Indapamide plus AmlodipiNe in high rISk hyperTensive patients (PIANIST) study demonstrated the safety and BP-lowering efficacy of perindopril and indapamide plus amlodipine [77]; in the ADVANCE trial, the combination of perindopril and indapamide plus a CCB was also shown to enhance cardiovascular and mortality risk reduction compared with perindopril–indapamide alone in patients with T2DM [78]. More recently, a subgroup analysis of the Brisighella Heart Study showed that triple combination therapy with a RAAS inhibitor (particularly an ACE inhibitor), CCB and thiazide or thiazide-like diuretic was associated with better long-term BP and lipid control than other three-drug combinations, while perindopril–amlodipine–indapamide in particular was associated with a better metabolic profile [79].
For cases of resistant hypertension despite three-drug SPC therapy, clinical guidelines recommend the addition of spironolactone or, if not tolerated, another diuretic, a β-blocker or an α-blocker (Fig. 5) [45, 47, 48]. Evidence supporting add-on spironolactone comes from the randomised PATHWAY-2 trial, which compared the safety and efficacy of spironolactone, bisoprolol, doxazosin and placebo when added to three-drug combination therapy in patients with treatment-resistant hypertension [80]. PATHWAY-2 showed that add-on spironolactone was the most effective BP-lowering agent, suggesting that excessive sodium retention plays key role in resistant hypertension [80].
Although clinical guidelines provide a preferred treatment algorithm for the management of hypertension, they also state that other combinations of the five major drug classes (i.e. ACE inhibitors, ARBs, CCBs, diuretics and β-blockers) can be used [47, 48]. For example, combination therapies including β-blockers (e.g. bisoprolol and perindopril) are advocated for patients with comorbidities where sympathetic nervous system activation is implicated, such as angina, post-myocardial infarction, heart failure and increased heart rate [47, 81]. It is hoped that these pragmatic guidelines, in conjunction with the wide range of SPC therapies available, will help to optimise treatment for patients with hypertension and improve BP control in the Middle East and worldwide.
Roadmap for Better T2DM and Hypertension Management in the Middle East
A plan of action is needed to optimise the management of T2DM and hypertension in the Middle East region (Fig. 6). Glucocentric and cardiocentric strategies are required, in conjunction with managing obesity (one of the main risk factors contributing to the high prevalence of both T2DM and hypertension) and early detection methods for at-risk individuals, to address the increasing prevalence of both diseases, low glycaemic and BP control rates, and diabetes- and cardiovascular-related complications. Several specific recommendations regarding the challenges faced in the management of T2DM are summarised in Table 2, and many may also be applicable to hypertension.
Overall, the following broad recommendations can be made, with the aim of helping improve the quality of care of patients with T2DM and/or hypertension in the Middle East: a roll-out of disease prevention strategies; increased public awareness (including early detection and screening); training of healthcare providers; individualised approaches to management; patient education initiatives, support and self-management of the disease(s); implementation of governmental plans and strategies to reduce the diabetes burden with defined outcomes; and advocating for greater investment in diabetes research. A collaborative effort by healthcare professionals, government departments and patients would ensure optimal outcomes in managing T2DM and hypertension effectively.
Conclusions
T2DM and hypertension are both highly prevalent, underdiagnosed and poorly controlled diseases in the Middle East. Patients frequently require treatment modification and/or escalation to achieve and maintain early glycaemic and BP control; however, clinical inertia among physicians and poor medication adherence among patients are major barriers to achieving optimal treatment outcomes. Recent advances, including individualised treatment algorithms, the T2DM Oral Agents Fact Checking programme and SPC antihypertensive therapies, aim to address these challenges by promoting personalised therapy and reducing the burden of treatment on patients and physicians. A recommended roadmap for the management of T2DM and hypertension in the Middle East includes disease prevention, public awareness and training of healthcare providers, individualised disease management, better patient education, targeted government policies and increased research (Fig. 6).
