Abstract
Introduction
Guidelines for the treatment of chronic kidney disease (CKD) recommend early intervention and management to slow disease progression. However, associations between diagnosis and CKD progression are not fully understood.
Methods
REVEAL-CKD (NCT04847531) is a retrospective observational study of patients with stage 3 CKD. Data were extracted from the US TriNetX database. Eligible patients had two consecutive estimated glomerular filtration rate (eGFR) measurements indicative of stage 3 CKD (≥ 30 and < 60 ml/min/1.73 m2) recorded 91–730 days apart from 2015 to 2020. Diagnosed patients were included if their first CKD diagnosis code was recorded at least 6 months after their second qualifying eGFR measurement. We assessed CKD management and monitoring practices for the 180 days before and after CKD diagnosis, annual eGFR decline in the 2 years before and after CKD diagnosis, and associations between diagnostic delay and post-diagnosis event rates.
Results
The study included 26,851 patients. After diagnosis, we observed significant increases in the prescribing rate of guideline-recommended medications such as angiotensin-converting enzyme inhibitors (rate ratio [95% confidence interval]: 1.87 [1.82, 1.93]), angiotensin receptor blockers (1.91 [1.85, 1.97]) and mineralocorticoid receptor antagonists (2.23 [2.13, 2.34]). Annual eGFR decline was significantly reduced following a CKD diagnosis, from 3.20 ml/min/1.73 m2 before diagnosis to 0.74 ml/min/1.73 m2 after diagnosis. Delayed diagnosis (by 1-year increments) was associated with elevated risk of CKD progression to stage 4/5 (1.40 [1.31–1.49]), kidney failure (hazard ratio [95% confidence interval]: 1.63 [1.23–2.18]) and the composite of myocardial infarction, stroke and hospitalization for heart failure (1.08 [1.04–1.13]).
Conclusions
A recorded CKD diagnosis was associated with significant improvements in CKD management and monitoring practices and attenuated eGFR decline. Recorded diagnosis of stage 3 CKD is an important first step to reduce the risk of disease progression and minimize adverse clinical outcomes.
Trial Registration
ClinicalTrials.gov identifier, NCT04847531.
Plain Language Summary
Chronic kidney disease (CKD) is a long-term condition in which the function of the kidneys is reduced. Kidney function is monitored using a measurement called the estimated glomerular filtration rate. CKD can be separated into stages of severity, ranging from 1 (mild) to 5 (severe), using estimated glomerular filtration rate. Mild to moderate CKD (stages 1–3) is difficult to diagnose because there are usually no symptoms. In this study from the REVEAL-CKD programme, we looked at the effects of having undiagnosed stage 3 (moderate) CKD and examined how a CKD diagnosis affects disease management and worsening of the condition. Using a database of medical records for patients in the USA called TriNetX, we looked at data from over 26,000 patients with stage 3 CKD who were identified using estimated glomerular filtration rate measurements. We found that healthcare teams prescribed significantly more guideline-recommended medications and did more clinical monitoring in the 180 days after a CKD diagnosis than they did before the diagnosis. Additionally, the rate of decline in kidney function slowed after a CKD diagnosis. Delaying diagnosis by 1 year increased the risk of deterioration of the condition by 40%, the risk of needing a kidney transplant or long-term dialysis treatment by 63% and the risk of major heart and blood vessel diseases (known as cardiovascular events) by 8%. Our findings suggest that diagnosis of stage 3 CKD is an important first step to reduce the risk of the disease worsening and other complications.
Video Abstract (MP4 82773 KB)

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Chronic kidney disease (CKD) is highly prevalent but remains under-diagnosed, leading to missed opportunities to slow disease progression with guideline-directed interventions. |
This analysis of patients with estimated glomerular filtration rate (eGFR) measurements indicative of stage 3 CKD aimed to investigate changes in CKD management and monitoring practices, eGFR decline and clinical outcomes following a recorded diagnosis of CKD. |
What was learned from the study? |
In patients with stage 3 CKD, presence of a CKD diagnosis code was followed by an improvement in CKD management and monitoring practices and an attenuation in eGFR decline. |
Conversely, delayed diagnosis was associated with an elevated risk of adverse cardiorenal outcomes, including kidney failure, CKD progression and the composite of myocardial infarction, stroke and hospitalization for heart failure. |
These findings suggest that a recorded diagnosis of stage 3 CKD is an important first step to reduce the risk of disease progression and associated complications. |
Digital Features
This article is published with digital features, including a video abstract, graphical PLS, and slide deck to facilitate understanding of the article. To view digital features for this article go to https://doi.org/10.6084/m9.figshare.22147376.
Introduction
Chronic kidney disease (CKD) is an established public health concern, affecting an estimated 11% of the global population [1]. The prevalence of CKD is rising, owing in part to increasing prevalence of risk factors for CKD, such as type 2 diabetes (T2D) and hypertension [2, 3]. However, the disease is often under-recognized by patients and healthcare practitioners [4]. This is particularly the case for stage 1–3 CKD, which is primarily asymptomatic and is usually identified through laboratory measurements of kidney function such as estimated glomerular filtration rate (eGFR) and urinary albumin-to-creatinine ratio (UACR) [5].
