Abstract
Introduction
Dose escalation is one of the treatment approaches studied and suggested in advanced therapies for Crohn’s disease (CD) and ulcerative colitis (UC). This study aimed to identify and characterize the dosing escalation patterns of advanced therapies in CD and UC.
Methods
Two systematic literature reviews (SLRs) were conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. MEDLINE®, Embase®, and Cochrane Library were searched for articles published between January 2011 and October 2021 and limited to non-interventional studies in English language. Congress and bibliographic searches were also conducted. Articles were screened by two independent researchers. Dose escalation patterns were described and summarized considering the regional regulatory label recommendation (in North America [NA] or outside of North America [ONA]).
Results
Among 3190 CD and 2116 UC articles identified in the Ovid searches, 100 CD and 54 UC studies were included in the SLR, with more studies conducted ONA. Most studies reported an initial maintenance dose pattern aligned with the lower starting dose per local regulatory label; however, several ONA studies (n = 13 out of 14) reported ustekinumab every 8 weeks as starting maintenance pattern in CD. In ONA studies, the median within-guideline escalation rates in CD and UC were 43% in ustekinumab (CD only), 33% and 32% for vedolizumab; 29% and 39% for adalimumab; and 14% and 10% for infliximab. Evidence regarding dose escalation patterns for tofacitinib, certolizumab pegol, and golimumab was limited. Some dose escalation patterns outside of label recommendations were observed including ustekinumab every 8 weeks to every 4 weeks and vedolizumab every 8 weeks to every 6 weeks.
Conclusion
Dose escalation strategies are widely documented in the literature. The reported dose escalation patterns and escalation rates vary by region and by CD and UC. Most escalation patterns reported were aligned with regulatory recommendations while some reported more diverse or aggressive dose escalation.
Prospero Registration
CRD42021289251.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
In this systematic review, most identified studies reported initial maintenance dosages aligned with local regulatory label indications for CD and UC, except for ustekinumab in countries ONA, where the majority of studies reported initial maintenance doses of 90 mg every 8 weeks | |
In ONA studies, the median within-guideline escalation rates in CD and UC were 43% in ustekinumab (CD only); 33% and 32% for vedolizumab; 29% and 39% for adalimumab; and 14% and 10% for infliximab | |
Some dose escalation patterns outside of label recommendations were observed including ustekinumab every 8 weeks to every 4 weeks and vedolizumab every 8 weeks to every 6 weeks | |
The most commonly reported reason for dose escalation in identified studies was a loss of response |
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing-remitting inflammatory disease that includes Crohn’s disease (CD) and ulcerative colitis (UC) [1,2,3]. Presenting symptoms of IBD most commonly include mild to moderate diarrhea and abdominal pain, nausea, and vomiting [1, 4,5,6]. CD impacts the entire digestive tract and is broadly characterized by the presence of a patchy network of transmural inflammatory lesions, whereas UC is an idiopathic disease of the colon and rectum characterized by mucosal inflammation and ulceration [1, 5,6,7,8].
IBD may occur at any age, yet peak diagnosis is typically between the ages of 15 and 35 for both CD and UC [5, 6, 9]. In 2017 alone, > 6.8 million cases of IBD (age-standardized prevalence: 84.3 per 100,000) were reported globally [4]. Though IBD is typically regarded as a disease with the greatest impact in developed countries, incidence rates have either declined or stabilized over the past two decades [4, 8]. In contrast, IBD incidence in developing countries continues to rise [5, 6, 8].
IBD is associated with significant impairment to the patient’s physical and emotional quality of life [9,10,11,12]. Characterized by intermittent periods of gastrointestinal inflammation and remission, uncontrolled IBD increases a patient’s risk of developing complications, frequently leading to a higher risk of oncogenesis and higher rates of mortality than the general population [9, 11, 12]. As there is no cure for CD or UC, the primary goal of treatment involves symptom resolution, inflammation reduction, and improvements in long-term prognostic outcomes and quality of life [1, 5, 6, 13].
Patients with mild symptoms are often treated with anti-inflammatory medications, yet more aggressive cases require lifelong use of advanced therapies, including infliximab, adalimumab, golimumab, vedolizumab, or ustekinumab as well as small molecular therapies such as tofacitinib, filgotinib, or ozanimod [5, 6, 14]. Healthcare costs for patients with IBD vary based upon disease severity, yet generally cost three times that of the general population [13, 15,16,17,18].
In the United States (US), total lifetime healthcare costs were $622,056 and $405,496 (2016 US dollars [USD]) per CD and UC patient, respectively [19]. IBD also imposes a considerable societal burden due to loss of schooling, work absenteeism, short-term disability, and early retirement [4, 15, 18, 20]. Indirect costs in patients with IBD account for 30–50% of all healthcare expenditure in the US, with recent estimates totaling $5.1 and $4.9 billion annually for CD and UC patients, respectively [4, 15, 18].
Even in the era of widespread advanced therapy availability, up to 65% of patients will experience treatment failure within 12 months of maintenance therapy initiation [16, 21,22,23,24]. Dose escalation has been observed as a common treatment strategy, even in the range outside of regulatory recommendations, to maintain or regain response [16, 21, 25, 26]. Though effective, dose escalation also comes at a cost to the healthcare systems and patients [17].