References
World Health Organization. Cardiovascular diseases (CVDs). 2021. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed Oct 24, 2022.
International Diabetes Federation. IDF diabetes atlas, 10th edition. 2021. Brussels, Belgium. https://www.diabetesatlas.org. Accessed Oct 18, 2022.
Meo SA, Usmani AM, Qalbani E. Prevalence of type 2 diabetes in the Arab world: impact of GDP and energy consumption. Eur Rev Med Pharmacol Sci. 2017;21:1303–12.
NCD Risk Factor Collaboration. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–80.
Abboud M, Karam S. Hypertension in the Middle East: current state, human factors, and barriers to control. J Hum Hypertens. 2022;36:428–36.
Al-Rasheedi AA. Glycemic control among patients with type 2 diabetes mellitus in countries of Arabic Gulf. Int J Health Sci (Qassim). 2015;9:345–50.
Hassanein M, Akbar MAJ, Al-Shamiri M, et al. Management of diabetes and hypertension within the Gulf Region: updates on treatment practices and therapies. Diabetes Ther. 2022;13:1253–80.
Aljulifi MZ. Prevalence and reasons of increased type 2 diabetes in Gulf Cooperation Council Countries. Saudi Med J. 2021;42:481–90.
Gomes MB, Rathmann W, Charbonnel B, et al. Treatment of type 2 diabetes mellitus worldwide: baseline patient characteristics in the global DISCOVER study. Diabetes Res Clin Pract. 2019;151:20–32.
Lind M, Imberg H, Coleman RL, Nerman O, Holman RR. Historical HbA1c values may explain the type 2 diabetes legacy effect: UKPDS 88. Diabetes Care. 2021;44:2231–7.
Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–12.
Laiteerapong N, Ham SA, Gao Y, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (The Diabetes & Aging Study). Diabetes Care. 2019;42:416–26.
Folz R, Laiteerapong N. The legacy effect in diabetes: are there long-term benefits? Diabetologia. 2021;64:2131–7.
International Diabetes Federation. IDF clinical practice recommendations for managing type 2 diabetes in primary care. 2017. https://idf.org/e-library/guidelines.html. Accessed Oct 19, 2022.
National Institute for Health and Care Excellence. Type 2 diabetes in adults: management. 2015. https://www.nice.org.uk/guidance/ng28. Accessed Oct 19, 2022.
Draznin B, Aroda VR, Bakris G, et al. Glycemic targets: standards of medical care in diabetes—2022. Diabetes Care. 2022;45:S83–96.
Davies MJ, Aroda VR, Collins BS, et al. Management of hyperglycaemia in type 2 diabetes, 2022. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2022;65:1925–66.
Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J. 2020;41:255–323.
Khunti K, Chen H, Cid-Ruzafa J, et al. Glycaemic control in patients with type 2 diabetes initiating second-line therapy: results from the global DISCOVER study programme. Diabetes Obes Metab. 2020;22:66–78.
Jabbar A, Abdallah K, Hassoun A, et al. Patterns and trends in insulin initiation and intensification among patients with type 2 diabetes mellitus in the Middle East and North Africa region. Diabetes Res Clin Pract. 2019;149:18–26.
Draznin B, Aroda VR, Bakris G, et al. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes—2022. Diabetes Care. 2022;45:S125–43.
Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020;43:487–93.
Giugliano D, Maiorino MI, Bellastella G, Chiodini P, Esposito K. Glycemic control, preexisting cardiovascular disease, and risk of major cardiovascular events in patients with type 2 diabetes mellitus: systematic review with meta-analysis of cardiovascular outcome trials and intensive glucose control trials. J Am Heart Assoc. 2019;8: e012356.
McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021;6:148–58.
Giugliano D, Scappaticcio L, Longo M, et al. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20:189.
Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020;173:278–86.
Maloney A, Rosenstock J, Fonseca V. A model-based meta-analysis of 24 antihyperglycemic drugs for type 2 diabetes: comparison of treatment effects at therapeutic doses. Clin Pharmacol Ther. 2019;105:1213–23.