Increased emphasis is being placed on early intervention in CKD. For example, recent guidance from the Kidney Disease: Improving Global Outcomes (KDIGO) workgroup stressed the importance of early intervention and appropriate management of CKD to delay disease progression and minimize associated complications [5, 6]. CKD is managed using a multifaceted approach that includes lifestyle changes, blood pressure control [7], lipid management [8], glycaemic control in patients with T2D [9, 10] and, more recently, use of new drugs with cardiovascular and renal benefits, such as sodium–glucose co-transporter-2 (SGLT-2) inhibitors [11, 12]. Despite efforts to improve healthcare practitioner awareness of CKD, as well as clear guidelines advocating for early intervention and the approval of new drugs shown to improve outcomes in patients with the disease, diagnosis rates of stage 1–3 CKD remain low [13,14,15,16,17,18,19]. These low diagnosis rates are driven in part by low rates of UACR testing in routine clinical practice [2, 20, 21] as well as a lack of clinical recognition of reduced eGFR values [22].
A recorded diagnosis represents the formal basis for the treatment a patient receives and is crucial for informing other members of the multidisciplinary teams often involved in the management of patients with CKD. Timely and accurate diagnosis allows healthcare practitioners to tailor decision-making to individual patients [23]. Furthermore, a diagnosis provides an opportunity to improve the health literacy of patients [24], supporting lifestyle changes that are often the foundation of disease management in early stage CKD [5, 25]. Recorded diagnoses can also inform healthcare policy, resource allocation and research priorities [26, 27].
Despite the high prevalence of CKD and the risk of complications and mortality associated with the disease, there has been little research on associations between a recorded CKD diagnosis and patient management or clinical outcomes. The REVEAL-CKD study programme (NCT04847531) is a multinational, observational study designed to investigate the prevalence and consequences of undiagnosed stage 3 CKD in large populations across several countries. Here, we investigate associations between a recorded diagnosis of CKD and the medical management of, and clinical outcomes in, adult patients in the USA with eGFR measurements indicative of stage 3 CKD.
Methods
The study design for REVEAL-CKD has been reported in detail elsewhere [28] and is briefly summarized below.
Data for this analysis were extracted from TriNetX [29], a database of integrated electronic medical records and insurance claims data from 35 healthcare organizations in the USA. TriNetX provides clinical patient data and prescription information from both inpatient and outpatient encounters. REVEAL-CKD used de-identified data from existing databases and did not require data collection beyond that of routine clinical care. No identifiable information was collected or examined as part of the study. All externally conducted analyses were completed in line with local ethics regulations/legislation. De-identified, internally licensed databases were shared with AstraZeneca by the licensee; therefore, ethics review and approval were not required for the use of these databases for this study.
Patients eligible for inclusion in REVEAL-CKD (aged ≥ 18 years) were identified by the presence of two consecutive eGFR measurements indicative of stage 3a/3b CKD (≥ 30 and < 60 ml/min/1.73 m2) recorded > 90 days apart (up to a maximum of 730 days), taken between 1 January 2015 and 31 December 2020 (Fig. 1). All patients had at least 12 months of continuous presence in the database before the first qualifying eGFR measurement. Patients with a solid organ transplant, renal replacement therapy or any evidence of advanced CKD (stages 4, 5 and end-stage renal disease) recorded before the second qualifying eGFR measurement were excluded. eGFR was calculated from serum creatinine, sex and age using the CKD Epidemiology Collaboration (CKD-EPI) equation [30]. In line with expert recommendations, race was not included in the calculation of eGFR [31]. Diagnoses of CKD were identified by the presence of an International Classification of Diseases (ICD)-9/10 code for CKD (any stage). The full list of ICD-9/10 codes used to determine diagnoses can be found in Supplementary Materials Table 1.
Study timeline. aeGFR was calculated using the CKD Epidemiology Collaboration equation from serum creatinine, sex and age values. bTwo consecutive eGFR measurements ≥ 30 and < 60 ml/min/1.73 m² recorded 91–730 days apart from 2015 to 2020. cEligible patients required ≥ 12 months of continuous database presence before the first qualifying eGFR measurement. dIncluded patients lacked a CKD diagnosis before and up to 6 months after the second qualifying eGFR measurement and were diagnosed with CKD 6 months or more after their second qualifying eGFR measurement. eCKD diagnosis status was determined by the presence of an International Classification of Diseases-9/10 code for CKD (any stage). CKD chronic kidney disease, eGFR estimated glomerular filtration rate
Patients eligible for REVEAL-CKD were included in this analysis if they did not have a CKD diagnosis any time before, and up to 6 months after the date of their second qualifying eGFR measurement, and subsequently received a CKD diagnosis (i.e. there was a period of ≥ 6 months between their second qualifying eGFR measurement and the first recorded CKD diagnosis; Fig. 1). The index date for this analysis was defined as the date of the CKD diagnosis. Patients were classified into CKD stage according to the most recent eGFR measurement available on, or within 12 months before, the index date.