In the European Medicines Agency (EMA) label of advanced therapies in CD/UC, dose escalation is usually allowed if patients lose their response to the initial lower dose of the regimen (e.g., increase in dosing frequency from 90 mg every 12 weeks to every 8 weeks in ustekinumab), although, in the US and certain countries like Canada, dose escalation may not be allowed in the regulatory label recommendation. It is unclear to what extent the maintenance dose or the dose escalation regimen is aligned with the label.
The objective of this study was therefore to identify and characterize dosing patterns of advanced therapies for CD and UC through a comprehensive assessment of the data from real-world (RW) evidence studies.
Methods
Two systematic literature reviews (SLRs), one in patients with CD and the other in patients with UC, were conducted and registered with PROSPERO (trial registration: CRD42021289251). The searches were done per Centre for Reviews and Dissemination (CRD), Cochrane Collaboration, and National Institute for Health and Care Excellence (NICE) guidelines on October 26, 2021.
Each SLR focused on non-interventional studies to capture all dosing pattern analyses conducted in RW settings. To reflect current CD/UC clinical practices, studies were restricted to those conducted between January 2011 and October 2021 and limited to English language studies of adults with active CD/UC undergoing advanced therapy maintenance treatment. Advanced therapies include infliximab, adalimumab, vedolizumab, ustekinumab, and certolizumab pegol in CD; infliximab, adalimumab, golimumab, vedolizumab, tofacitinib, ustekinumab, and ozanimod in UC. The full scope of each SLR was defined in terms of the patient population, intervention, comparators, outcome measures, and study design (PICOS) statement for study inclusion and exclusion (Table 1).
Search Strategy
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. The Ovid platform was used to conduct searches of MEDLINE® (Medical Literature Analysis and Retrieval System Online), Embase® (Excerpta Medica Database), and Cochrane collaboration. Secondary searches were conducted in EconLit, MEDLINE® Epub Ahead of Print, and In-Process to identify relevant economic studies or non-indexed citations. Studies, systematic reviews, and meta-analyses identified through the database searches underwent bibliography review to identify relevant studies and capture articles or papers not identified during the initial search. All publication types, including conference abstracts and pre-prints, were considered for inclusion. Conference abstracts from the past two years, and those indexed via Ovid, were searched to retrieve the latest studies. Please refer to the Supplementary Material for CD and UC search strategies.
Search Results and Screening
The Ovid searches identified 3190 CD and 2116 UC publications. Upon completion of database and bibliographic searches, all publications underwent an abstract/title review resulting in 1809 CD and 1233 UC records being excluded based upon PICOS criteria. Three hundred eighty-one CD and 323 UC publications were retained for full-text review by two independent researchers (Stacy Grieve and Rhiannon Campden). Based upon the SLR’s inclusion/exclusion criteria, 100 studies in CD and 54 in UC met the inclusion criteria (Figs. 1a, b) and were fully extracted. Fully extracted studies were those that contained information on proportions of individuals on any dose escalation, escalated maintenance patterns, or any reports of re-induction and/or de-escalation.
Data Extraction
All data were extracted into a pre-defined Excel-based template by one independent reviewer (Stacy Grieve) and cross-checked by a second senior reviewer (Rhiannon Campden) in compliance with CRD’s guidelines for Undertaking Reviews in Healthcare. A third reviewer was consulted to resolve any disagreements and/or make final decisions (Sharada Harricharan). Search findings are presented in Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagrams and systematic review reports.
Risk of Bias Assessment
The Risk of Bias in Non-randomized Studies-of Interventions (ROBINS-I) tool was utilized to address any possible biases introduced by studies that did not randomize participants into comparison groups. Overall estimates of bias were calculated based on the seven domains of bias addressed by the ROBINS-I tool.
Outcomes
All treatment patterns were evaluated by disease (CD or UC) and treatment. A cross-sectional view of the regimen dose at the beginning of the maintenance therapy and the last dose at the end of follow-up were recorded. Dose escalation was categorized into shortening of the interval/frequency or increase in dosage/strength through a longitudinal view of the initial starting dose and the dose to which the regimen later escalated. For a given treatment pattern, a dose escalation median rate was reported. Given the variation of regulatory recommendations in maintenance dose (Table 2) and allowance of dose escalation, results were reported by region as the US and Canada (e.g., North America [NA]) and outside of North America (ONA) for all other studies.
Results
Study Characteristics and Patient Characteristics
The included CD/UC studies were conducted in the US, Canada, Europe, Australia (CD only), Israel, Japan, and Korea. Overall, interval shortenings were most often reported in patients treated with ustekinumab, vedolizumab, and adalimumab (CD/UC), while infliximab (CD/UC) and golimumab (UC) treatment were more commonly associated with dose strength increases (Table 3).