Al Sifri S, Basiounny A, Echtay A, et al. The incidence of hypoglycaemia in Muslim patients with type 2 diabetes treated with sitagliptin or a sulphonylurea during Ramadan: a randomised trial. Int J Clin Pract. 2011;65:1132–40.
Aravind SR, Ismail SB, Balamurugan R, et al. Hypoglycemia in patients with type 2 diabetes from India and Malaysia treated with sitagliptin or a sulfonylurea during Ramadan: a randomized, pragmatic study. Curr Med Res Opin. 2012;28:1289–96.
Zaccardi F, Jacquot E, Cortese V, et al. Comparative effectiveness of gliclazide modified release versus sitagliptin as second-line treatment after metformin monotherapy in patients with uncontrolled type 2 diabetes. Diabetes Obes Metab. 2020;22:2417–26.
Simpson SH, Lee J, Choi S, Vandermeer B, Abdelmoneim AS, Featherstone TR. Mortality risk among sulfonylureas: a systematic review and network meta-analysis. Lancet Diabetes Endocrinol. 2015;3:43–51.
Perkovic V, Heerspink HL, Chalmers J, et al. Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83:517–23.
Al-Saleh Y, Sabico S, Al-Furqani A, et al. Sulfonylureas in the current practice of type 2 diabetes management: are they all the same? Consensus from the Gulf Cooperation Council (GCC) Countries Advisory Board on sulfonylureas. Diabetes Ther. 2021;12:2115–32.
Tomlinson B, Li YH, Chan P. Evaluating gliclazide for the treatment of type 2 diabetes mellitus. Expert Opin Pharmacother. 2022;23:1869–77.
Zoungas S, Chalmers J, Kengne AP, et al. The efficacy of lowering glycated haemoglobin with a gliclazide modified release-based intensive glucose lowering regimen in the ADVANCE trial. Diabetes Res Clin Pract. 2010;89:126–33.
Schrijnders D, Wever R, Kleefstra N, et al. Addition of sulphonylurea to metformin does not relevantly change body weight: a prospective observational cohort study (ZODIAC-39). Diabetes Obes Metab. 2016;18:973–9.
Servier. Type 2 diabetes mellitus oral agent's fact checking. 2022. Servier. https://medicallearninghub.com/course/type-2-diabetes-mellitus-oral-agents-fact-checking. Accessed Oct 25, 2022.
GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–94.
Alsheikh-Ali AA, Omar MI, Raal FJ, et al. Cardiovascular risk factor burden in Africa and the Middle East: the Africa Middle East Cardiovascular Epidemiological (ACE) study. PLoS ONE. 2014;9: e102830.
Yusufali AM, Khatib R, Islam S, et al. Prevalence, awareness, treatment and control of hypertension in four Middle East countries. J Hypertens. 2017;35:1457–64.
Wright JT Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–31.
Oparil S. Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial (ALLHAT): practical implications. Hypertension. 2003;41:1006–9.
Zhang W, Zhang S, Deng Y, et al. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med. 2021;385:1268–79.
JATOS Study Group. Principal results of the Japanese trial to assess optimal systolic blood pressure in elderly hypertensive patients (JATOS). Hypertens Res. 2008;31:2115–27.
Alhabeeb W, Tash AA, Alshamiri M, et al. National Heart Center/Saudi Heart Association 2023 guidelines on the management of hypertension. J Saudi Heart Assoc. 2023;35:16–39.
Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Hypertension. 2018;71:e13–115.
Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39:3021–104.
Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75:1334–57.
World Health Organization. Guideline for the pharmacological treatment of hypertension in adults. 2021. World Health Organization, Geneva. https://apps.who.int/iris/bitstream/handle/10665/344424/9789240033986-eng.pdf. Accessed Oct 24, 2022.
Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13.
Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387:957–67.
Wright JT Jr, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16.