Statistical Analysis
Demographic and clinical characteristics of patients at index are presented descriptively. The incidence of CKD management practices and treatment patterns (identified using pharmacy claims) is presented as events per person-year with exact Poisson confidence intervals (CIs) and compared between the 180 days pre-and post-diagnosis using a Poisson regression model.
Estimated eGFR trajectories as a function of time to (and from) CKD diagnosis were estimated by applying a generalized additive model [32].
The annual eGFR decline before and after a CKD diagnosis was estimated by fitting individual linear regression models with time to diagnosis as the only independent variable for the 2-year period before, and up to 2-year period after, a CKD diagnosis. Estimated annual eGFR decline was summarized using medians and compared before and after CKD diagnosis using the Wilcoxon rank sum test. To minimize the observed effect of regression to the mean, calculation of eGFR slopes excluding eGFR measurements taken within 0.5 years before or after the date of CKD diagnosis was also performed.
The incidence of clinical outcomes was estimated as the number of events per 100 person-years and presented with 95% exact Poisson CIs.
Associations between diagnostic delay and post-diagnosis event rates were assessed using a Cox regression model. Diagnostic delay was defined as the time from 6 months after the second qualifying eGFR measurement to CKD diagnosis. This Cox regression model was adjusted for sex, age, history of diabetes (type 1 and type 2), number of pre-index visits, eGFR change rate (difference between eGFR at second qualifying measurement and eGFR at or within 12 months before CKD diagnosis divided by the time between those measurements) and eGFR at second qualifying eGFR measurement. Hazard ratios from this model estimated the relative increase in risk for each year of diagnostic delay; a hazard ratio > 1 indicates that greater diagnostic delay increased the risk of an event.
All hypothesis tests were performed at the 5% significance level. Missing data were not imputed. All statistical analyses were performed in R version 4.0.2.
Results
In total, 26,851 patients were included in the analysis (Supplementary Materials Fig. 1), with a median (interquartile range) follow-up time after CKD diagnosis of 1.01 (0.39–1.89) years. Demographic and clinical characteristics of patients at study index, overall and by CKD stage 3a/3b at study index (time of CKD diagnosis), are shown in Table 1. Overall, the mean age was 71.3 years, and 57.4% of patients were female. The mean (standard deviation) diagnostic delay (time from second qualifying eGFR measurement to CKD diagnosis) was 1.58 (0.90) years. When grouping patients by CKD stage based on their most recent eGFR measurement at or within 12 months before diagnosis, 10.0% of patients had stage 1/2 CKD and 10.1% of patients had stage 4/5 CKD; the remaining patients had stage 3a/b CKD. The proportions of patients with comorbidities such as hypertension, T2D, heart failure or a history of stroke or myocardial infarction tended to be higher in patients with stage 3b CKD at diagnosis than in those with stage 3a CKD.
CKD Management and Monitoring Practices
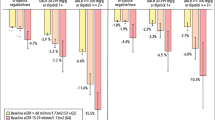
CKD management and monitoring practices, including select care quality indicators and prescribing of recommended medications in the 180 days before and after CKD diagnosis, were significantly improved following a CKD diagnosis (Fig. 2 and Supplementary Materials Table 2). The rate of blood pressure and creatinine monitoring doubled after a CKD diagnosis; more modest improvements in UACR testing and glycated haemoglobin (HbA1c) monitoring were also observed. Of note, while the rate of UACR testing did increase after a CKD diagnosis, it remained extremely low (0.05 measurements per person-year or 5 measurements per 100 person-years).
Rate ratios for CKD management and monitoring care quality indicators and prescription fills of guideline-recommended medications in the 180 days before and after a CKD diagnosis. Error bars indicate 95% CIs. ap values comparing rates of prescriptions per person-year for the 180 days before, and up to 180 days after, a CKD diagnosis are calculated using a Poisson regression model. ACE angiotensin-converting enzyme, ARB angiotensin II receptor blocker, CI confidence interval, CKD chronic kidney disease, DPP-4 dipeptidyl peptidase-4, GLP-1 glucagon-like peptide 1, HbA1c glycated haemoglobin, MRA mineralocorticoid receptor antagonist, SGLT-2 sodium-glucose cotransporter-2, T2D type 2 diabetes, UACR urinary albumin-to-creatinine ratio
CKD diagnosis was associated with significant increases in the prescribing rates of guideline-recommended medications such as angiotensin-converting enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), mineralocorticoid receptor antagonists and SGLT-2 inhibitors. Similar trends were observed for glucose-lowering drugs in the subgroup of patients with T2D at study index (Fig. 2 and Supplementary Materials Table 2).