The CD study populations ranged from 10 to 2904 patients with a mean age range of 25–48.5 years. The proportion of males varied widely (25–85.4%) between studies, as did the proportion of smokers (3.5–52.4%). The mean disease duration ranged from 3.2 to 32.5 years. Population size for the UC studies ranged from 18 to 2968 patients; the mean age ranged from 31.5 to 49.8 years. The proportion of males varied between 25% and 73.1% across studies, with the proportion of smokers ranging from 3% to 25%. The mean disease duration ranged from 4.1 to 11.2 years.
Results in CD
Overview
In 69 studies reporting reasons for dose escalation, the most common reason was related to partial response, no response, or loss of response; some were related to low drug serum levels (Supplementary Material) [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87]. The most common initial maintenance doses (Fig. 2), dose escalation patterns (Fig. 3), and last dose pattern at the end of follow-up (Fig. 4) are described by region below, with full details in the Supplementary Material.
Most reported initial maintenance dose patterns, Crohn’s disease. EOW, every other week; EU, European Union; kg, kilogram; mg, milligram; N, number within the overall population; q4w, every 4 weeks; q8w, every 8 weeks; q12w, every 12 weeks. ∆Includes Biemans et al.'s 2020 [104] study where the maintenance pattern proportions were calculated based on the number of individuals at 12 weeks instead of baseline. ¤Studies reporting 5 mg/kg were assumed to have dosing every 8 weeks. Source: [27,28,29,30,31, 33,34,35,36,37, 39,40,41,42,43,44,45,46,47,48,49, 51,52,53,54,55,56, 59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80, 82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104, 106,107,108,109,110, 112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133, 198,199,200,201,202]
Most reported dose escalation patterns, Crohn’s disease. EOW, every other week; EU, European Union; EW, every week; kg, kilogram; mg, milligram; N, number within the overall population; q2w, every 2 weeks; q4w, every 4 weeks; q6w, every 6 weeks; q8w, every 8 weeks; q12w, every 12 weeks. €Includes Biemans et al.'s 2020 [104] study where the maintenance pattern proportions were calculated based on the number of individuals at 12 weeks instead of baseline. †Includes IV reinduction plus 90 mg q4w dosing. ‡Including maintenance patterns where only the interval (e.g., q8w) was reported. Source [29,30,31, 33, 35,36,37, 39, 40, 44,45,46,47,48,49, 51,52,53,54, 59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75, 77,78,79,80,81,82,83, 85,86,87,88,89,90,91, 95,96,97, 99, 101, 103,104,105,106,107,108, 110, 111, 114,115,116,117,118, 120,121,122,123,124,125,126,127,128,129,130,131, 133,134,135,136,137,138,139, 155, 209,210,211, 214]
Most reported dose patterns at the end of follow-up in Crohn’s disease. EOW, every other week; EU, European Union; EW, every week; kg, kilogram; mg, milligram; N, number within the overall population; q2w, every 2 weeks; q4w, every 4 weeks; q4-7w, every 4–7 weeks; q6w, every 6 weeks; q8w, every 8 weeks; q12w, every 12 weeks. ¤Studies reporting 90 mg q2-8w include interval shortenings and re-inductions if they occurred together. ∆Includes one study with ‘NR’ reported as the patient proportion for 90 mg q8w [36, 37]. *Including Reinglas et al. [107] who did not report the number of individuals on 300 mg q8w or 300 mg q4w at the end of follow-up. €Studies reporting 10 mg/kg were assumed to have dosing every 8 weeks. Source: [21, 28,29,30,31,32,33, 35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54, 58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76, 79, 80, 82,83,84,85,86,87,88,89, 92,93,94,95,96,97,98,99,100,101,102,103,104, 106, 107, 109,110,111,112,113, 115,116,117,118,119,120,121,122,123,124,125,126, 128,129,130,131,132, 198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215]
Ustekinumab
Most studies conducted within the US and Canada (n = 10) reported initial dosing in alignment with local regulatory guidelines (e.g., 90 mg every 8 weeks) in a median of 100% of patients (range: 74–100%) [27, 30, 31, 34, 39, 88,89,90,91,92,93,94,95,96,97]. Among studies ONA that reported an initial maintenance dose (n = 14), six reported a median of 29% of patients (range 9–100%) started on every-12-week dosing, while 13 out of 14 studies reported a median of 100% (range 63–100%) of patients initially started on every-8-week maintenance dosing (Fig. 2) [28, 29, 33, 35,36,37, 40, 41, 98,99,100,101,102,103,104]. The EMA recommendation, however, is to start with a lower dose regimen (e.g., 90 mg every 12 weeks) [105].
During follow-up, interval shortening to 90 mg every 4 weeks was reported in a median patient proportion of 27% (range 17–77%) in NA and 24% (range 11–33%) in studies ONA when started on every-8-week dosing (Fig. 3) [29,30,31, 33, 35, 39, 40, 88, 89, 92,93,94, 96, 98, 100,101,102,103,104]. Dose strength increases were reported in two studies and ranged from 108 to 180 mg every 8 weeks in 18% to 23% of patients in NA studies [90, 91, 97].