Collaboration BPLTT. Pharmacological blood pressure lowering for primary and secondary prevention of cardiovascular disease across different levels of blood pressure: an individual participant-level data meta-analysis. Lancet. 2021;397:1625–36.
Brguljan-Hitij J, Thijs L, Li Y, et al. Risk stratification by ambulatory blood pressure monitoring across JNC classes of conventional blood pressure. Am J Hypertens. 2014;27:956–65.
Mehlum MH, Liestøl K, Kjeldsen SE, et al. Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J. 2018;39:2243–51.
Campana E, Cunha V, Glaveckaite S, et al. The use of single-pill combinations as first-line treatment for hypertension: translating guidelines into clinical practice. J Hypertens. 2020;38:2369–77.
Parati G, Lombardi C, Pengo M, Bilo G, Ochoa JE. Current challenges for hypertension management: from better hypertension diagnosis to improved patients’ adherence and blood pressure control. Int J Cardiol. 2021;331:262–9.
Redon J, Erdine S, Böhm M, et al. Physician attitudes to blood pressure control: findings from the Supporting Hypertension Awareness and Research Europe-wide survey. J Hypertens. 2011;29:1633–40.
Ali DH, Kiliç B, Hart HE, et al. Therapeutic inertia in the management of hypertension in primary care. J Hypertens. 2021;39:1238–45.
Burnier M, Egan BM. Adherence in hypertension: a review of prevalence, risk factors, impact, and management. Circ Res. 2019;124:1124–40.
Bramlage P, Böhm M, Volpe M, et al. A global perspective on blood pressure treatment and control in a referred cohort of hypertensive patients. J Clin Hypertens (Greenwich). 2010;12:666–77.
Thoenes M, Neuberger HR, Volpe M, Khan BV, Kirch W, Böhm M. Antihypertensive drug therapy and blood pressure control in men and women: an international perspective. J Hum Hypertens. 2010;24:336–44.
Gupta P, Patel P, Štrauch B, et al. Risk factors for nonadherence to antihypertensive treatment. Hypertension. 2017;69:1113–20.
Lévy BI, Mourad JJ. Renin angiotensin blockers and cardiac protection: from basics to clinical trials. Am J Hypertens. 2022;35:293–302.
Ancion A, Tridetti J, Nguyen Trung ML, Oury C, Lancellotti P. A review of the role of bradykinin and nitric oxide in the cardioprotective action of angiotensin-converting enzyme inhibitors: focus on perindopril. Cardiol Ther. 2019;8:179–91.
van Vark LC, Bertrand M, Akkerhuis KM, et al. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: a meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone system inhibitors involving 158,998 patients. Eur Heart J. 2012;33:2088–97.
Strauss MH, Hall AS. Angiotensin receptor blockers do not reduce risk of myocardial infarction, cardiovascular death, or total mortality: further evidence for the ARB-MI paradox. Circulation. 2017;135:2088–90.
Strauss MH, Hall AS. Angiotensin receptor blockers may increase risk of myocardial infarction: unraveling the ARB-MI paradox. Circulation. 2006;114:838–54.
Hall AS, Strauss MH. More about the “ARB MI paradox.” Heart. 2007;93:1011–4.
Taddei S, Bortolotto L. Unraveling the pivotal role of bradykinin in ACE inhibitor activity. Am J Cardiovasc Drugs. 2016;16:309–21.
Flack JM, Nasser SA. Benefits of once-daily therapies in the treatment of hypertension. Vasc Health Risk Manag. 2011;7:777–87.
Dahlöf B, Sever PS, Poulter NR, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906.
Gupta A, Mackay J, Whitehouse A, et al. Long-term mortality after blood pressure-lowering and lipid-lowering treatment in patients with hypertension in the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Legacy study: 16-year follow-up results of a randomised factorial trial. Lancet. 2018;392:1127–37.