eGFR Decline Before and After a CKD Diagnosis
Models of annual eGFR decline before and after CKD diagnosis (excluding eGFR measurements taken within 0.5 years before or after a CKD diagnosis) showed a significant attenuation of annual eGFR decline following a CKD diagnosis, from 3.20 ml/min/1.73 m2 before diagnosis to 0.74 ml/min/1.73 m2 after diagnosis. In the subgroup of patients with T2D, the estimated annual eGFR decline decreased from 3.60 ml/min/1.73 m2 before diagnosis to 1.42 ml/min/1.73 m2 after diagnosis (Table 2). Furthermore, the overall proportion of patients who showed a rapid decline in eGFR (≥ 4 ml/min/1.73 m2 per year) was higher before a diagnosis (47.1% of patients) than after a diagnosis (39.2%; Table 2). Individual linear regression models that did not exclude values from within 0.5 years before or after a CKD diagnosis showed that the annual decline in eGFR decreased from 4.12 ml/min/1.73 m2 before diagnosis to 0.30 ml/min/1.73 m2 after diagnosis in the overall cohort and from 4.49 ml/min/1.73 m2 before diagnosis to 0.76 ml/min/1.73 m2 after diagnosis in the subgroup of patients with T2D (Supplementary Materials Table 3). The generalized additive model estimating eGFR trajectories also demonstrated this attenuation of eGFR decline after a CKD diagnosis (Fig. 3).
Associations Between CKD Diagnostic Delay and Clinical Outcomes
Adjusted HRs estimating the relative change in risk of selected clinical outcomes after CKD diagnosis for each year of diagnostic delay are shown in Fig. 4. Diagnostic delay was associated with a significant increase in the incidence of kidney failure (including kidney transplant and chronic dialysis), progression to CKD stage 4/5, major adverse cardiovascular events (MACE; a composite of myocardial infarction and stroke) and MACE + (a composite of myocardial infarction, stroke and hospitalization for heart failure). Similarly, Kaplan-Meier curves of cumulative event rates by CKD stage at time of diagnosis indicated that event rates were typically higher in patients who received a diagnosis for CKD at a higher CKD stage (Supplementary Materials Fig. 2).
Associations between delayed CKD diagnosis and risk of clinical outcomes per year of diagnostic delay, evaluated using Cox regression analysis. Error bars indicate 95% CIs. Diagnostic delay is defined as the length of time between a patient’s second qualifying eGFR measurement and the date of first recorded ICD-9/10 diagnosis code for CKD (any stage). Hazard ratios estimate the relative increase in risk for each year of diagnostic delay; a hazard ratio > 1 indicates that longer diagnostic delay increases the risk of an event. aHazard ratios calculated using Cox regression adjusting for sex, age, history of diabetes (type 1 and type 2), number of pre-index visits, eGFR change rate and eGFR at date of second qualifying eGFR measurement. bComposite of cardiorenal outcomes including chronic dialysis, kidney transplant, CKD progression to stage 4/5, myocardial infarction, stroke and hospitalization for heart failure. cIncludes patients who underwent kidney transplant or chronic dialysis during follow-up, identified using recorded procedure codes (CPT, HCPCS, ICD-9-PCS or ICD-10-PCS). dIncludes patients who have a recorded ICD-9/10 code for stage 4 or 5 CKD (< 30 ml/min/1.73 m2) during follow-up. eIncludes patients who experienced myocardial infarction and/or stroke during follow-up, identified using ICD-9/10 codes. fIncludes patients who experienced myocardial infarction, stroke and/or hospitalization for heart failure during follow-up, identified using ICD-9/10 codes. CI confidence interval, CKD chronic kidney disease, CPT current procedural terminology, eGFR estimated glomerular filtration rate, HCPCS Healthcare Common Procedure Coding System, HR hazard ratio, ICD International Classification of Diseases, ICD-9/10-PCS International Classification of Diseases 9/10 Procedure Coding System, MACE major adverse cardiovascular events
Discussion
This analysis of a large US cohort of patients with eGFR measurements indicative of stage 3 CKD showed that a recorded CKD diagnosis was associated with improvements in CKD management and monitoring and prescribing of guideline-recommended medications. Furthermore, an attenuation of annual eGFR decline was observed following a diagnosis. Conversely, delayed diagnosis was associated with a greater incidence of CKD progression and adverse cardiovascular outcomes. Taken together, these findings suggest that recording of a diagnosis is an important first step in improving quality of care and outcomes for patients with stage 3 CKD.
Early disease detection is important to avoid delayed treatment of CKD and prevent progression to end-stage renal disease [33]. Previous studies have shown that CKD diagnosis is often delayed, especially for stage 1–3 CKD [13, 14, 18, 19, 34, 35]. Low rates of diagnosis may be driven by suboptimal monitoring of UACR, which is an important indicator of early kidney damage. For example, despite clinical guidelines recommending annual testing of UACR and eGFR in patients with T2D, a study of 24 healthcare organizations in the USA showed that UACR tests were frequently underused in routine clinical practice [20]. Furthermore, a study of patients in the USA at risk for CKD (diagnosed with hypertension, diabetes or both) showed that 80% did not receive guideline-appropriate assessment of kidney function during the study period [21]. In line with these studies, we observed very low rates of UACR testing even among patients with a recorded CKD diagnosis, highlighting shortcomings in the detection of mild to moderate kidney damage that must be addressed to improve patient care.