Vedolizumab
All studies conducted within NA (n = 3/3) [46, 47, 106, 107] or ONA (n = 8/8) [42,43,44,45, 48, 49, 51, 52, 103, 108,109,110] reported initial maintenance dosing of 300 mg every 8 weeks with a median proportion of 100% (Fig. 2). The most common dose escalation pattern observed during follow-up was from 300 mg every 8 weeks to every 4 weeks [median proportion: 18% (range 8–27%) in NA studies; 33% (range 12–79%) in studies ONA] (Fig. 3) [44,45,46,47,48,49, 51, 52, 103, 106, 107, 109,110,111].
Certolizumab Pegol
The initial maintenance pattern reported in one study was 400 mg every 4 weeks, per US Food & Drug Administration (FDA) guidelines, in 88% of patients (Fig. 2) [86, 87]. Dose escalation of certolizumab pegol in one study was reported as an interval shortening to 400 mg every 2 weeks in 17% of patients from 400 mg every 4 weeks (Fig. 3) [86, 87]. The study also reported a dose strength increase, from 200 mg every 2 weeks to 400 mg every 2 weeks, in 18% of patients (Supplementary Material) [86, 87].
Adalimumab
Most studies reported an initial maintenance dose of 40 mg every other week (EOW): four NA studies with a median proportion of 100% [69, 90, 91, 112,113,114] and 17 ONA studies with a median of 100% (range 38–100%) [53,54,55,56, 59,60,61,62,63,64,65,66,67,68, 70, 115,116,117,118,119,120,121,122,123] (Fig. 2). Follow-up escalation patterns in NA and ONA were most commonly interval shortenings to 40 mg every week (EW) dosing from 40 mg EOW in a median proportion of 43% (range 40–45%) and 29% (range 14–77%) of patients, respectively (Fig. 3) [53, 54, 59,60,61,62,63,64,65,66,67,68,69,70, 112, 113, 115,116,117,118,119,120,121,122,123].
Infliximab
An initial maintenance dose of 5 mg/kg every 8 weeks was used (Fig. 2), per FDA/EMA recommendations, in three NA studies (median proportion: 100%; range 76–100%) and 17 studies ONA (median proportion: 100%; range 100–100%) [53, 54, 63, 64, 66, 68, 71,72,73,74,75,76,77,78,79,80, 82,83,84,85, 123,124,125,126,127,128,129,130,131,132]. Dose strength increases from 5 to 10 mg/kg were the most common dose escalation pattern observed [proportion: 8% in one NA study; 14% (range 3–67%) in 15 studies ONA] during follow-up (Fig. 3) [53, 54, 63, 64, 66, 68, 71, 72, 74,75,76,77,78,79,80,81,82,83,84,85, 123,124,125,126, 128,129,130,131,132,133]. Studies reporting only “10 mg/kg” without an interval were assumed to have dosing 10 mg/kg every 8 weeks.
Figure 4 presents the most common maintenance pattern for each selected therapy at the end of follow-up in CD, regardless of the initial dose.
Results in UC
Overview
Nineteen UC studies reported reasons for dose escalation. The most commonly reported reasons (79%) for dose escalation were partial response, no response, or loss of response; few were related to evidence of inflammation assessed by endoscopy or biomarkers (Supplementary Material) [44,45,46,47, 51, 52, 110, 123, 132, 134,135,136,137,138,139,140,141,142,143,144,145,146,147,148]. The most common initial maintenance dose (Fig. 5), dose escalation patterns (Fig. 6), and last dose at the end of follow-up (Fig. 7) are described by region below, with full details in the Supplementary Material.
Most reported initial maintenance dose patterns, ulcerative colitis. EOW, every other week; kg, kilogram; mg, milligram; N, number within the overall population; NR, not reported; q4w, every 4 weeks; q8w, every 8 weeks; q12w, every 12 weeks. *Includes studies that reported 300 mg dosing and every-8-week dosing. ∆Includes two studies that did not report on the number of individuals on the recorded maintenance dose [144,145,146, 159,160,161]. Source: [7, 42,43,44,45,46, 48, 49, 51, 52, 61, 107, 110, 123,124,125, 127, 132, 134,135,136,137,138,139,140,141,142,143,144,145,146,147,148, 151,152,153,154, 156, 157, 159,160,161, 163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179, 216]
Most reported dose escalation patterns, ulcerative colitis. EOW, every other week; EW, every week; kg, kilogram; mg, milligram; N, number within the overall population; NR, not reported; q4w, every 4 weeks; q6w, every 6 weeks; q8w, every 8 weeks. Source: [7, 44,45,46,47,48,49, 51, 52, 61, 89, 107, 110, 123,124,125, 127, 132, 134,135,136,137,138,139,140,141,142,143,144,145,146,147,148, 151,152,153,154,155,156,157, 160,161,162,163,164,165,166,167, 169,170,171,172, 175,176,177,178,179, 217, 218]
Most reported dose patterns at the end of follow-up, ulcerative colitis. BID, every 12 h; EOW, every other week; EW, every week; kg, kilogram; mg, milligram; N, number within the overall population; q2w, every 2 weeks; q4w, every 4 weeks; q6w, every 6 weeks; q8w, every 8 weeks. €Includes reported dosing of every 4–6 weeks; *Includes studies that only reported q6w and q4w dosing, respectively. ∆Includes two studies that did not report the number of individuals dose escalated [111, 164]. ¤Reported dosages include 40 mg q7-10d, biweekly dose ≥ 50 mg, 40 mg q10d, ≥ 4 mg q1d, an increase of ≥ 50% compared to label-recommended daily dose, and a doubling of the average daily dose. Source: [21, 42,43,44,45,46,47,48,49, 51, 52, 62, 92, 110,111,112, 115, 128, 141,142,143,144,145, 152,153,154, 157,158,159,160, 164, 171, 173,174,175,176,177,178,179,180,181,182,183, 196]
Tofacitinib
One tofacitinib study, conducted in Spain, did not report initial maintenance dosages, yet EMA label indications are 5 mg two times a day (BID) for the treatment of UC [149, 150]. Though patterns of dose escalation could not be ascertained, it was reported that 8% of patients were on 10 mg of tofacitinib BID at the end of follow-up (Fig. 7) [150].