Bahl VK, Jadhav UM, Thacker HP. Management of hypertension with the fixed combination of perindopril and amlodipine in daily clinical practice: results from the STRONG prospective, observational, multicenter study. Am J Cardiovasc Drugs. 2009;9:135–42.
Glezer MG. Antihypertensive effect of switching to a fixed perindopril/amlodipine combination in patients ineffectively treated by free sartan-containing combinations. Results of the AVANGARD Study [in Russian]. Kardiologiia. 2019;59:31–8.
Dézsi CA, Glezer M, Karpov Y, Brzozowska-Villatte R, Farsang C. Effectiveness of perindopril/indapamide single-pill combination in uncontrolled patients with hypertension: a pooled analysis of the FORTISSIMO, FORSAGE, ACES and PICASSO observational studies. Adv Ther. 2021;38:479–94.
Tóth K. Antihypertensive efficacy of triple combination perindopril/indapamide plus amlodipine in high-risk hypertensives: results of the PIANIST study (Perindopril-Indapamide plus AmlodipiNe in high rISk hyperTensive patients). Am J Cardiovasc Drugs. 2014;14:137–45.
Chalmers J, Arima H, Woodward M, et al. Effects of combination of perindopril, indapamide, and calcium channel blockers in patients with type 2 diabetes mellitus: results from the Action in Diabetes and Vascular Disease: Preterax and Diamicron Controlled Evaluation (ADVANCE) trial. Hypertension. 2014;63:259–64.
Cicero AFG, Fogacci F, Rizzoli E, D’Addato S, Borghi C. Long-term impact of different triple combination antihypertensive medications on blood pressure control, metabolic pattern and incident events: data from the Brisighella Heart Study. J Clin Med. 2021;10(24):5921.
Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–68.
Abeel M, Gupta A, Constance C. Concomitant treatment of hypertensive patients with bisoprolol and perindopril in routine clinical practice: a post hoc analysis of the CONFIDENCE II, PROTECT I, and PROTECT III observational studies. Adv Ther. 2022;39:391–404.
Acknowledgements
Funding
Servier provided funding for the Evidence in Diabetes and Hypertension Summit (EVIDENT) and an unrestricted educational grant to develop the T2DM Oral Agents Fact Checking programme. The sponsor also funded the preparation of this article and the journal’s Rapid Service and Open Access Fees.
Medical Writing, Editorial, and Other Assistance
Editorial assistance in the preparation of this article was provided by Karina Hamilton-Peel of Springer Healthcare Communications, who wrote the first draft of this article. Support for this assistance was funded by Servier.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Author Contributions
All named authors prepared and gave presentations during the EVIDENT summit, held in September 2022. All named authors participated in the drafting, critical review, and approval of this article, which is based on their presentations at the EVIDENT summit.
Prior Presentation
This article is based on presentations given by the authors during the EVIDENT summit, held on September 9, 2022, in Jeddah, Kingdom of Saudi Arabia.
Disclosures
Yousef Al Saleh received honoraria from and was on advisory boards for Lilly, Novo Nordisk, Sanofi Aventis and Servier. Siew Pheng Chan received honoraria as a speaker or member of advisory boards for AstraZeneca, Boehringer Ingelheim, Merck, Novo Nordisk, Novartis, Sanofi Aventis, Servier and Zuellig Pharma. Noor Al Busaidi, Waleed Al Dahi, Munawar Almajnoni, Al Saeed Mohammed, Khalid Alshali, Mostafa Al-Shamiri, Saud Al Sifri, Mohammed Arafah, Hassan El-Tamimi, Khadija Hafidh, Mohamed Hassanein, Ashraf Shaaban, Ali Sultan and Guido Grassi declare no conflicts of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no data sets were generated or analysed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Al Saleh, Y., Al Busaidi, N., Al Dahi, W. et al. Roadmap for the Management of Type 2 Diabetes and Hypertension in the Middle East: Review of the 2022 EVIDENT Summit. Adv Ther 40, 2965–2984 (2023). https://doi.org/10.1007/s12325-023-02529-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02529-7