Late diagnosis of end-stage renal disease has been associated with higher mortality risk [36], although little research has been done on the impact of diagnostic delay in early-stage CKD. In the present analysis, long delays in CKD diagnosis were common, and eGFR measurements indicated that some patients progressed to higher CKD stages before receiving a diagnosis. We found that both greater diagnostic delay and diagnosis at a later CKD stage at diagnosis were associated with significant increases in the risks of adverse cardiorenal outcomes, including CKD progression, kidney failure and MACE/MACE+. Given the progressive nature of CKD, it is important that healthcare practitioners act quickly to minimize deterioration of kidney function and delay the onset of complications.
Guideline-directed therapies to slow progression and improve outcomes are a core component of CKD management, and treatment is often modified depending on individual patient circumstances and comorbidities. For example, ACE inhibitor/ARB therapy has been shown to lower the risk of kidney failure and cardiovascular events significantly [37], glycaemic control in patients with T2D reduces the risk for microalbuminuria and macroalbuminuria [38], and treatment with SGLT-2 inhibitors is associated with a significant reduction in CKD progression in patients with or without T2D [39]. It is reassuring that we observed increases in the prescribing rates of all guideline-recommended treatments investigated following a CKD diagnosis. However, despite these improvements, Kaplan-Meier analysis of cumulative event rates after CKD diagnosis showed that many patients were still at risk of CKD progression and cardiovascular complications. Furthermore, previous findings from the REVEAL-CKD study have identified that many patients with stage 3 CKD do not receive appropriate treatment regardless of diagnosis status [13]. In the present study, the absolute rate of prescribing of guideline-directed therapies, in particular SGLT-2 inhibitors, remained low even after a diagnosis. This is likely explained by the small overlap between the period following US Food and Drug Administration (FDA) approval of SGLT-2 inhibitors for the prevention of kidney function decline and the data collection period for the present study. We identified patients using serum creatinine measurements taken between 2015 and 2020; the first FDA approval for the use of SGLT-2 inhibitors to limit kidney function decline in diabetic kidney disease was granted to canagliflozin in 2019 [40], whereas other SGLT-2 inhibitors such as dapagliflozin had yet to receive FDA approvals for use in CKD (with or without T2D) at this point in time. Results from clinical trials of these agents provide a clear rationale for prescribing these newer therapies to improve outcomes in patients with CKD [39]. There is a need for healthcare practitioners to accelerate the uptake of newer evidence-based therapies for CKD management to reduce the risk of CKD progression and complications.
eGFR decline has been implicated as a predictor for poor clinical outcomes [41]. An analysis of eGFR slopes in from multiple prospective cohorts, clinical trials and simulations showed that even a modest decrease in eGFR (0.75 ml/min/1.73 m2 over 2 years) had a meaningful effect on the likelihood of end-stage kidney disease and concluded that such a decline is a suitable surrogate endpoint for trials in CKD [42]. In the present study, the significant attenuation in eGFR decline observed following a CKD diagnosis exceeded this threshold, providing further evidence to support that recording of a CKD diagnosis is a key first step in the management of the disease. While the observed reduction in eGFR decline after diagnosis may be partially attributable to lifestyle changes, regression to the mean or other unidentified confounders, it is plausible that improvements in CKD monitoring practices and guideline-directed prescribing had a positive impact on slowing CKD progression.
A diagnosis presents opportunities for improved clinical transparency and multidisciplinary care. As an example, a diagnosis of CKD from a primary care practitioner is likely to influence the approach taken by an endocrinologist in the management of T2D or the approach taken by a cardiologist in the management of heart failure given that guideline-directed management of CKD in patients with such concomitant conditions often differs [9, 43]. Without a documented diagnosis, opportunities to manage CKD may be missed, putting patients at unnecessary risk of CKD progression and complications. Primary care practitioners may be hesitant to diagnose CKD because they are concerned about emotionally overwhelming patients by diagnosing them with a chronic condition with potentially severe consequences [22]. However, this should not be a reason to avoid diagnosing the condition; indeed, it has been shown that patients have a strong desire to be informed and educated about their CKD [44].
Of note, our study population consisted mainly of elderly patients (mean age, 71.3 years). In these patients, healthcare practitioners may consider eGFR decline to be age-related [45], leading to underdiagnosis of CKD. However, our results demonstrate that in this elderly population, diagnosis may still have a positive impact. It is important that healthcare practitioners diagnose and treat CKD early in patients of all ages, even if the treatment of elderly patients may require adjustment to account for their age, frailty and comorbidities.
REVEAL-CKD uses a strict, consistent and internationally recognized definition of stage 3 CKD to ensure accurate identification of patients. Values for eGFR were calculated from serum creatinine according to the CKD-EPI equation, which is routinely used to calculate eGFR in real-world clinical practice. The present study examined a large cohort of patients from a wide range of healthcare organizations across the USA. Many of the patients in the present study were elderly, providing evidence of improvements following a CKD diagnosis in patients whose kidney function decline may be considered age-related rather than resulting from a disease state.