Ustekinumab
Two studies conducted in NA reported initial maintenance dosing of 90 mg every 8 weeks in all patients (100%; range 100–100%), per FDA recommendations (Fig. 5) [151,152,153]. Initial dosing of 90 mg every 12 weeks was reported in only one of the two studies conducted ONA (7% of patients) [154]. When initially started on 90 mg every 8 weeks, the most common dose escalation pattern in NA studies was an interval shortening to 90 mg every 4 weeks (47% of patients) or 90 mg every 6 weeks (12% of patients) (Fig. 6) [151,152,153]. In studies ONA, interval shortening to every 6 weeks or every 4 weeks was reported in 27% of patients who were started on an initial interval of every 12 weeks or every 8 weeks (Supplementary Material) [154, 155].
Vedolizumab
Studies in NA (N = 6) and ONA (N = 5) reported an initial starting maintenance dose of vedolizumab per local regulatory recommendations (e.g., 300 mg every 8 weeks) in a median of 100% of patients (Fig. 5) [7, 42,43,44,45,46, 48, 49, 51, 52, 107, 110, 134,135,136, 147, 148, 156, 157]. The most common dose escalation pattern for UC patients on vedolizumab was an interval shortening from 300 mg every 8 weeks to 300 mg every 4 weeks (Fig. 6) [44,45,46,47,48,49, 51, 52, 89, 107, 110, 134,135,136, 147, 148, 156]. Studies that reported dosing “every 8 weeks” without strength were assumed to have 300 mg/kg every 8 weeks.
Golimumab
All included studies (n = 6) were from ONA where local regulatory guidelines recommend initial dosing based upon patient weight (50 mg every 4 weeks if < 80 kg; 100 mg every 4 weeks if > 80 kg) [144,145,146, 157,158,159,160,161,162]. The most common initial maintenance pattern was 100 mg every 4 weeks in a median of 47% of patients (range 43–100%) (Fig. 5) [7, 144,145,146, 157, 159,160,161]. A dose strength increase from 50 to 100 mg was observed in a median of 76% of patients in four studies conducted ONA (Fig. 6) [144,145,146, 160,161,162].
Adalimumab
The studies (N = 10) conducted ONA reported initial maintenance dosing according to the EMA label recommendation (e.g., 40 mg EOW) in a median of 100% (range 94–100%) of patients (Fig. 5) [7, 61, 123, 137, 138, 157, 163,164,165,166,167,168,169,170,171,172]. Dose escalation patterns were not reported in NA studies, yet were most commonly interval shortenings to 40 mg EW (median proportion 39%; range 30–56%) in studies ONA (Fig. 6) [61, 123, 137, 138, 163,164,165,166,167, 169,170,171,172]. A dose strength increase to either 80 mg EOW from baseline (2%) or to an average biweekly dose ≥ 50 mg (median proportion 22%; range 2–74%) was also reported in patients ONA (Supplementary Material) [7, 137, 138, 157, 168, 169].
Infliximab
Two studies conducted in NA reported an initial maintenance pattern in alignment with FDA label recommendations (5 mg/kg every 8 weeks) in a median of 89% of patients (range 78–100%) [124, 125, 127, 173, 174]. Outside of NA, most studies (N = 8) reported an initial maintenance dose of 5 mg/kg every 8 weeks in a median of 100% of patients (range 68–100%) [7, 123, 132, 139,140,141,142,143, 157, 175,176,177,178,179].
Infliximab studies conducted ONA reported an interval shortening to either 5 mg/kg every 6 weeks (median proportion 20%; range 15–25%) or 5 mg/kg every 4 weeks (proportion 6%) from a starting dose of 5 mg/kg every 8 weeks (Fig. 6) [123, 140, 141, 143, 177, 178]. A dose strength increase from 5 to 10 mg/kg was reported in a median 10% of patients (range 5–38%) in five studies conducted ONA [132, 139,140,141,142,143, 175, 176, 178, 179]. Six studies ONA also reported a dose strength increase to > 5 mg/kg in a median of 33% of patients (Supplementary Material) [7, 127, 132, 140,141,142,143, 157, 175, 176, 178, 179]. One NA study reported a dose strength increase from 5 to > 5 mg/kg in 27% of patients [124, 125, 127].
Figure 7 presents the most common maintenance pattern for each selected therapy at the end of follow-up in UC, regardless of the initial dose.
Discussion
Two SLRs were conducted to evaluate the magnitude of dose escalations in non-interventional studies of patients receiving maintenance therapies for CD and UC. Data were extracted to identify any interval shortening or dose-strength increase among individuals treated with ustekinumab (CD/UC), vedolizumab (CD/UC), adalimumab (CD/UC), infliximab (CD/UC), certolizumab pegol (CD), golimumab (UC), and tofacitinib (UC). Overall, 100 CD and 54 UC publications were fully extracted for inclusion in this study.
All included studies, except those reporting on ustekinumab, reported patients starting maintenance dosing per label indications. In studies of ustekinumab conducted ONA, a median of 100% of patients with CD and 56% of patients with UC started on maintenance at 90 mg every 8 weeks. During the follow-up, there was a significant dose creep (e.g., increased doses or shortened intervals) for each selected advanced therapy, yet the escalations were generally aligned with the EMA escalation allowances. However, some dose patterns outside of label recommendations were observed, such as ustekinumab from 90 mg every 8 weeks to every 4 weeks; vedolizumab from every 8 weeks to every 6 weeks. Dose strength increases were commonly reported for infliximab in CD and UC studies, and evidence regarding dose escalation patterns for certolizumab pegol, golimumab, and tofacitinib was limited.
Despite more frequent reliance on dose escalation to regain clinical response and induce remission, studies evaluating the effectiveness of standard vs. escalated doses are scarce and outcomes were assessed differently. As such, an assessment of clinical outcomes was outside of the initial scope of the SLR and synthesis of outcome data was not feasible. In a critical appraisal of advanced therapy dose escalation in IBD treatment, the Canadian Agency for Drugs and Technologies in Health (CADTH) found limited evidence to demonstrate the incremental efficacy of escalated vs. standard dosing [180]. Report findings showed that clinical effectiveness and safety between populations on standard and escalated doses were similar [180]. However, patients who dose escalated due to loss of response might have different disease characteristics than those without dose escalation, which could potentially confound the comparison of efficacy between the two groups. In addition, the use of escalated doses of advanced therapeutics often results in increased costs [7, 16, 17]. In general, compared to standard of care, the cost of advanced therapies is thought to be offset in the long term by reductions in healthcare resource utilization as well as improved quality of life, leading to reductions in indirect costs [7, 16, 21, 181]. However, the cost-effectiveness of escalated vs. standard dosing remains unclear and could not be assessed robustly without proper quantification of the incremental efficacy associated with escalated dosing. More studies are needed to evaluate effectiveness, particularly in terms of clinical outcomes, safety, and health care resource utilization, associated with dose escalation to enable a more robust cost-effectiveness evaluation on dose escalation as well as to inform therapeutic decision making.
Even though improved treatment strategies and the use of more effective therapeutics have greatly enhanced the management of CD and UC, there are still many unmet needs in IBD. Recent research has shown that a substantial number of patients experience treatment failure and/or loss of response with first- or second-line advanced therapies [182,183,184,185,186]. Loss of response, though a common occurrence in IBD treatment, is multifactorial and can be caused by inadequate drug concentrations, antidrug antibody formation, sub-optimal adherence to treatment, or uncontrolled inflammation [24, 26, 184, 187,188,189,190,191]. Loss of response presents a major challenge in clinical practice due to the limited availability of effective treatment alternatives and adverse impacts on patient quality of life [23, 182, 184, 186, 188, 192]. Clinicians thus have a pressing need to use treatment strategies such as dose escalation to reclaim or maintain clinical response [25, 84, 182, 183, 186]. The frequent occurrence of dose escalations, therefore, underscores the need for effective treatment with long-term durability [182, 186].
Although reported less frequently, other reasons for dose escalation include continued endoscopic inflammation, elevated levels of biomarkers (C-reactive protein, fecal calprotectin), and lower level of drug serum. This might indicate that dose escalation is used in a tight control or treat-to-target management approach. As recommended by the updated STRIDE II consensus recommendation, long-term treatment goals including lower inflammatory biomarker levels or achievement of endoscopic healing could lead to long-term remission [25, 182, 186, 193,194,195]. The CALM study has demonstrated timely escalation with adalimumab dose in a tight control approach is associated with greater clinical and endoscopic improvements as well as reduced hospitalization events than conventional management in CD [196, 197]. Therefore, reason for dose escalation might also play a role in understanding the associated clinical benefit of dose escalation.
Limitations
There are several limitations of the SLRs and included studies. First, the inclusion of non-interventional observation studies may contribute to heterogeneity due to the wide variability in observed study methods, outcome measures, and patient characteristics. Second, study design of included articles may have also contributed to the increased frequency of dose escalation reporting. For example, some publications reported on dose escalation in patient populations where a therapeutic drug monitoring (TDM) algorithm was used to optimize treatment. The use of TDM algorithms to guide dosing often results in dose adjustments to levels much higher than those in routine practice. In addition, some included studies focused on populations that were likely to experience dose escalation or specifically aimed to report on the efficacy of dose escalation, thereby increasing the likelihood of dose escalation overestimation. To mitigate this, studies that included patients who were on an escalated dose at study onset were excluded. Finally, the search strategy was limited to the availability of indexed terms involving dose escalation, which may have introduced selection bias. Bibliographic and hand searches were employed to capture any previously unidentified studies.