A limitation of the available data was short follow-up time after a CKD diagnosis. CKD is a chronic condition that tends to have a slow disease course, with deterioration in eGFR typically happening over the course of many years [46]. The short follow-up makes conclusions about the long-term impact of a diagnosis on eGFR and kidney function decline difficult to estimate. However, the observed improvements in CKD monitoring practices and prescribing of guideline-recommended medications were clear and immediate, and these were coupled with significant improvements in medium-term (2-year) eGFR decline. Although diagnosis of early-stage CKD (stage 1 and 2) typically requires confirmatory UACR testing [5], this analysis relied solely on eGFR values to identify patients with different CKD stages. REVEAL-CKD was designed specifically to evaluate stage 3 CKD, which is the earliest CKD stage identifiable by eGFR measurements alone [5]. This design choice was beneficial because very few patients had UACR data available at the time of CKD diagnosis (556 patients, 2.1%). However, because eGFR values were calculated from serum creatinine, individual readings may fluctuate slightly [47]. To minimize the impact of regression to the mean on annual eGFR decline before and after the diagnosis of CKD, eGFR values from the 0.5 years before and after CKD diagnosis were excluded from the regression models used to estimate eGFR slopes. However, regression to mean eGFR values may still account for some of the observed changes in eGFR decline. The individual linear regression models to estimate eGFR decline were adjusted for time to diagnosis only; other unidentified factors may have confounded these analyses.
Diagnoses of CKD made in environments that did not contribute to the database used will not have been captured. It was not possible to separate inpatient and outpatient encounters in this dataset; therefore, conclusions on potential differences in management and care quality between these two settings could not be drawn. Mortality data were not available in the dataset. This study does not capture lifestyle changes as a consequence of reduced eGFR or the presence of a CKD diagnosis, which would typically be the first step in the management of CKD. The findings from this study may not be generalizable to other countries which may have substantially different healthcare systems or CKD treatment policies. Finally, a high proportion of patients in the TriNetX dataset had commercial health insurance (including Medicare Advantage plans); therefore, this population may not be representative of the general population of the USA, in which a larger proportion may lack health insurance or be insured only through a basic Medicare plan.
Conclusions
The results of this analysis of patients from the USA with stage 3 CKD demonstrated that a recorded CKD diagnosis was associated with a significant improvement in measures of care quality and prescribing of guideline-recommended drugs. A recorded CKD diagnosis was also associated with a reduction in eGFR decline, whereas diagnostic delay was associated with an increased risk of adverse clinical outcomes. These findings provide compelling evidence indicating that diagnosis of stage 3 CKD is an important first step to reduce the risk of disease progression, thereby delaying adverse clinical outcomes. However, it should also be noted that diagnosis of stage 3 CKD can still be considered late identification of the disease: by this point a large portion of kidney function has already been lost. We observed very low rates of UACR testing, an important step in the diagnosis of stage 1/2 CKD, which should be addressed to improve early detection of CKD. Despite the improvements observed following a CKD diagnosis, it should be noted that many patients remain at risk of CKD progression and complications owing to diagnostic delay and under-prescribing of evidence-based therapies. Effective implementation of screening programmes, improved rates of UACR testing and recorded diagnosis and prescribing of newer therapies to treat patients with CKD are all important opportunities to lessen the clinical and economic burden of late-stage CKD on healthcare systems and improve patient outcomes.
References
Jager KJ, Kovesdy C, Langham R, et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant. 2019;34(11):1803–5.
United States Renal Data System. 2022 USRDS Annual Data Report: epidemiology of kidney disease in the United States. In: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD; 2022.
Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382(9889):339–52.
Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–72.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
Shlipak MG, Tummalapalli SL, Boulware LE, et al. The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2021;99(1):34–47.
Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021;99(3s):S1–87.
Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group. KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int Suppl. 2013;3:259–305.
Rossing P, Caramori ML, Chan JCN, et al. KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2022;102(5):S1–127.
Draznin B, Aroda VR, Bakris G, et al. 11. Chronic kidney disease and risk management: standards of medical care in diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S175–84.
Fontes-Carvalho R, Santos-Ferreira D, Raz I, et al. Protective effects of SGLT-2 inhibitors across the cardiorenal continuum: two faces of the same coin. Eur J Prev Cardiol. 2021;29(9):1352–60.
Rangaswami J, Bhalla V, Boer IHD, et al. Cardiorenal protection with the newer antidiabetic agents in patients with diabetes and chronic kidney disease: a scientific statement from the American Heart Association. Circulation. 2020;142(17):e265–86.
Tangri N, Moriyama T, Schneider MP, et al. REVEAL-CKD: management and monitoring of patients with stage 3 CKD in France, Germany, Italy, Japan and the USA [poster]. Presented at the American Society of Nephrology (ASN) Kidney Week 2022, November 3–6 2022, Orlando, Florida, USA.