Conclusion
Dose escalation in CD and UC is common. The reported dose escalation patterns and escalation rates vary by region and by CD and UC. Most escalation patterns reported were aligned with regulatory recommendations while some reported more diverse or aggressive dose escalation. Future studies are needed to evaluate optimal approaches for maintaining treatment effectiveness and durability in CD and UC patients.
References
Bernstein CN, et al. World gastroenterology organisation global guidelines inflammatory bowel disease: update august 2015. J Clin Gastroenterol. 2016;50(10):803–18.
Denson LA, et al. Challenges in IBD research: precision medicine. Inflamm Bowel Dis. 2019;25(Suppl 2):S31–9.
Tabib NSS, et al. Big data in IBD: big progress for clinical practice. Gut. 2020;69(8):1520–32.
Alatab S, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17–30.
Feuerstein JD, Cheifetz AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc. 2014;89(11):1553–63.
Feuerstein JD, Cheifetz AS. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin Proc. 2017;92(7):1088–103.
Dignass A, et al. Living with ulcerative colitis in Germany: a retrospective analysis of dose escalation, concomitant treatment use and healthcare costs. J Med Econ. 2020;23(4):415–27.
da Silva BC, et al. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J Gastroenterol. 2014;20(28):9458–67.
Crohn's & Colitis Foundation of America. The facts about Inflammatory Bowel Diseases. 2014.
Cohen R, et al. Direct and indirect healthcare resource utilization and costs associated with ulcerative colitis in a privately-insured employed population in the US. J Med Econ. 2015;18(6):447–56.
Ananthakrishnan AN, Kaplan GG, Ng SC. Changing global epidemiology of inflammatory bowel diseases: sustaining health care delivery into the 21st century. Clin Gastroenterol Hepatol. 2020;18(6):1252–60.
Matos R, et al. Quality of life in patients with inflammatory bowel disease: the role of positive psychological factors. Health Psychol Behav Med. 2021;9(1):989–1005.
Mayo Clinic. Crohn's disease. 2020 [cited 2022 February 15]; Available from: https://www.mayoclinic.org/diseases-conditions/crohns-disease/symptoms-causes/syc-20353304
Cleveland Clinic. Ulcerative Colitis. 2020 [cited 2022 February 15]; Available from: https://my.clevelandclinic.org/health/diseases/10351-ulcerative-colitis
Click B, Binion DG, Anderson AM. Predicting costs of care for patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2017;15(3):393–5.
Annese V, et al. Optimizing biologic therapy in inflammatory bowel disease: a Delphi consensus in the United Arab Emirates. Therap Adv Gastroenterol. 2021;14:17562848211065328.
Ehrenberg R., et al. Dose escalation assessment among targeted immunomodulators in the management of inflammatory bowel disease. J Manag Care Spec Pharm. 2020;26(6): 758–765
van der Valk ME, et al. Healthcare costs of inflammatory bowel disease have shifted from hospitalisation and surgery towards anti-TNFalpha therapy: results from the COIN study. Gut. 2014;63(1):72–9.
Lichtenstein, GR, et al. Lifetime economic burden of Crohn's disease and ulcerative colitis by age at diagnosis. Clin Gastroenterol Hepatol. 2020;18(4): 889–897 e10.
Gibson TB, et al. The direct and indirect cost burden of Crohn’s disease and ulcerative colitis. J Occup Environ Med. 2008;50(11):1261–2122.
Rubin DT, et al. Real-world assessment of therapy changes, suboptimal treatment and associated costs in patients with ulcerative colitis or Crohn’s disease. Aliment Pharmacol Ther. 2014;39(10):1143–55.
Long MD, et al. Retrospective database analysis: dose escalation and adherence in patients initiating biologics for ulcerative colitis. Dig Dis. 2021;40(5):553–64.
Guberna L., et al. Frequency and effectiveness of empirical anti-tnf dose intensification in inflammatory bowel disease: systematic review with meta-analysis. J Clin Med, 2021. 10(10).
Khan S, et al. Real-world evidence on adherence, persistence, switching and dose escalation with biologics in adult inflammatory bowel disease in the United States: a systematic review. J Clin Pharm Ther. 2019;44(4):495–507.
Zittan E, Gralnek IM, Berns MS. The new proactive approach and precision medicine in Crohn's disease. Biomedicines. 2020;8(7).
Sanchez-Hernandez JG, et al. A 3-year prospective study of a multidisciplinary early proactive therapeutic drug monitoring programme of infliximab treatments in inflammatory bowel disease. Br J Clin Pharmacol. 2020;86(6):1165–75.
Ma C, et al. Ustekinumab effective for maintaining clinical response in refractory moderate-to-severe Crohn’s disease: a multicentre cohort study [abstract]. In: Journal of the Canadian Association of Gastroenterology. Oxford University Press; 2018. p. 182–4.