De Nicola L, Peach E, Barone S, et al. MO509: REVEAL-CKD: prevalence of undiagnosed stage 3 chronic kidney disease in Italy [PowerPoint presentation]. Presented at the 59th European Renal Association Congress, May 19–22, 2022, Paris, France.
Chronic kidney disease often undiagnosed in Medicare beneficiaries. 2020. Accessed September 22, 2022, at https://www.cms.gov/files/document/ckd-data-highlight102020-2.pdf.
Bakris G, Coresh J, Vassalotti JA, et al. Prevalence and factors associated with undiagnosed chronic kidney disease in diabetes mellitus. In: National Kidney Foundation 2019 Spring Clinical Meetings. Boston, MA, USA; 2019.
Ryan TP, Sloand JA, Winters PC, et al. Chronic kidney disease prevalence and rate of diagnosis. Am J Med. 2007;120(11):981–6.
Sultan AA, Barone S, Kumar S, et al. 998-P: REVEAL-CKD: prevalence of and patient characteristics associated with undiagnosed stage 3 chronic kidney disease [poster]. Presented at the American Diabetes Association 81st Scientific Sessions, June 25–29, 2021 (virtual).
Schneider M, Peach E, Barone S, et al. POS-213 REVEAL-CKD: prevalence of undiagnosed early chronic kidney disease in Germany. Kidney Int Rep. 2022;7(2):S93.
Stempniewicz N, Vassalotti JA, Cuddeback JK, et al. Chronic kidney disease testing among primary care patients with type 2 diabetes across 24 US Health Care Organizations. Diabetes Care. 2021;44(9):2000–9.
Alfego D, Ennis J, Gillespie B, et al. Chronic kidney disease testing among at-risk adults in the US remains low: real-world evidence from a national laboratory database. Diabetes Care. 2021;44(9):2025–32.
Greer RC, Crews DC, Boulware LE. Challenges perceived by primary care providers to educating patients about chronic kidney disease. J Ren Care. 2012;38(4):174–81.
Holmboe ES, Durning SJ. Assessing clinical reasoning: moving from in vitro to in vivo. Diagnosis. 2014;1(1):111–7.
Paterick TE, Patel N, Tajik AJ, et al. Improving health outcomes through patient education and partnerships with patients. Proc (Bayl Univ Med Cent). 2017;30(1):112–3.
Evangelidis N, Craig J, Bauman A, et al. Lifestyle behaviour change for preventing the progression of chronic kidney disease: a systematic review. BMJ Open. 2019;9(10): e031625.
Jutel A. Sociology of diagnosis: a preliminary review. Sociol Health Illn. 2009;31(2):278–99.
O’Malley KJ, Cook KF, Price MD, et al. Measuring diagnoses: ICD code accuracy. Health Serv Res. 2005;40(5 Pt 2):1620–39.
Kushner P, Peach E, Wittbrodt E, et al. Investigating the global prevalence and consequences of undiagnosed stage 3 chronic kidney disease: methods and rationale for the REVEAL-CKD study. Clin Kidney J. 2021;15(4):738–46.
TriNetX Real World Data. 2021. Accessed September 22, 2022, at https://trinetx.com/real-world-data/linked/.
Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
Delgado C, Baweja M, Crews DC, et al. A unifying approach for GFR estimation: recommendations of the NKF-ASN Task Force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79(2):268-88.e1.
Hastie TJ. Generalized additive models. In: Statistical models in S. London: Routledge; 2017. p. 249–307.
Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA. 2011;305(15):1553–9.
Harindhanavudhi T, Freese RL, Caramori L. 396-P: delayed chronic kidney disease (CKD) diagnosis in type 2 diabetes (T2D) [poster]. Presented at the American Diabetes Association 81st Scientific Sessions, June 25–29, 2021 (virtual).
Kim V, Gusmanov A, Sakko Y, et al. POS-296 late diagnosis of CKD and associated survival after initationt of renal replacement therapy in Kazakhstan: analysis of nationwide electronic healthcare registry 2014–2020. Kidney Int Rep. 2022;7(2):S132–3.
Sesso R, Belasco AG. Late diagnosis of chronic renal failure and mortality on maintenance dialysis. Nephrol Dial Transplant. 1996;11(12):2417–20.
Xie X, Liu Y, Perkovic V, et al. Renin-angiotensin system inhibitors and kidney and cardiovascular outcomes in patients with CKD: a Bayesian network meta-analysis of randomized clinical trials. Am J Kidney Dis. 2016;67(5):728–41.
Coca SG, Ismail-Beigi F, Haq N, et al. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med. 2012;172(10):761–9.
Mende CW. Chronic kidney disease and SGLT2 inhibitors: a review of the evolving treatment landscape. Adv Ther. 2022;39(1):148–64.
US FDA approves INVOKANA® (canagliflozin) to treat diabetic kidney disease (DKD) and reduce the risk of hospitalization for heart failure in patients with type 2 diabetes (T2D) and DKD. 2019. Accessed January 12, 2023, at https://www.jnj.com/u-s-fda-approves-invokana-canagliflozin-to-treat-diabetic-kidney-disease-dkd-and-reduce-the-risk-of-hospitalization-for-heart-failure-in-patients-with-type-2-diabetes-t2d-and-dkd.
Zhang L, Hauske S, Ono Y, et al. Analysis of eGFR index category and annual eGFR slope association with adverse clinical outcomes using real-world Japanese data: a retrospective database study. BMJ Open. 2022;12(2): e052246.
Levey AS, Gansevoort RT, Coresh J, et al. Change in albuminuria and GFR as end points for clinical trials in early stages of CKD: a scientific workshop sponsored by the National Kidney Foundation in collaboration with the US Food and Drug Administration and European Medicines Agency. Am J Kidney Dis. 2020;75(1):84–104.
House AA, Wanner C, Sarnak MJ, et al. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2019;95(6):1304–17.
Wright Nunes J, Roney M, Kerr E, et al. A diagnosis of chronic kidney disease: despite fears patients want to know early. Clin Nephrol. 2016;86(2):78–86.
Glassock RJ, Rule AD. Aging and the kidneys: anatomy, physiology and consequences for defining chronic kidney disease. Nephron. 2016;134(1):25–9.
Rosansky SJ. Renal function trajectory is more important than chronic kidney disease stage for managing patients with chronic kidney disease. Am J Nephrol. 2012;36(1):1–10.
Kao SS, Kim SW, Horwood CM, et al. Variability in inpatient serum creatinine: its impact upon short- and long-term mortality. QJM. 2015;108(10):781–7.
Acknowledgements
Funding
REVEAL-CKD is funded by AstraZeneca. It is a non-interventional observational study, and as such, no drugs are supplied or funded. AstraZeneca designed the REVEAL-CKD study with input and guidance from external experts. AstraZeneca provided funding for data management and analysis. An AstraZeneca team reviewed this manuscript for scientific accuracy during its development and was allowed to make suggestions. However, the final content, analysis and interpretation of the data were determined by the authors. The decision to submit the data for publication was made by the authors. AstraZeneca provided funding for the Advances in Therapy Rapid Service and Open Access Fees.
Medical Writing and/or Editorial Assistance
Under the direction of the authors, medical writing support for this manuscript was provided by Bobby Thompson, MSc (Res), of Oxford PharmaGenesis, Oxford, UK, and was funded by AstraZeneca.
Author Contributions
Stefan Franzén analysed the data and Emily J Peach reviewed the data; Stefan Franzén and Emily J Peach take responsibility for the integrity of the data and the accuracy of the data analysis. All authors, Navdeep Tangri, Emily J Peach, Stefan Franzén, Salvatore Barone and Pamela R Kushner, agreed on the general content of the manuscript and were involved in drafting and critical revision of the manuscript during its development. All authors approved the final version of the manuscript before its submission. The corresponding author, Navdeep Tangri, attests that all listed authors meet International Committee of Medical Journal Editors authorship criteria and that no authors meeting the criteria have been omitted.
Prior Presentation
A portion of the results presented in this manuscript were previously presented at the American Society of Nephrology Kidney Week 2022, Orlando, FL, USA, 3–6 November 2022.
Disclosures
Emily J Peach, Stefan Franzén and Salvatore Barone are employees of AstraZeneca. Navdeep Tangri has received grants from AstraZeneca, Boehringer Ingelheim/Eli Lilly and Company, Janssen Pharmaceuticals, Otsuka Pharmaceutical Co., Ltd., and Tricida, Inc., has received honoraria from AstraZeneca, Boehringer Ingelheim/Eli Lilly and Company, Janssen Pharmaceuticals, Otsuka Pharmaceutical Co, Ltd., and Tricida, Inc., and holds stock options from Mesentech, Inc., Rénibus Therapeutics, Inc., pulseData and Tricida, Inc. Pamela R Kushner has received speaker’s bureau and advisory board fees from AstraZeneca, Eli Lilly and Company and Novo Nordisk A/S, speaker’s fees from Bayer AG and honoraria from AstraZeneca and Eli Lilly and Company.
Compliance with Ethics Guidelines
REVEAL-CKD (NCT04847531) used de-identified data from existing databases and did not require data collection beyond that of routine clinical care. No identifiable information was collected or examined as part of the study. All externally conducted analyses were completed in line with local ethics regulations/legislation. De-identified, internally licensed databases were shared with AstraZeneca by the licensee; therefore, ethics review and approval were not required for the use of these databases for this study.
Data Availability
The datasets analysed during the current study are not publicly available due to being granted under license from TriNetX, who are responsible for the collection of the data used.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Tangri, N., Peach, E.J., Franzén, S. et al. Patient Management and Clinical Outcomes Associated with a Recorded Diagnosis of Stage 3 Chronic Kidney Disease: The REVEAL-CKD Study. Adv Ther 40, 2869–2885 (2023). https://doi.org/10.1007/s12325-023-02482-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02482-5