Eberl A, et al. Ustekinumab for Crohn’s disease: a nationwide real-life cohort study from Finland (FINUSTE). Scand J Gastroenterol. 2019;54(6):718–25.
Yadav A, et al. Dose escalation (Q4) of ustekinumab should be considered for Crohn's disease patients who fail standard dosing [abstract]. In: Inflammatory bowel diseases. 2020, Crohn’s & Colitis Foundation and the AGA Institute. p. S72–S73.
Battat R, et al. Association between ustekinumab trough concentrations and clinical, biomarker, and endoscopic outcomes in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2017; 15(9): 1427–1434 e2.
Heron V, et al. Ustekinumab therapeutic drug monitoring in Crohn’s disease patients with loss of response. J Crohns Colitis. 2019;13:S379–80.
Olmedo-Martín R, et al. Effectiveness of ustekinumab dose escalation in Crohn’s disease patients with insufficient response to standard-dose subcutaneous maintenance therapy: an observational multicentre study. J Crohns Colitis. 2021;15:S467–8.
Mateos B, et al. Ustekinumab levels correlate with induction fecal calprotectin drop-slope and discriminate the need for intensification at week 52 in Crohn’s Disease patients. J Crohns Colitis. 2021;15:S533–4.
Ma C, et al. Long-term maintenance of clinical, endoscopic, and radiographic response to ustekinumab in moderate-to-severe Crohn’s disease: real-world experience from a multicenter cohort study. Inflamm Bowel Dis. 2017;23(5):833–9.
Khorrami S, et al. Ustekinumab for the treatment of refractory Crohn’s disease: the spanish experience in a large multicentre open-label cohort. Inflamm Bowel Dis. 2016;22(7):1662–9.
Chaparro M, et al. Long-term durability and safety of Ustekinuman (UST) in patients with active Crohn's disease (CD) in real life: SUSTAIN study [abstract]. In: 28th United European Gastroenterology Week. 2020: Virtual. 407–408.
Chaparro M, et al. Effectiveness and safety of Ustekinumab (UST) in patients with active Crohn's disease (CD) in real life: SUSTAIN study [abstract]. In: 28th United European Gastroenterology Week. 2020: Virtual. 406–407.
Truyens M, et al. Efficacy of Ustekinumab intensification and re-induction in Crohn’s disease patients with insufficient or loss of response. Unit Eur Gastroenterol J. 2019;7(8 suppl): 645–645
Glassner K, et al. Low levels of ustekinumab despite dose escalation is a risk factor for surgery. Am Coll Gastroenterol. 2019;114:S405.
Ramaswamy PK, et al. MO1869 Efficacy of dose intensification of ustekinumab in Crohn's disease. Gastroenterology. 2020;S-956.
Sipponen T, et al. A nationwide real-world study on dynamic ustekinumab dosing and concomitant medication use among Crohn’s disease patients in Finland. Scand J Gastroenterol. 2021;56(6):661–70.
Biemans VBC, et al. Vedolizumab for inflammatory bowel disease: two-year results of the initiative on Crohn and Colitis (ICC) registry, a nationwide prospective observational cohort study. Clin Pharmacol Ther. 2020;107:1189–99.
Biemans V, et al. Two year experience with vedolizumab in inflammatory bowel disease patients: results of the ICC case series, a nationwide prospective observational cohort study. J Crohns Colitis. 2019;13:S363–4.
Attauabi M, et al. Short and long-term effectiveness and safety of vedolizumab in treatment-refractory patients with ulcerative colitis and Crohn's disease—a real-world two-center cohort study. Eur J Gastroenterol Hepatol. 2021;33(1S Suppl 1): e709-e718.
Attauabi M, et al. Treatment optimization with vedolizumab in treatment-refractory patients with ulcerative colitis and Crohn’s disease—a real-world two-center cohort study [abstract]. In: 29th United European Gastroenterology Week. 2021:550.
Shivashankar R, et al. Effect of vedolizumab dose escalation on recapturing response in patients with inflammatory bowel disease. Gastroenterol. 2017;152(5).
Mendoza Ladd AH, et al. Sa1086 Dose Escalation of Vedolizumab From Every 8 Weeks to Every 4 or 6 Weeks Enables Patients With Inflammatory Bowel Disease to Recapture Response. In: Gastroenterology. 2016:S235-S236.
Iborra M, et al. Vedolizumab, an option in patients with inflammatory bowel disease intolerant to thiopurines and refractory to biological agents. Gastroenterología y Hepatología (English Edition). 2018;41(9):535–43.
Iborra M, et al. Vedolizumab, an adequate option in medically refractory and thiopurine-intolerant inflammatory bowel disease patients. J Crohns Colitis. 2018;12:S472–3.
Dulai PS, et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY consortium. Am J Gastroenterol. 2016;111(8):1147–55.
Amiot A, et al. One-year effectiveness and safety of Vedolizumab therapy for inflammatory bowel disease: a prospective multicentre cohort study. Aliment Pharmacol Ther. 2017;46(3):310–21.
Amiot A, et al. Three-year effectiveness and safety of Vedolizumab therapy for inflammatory bowel disease: a prospective multi-centre cohort study. Aliment Pharmacol Ther. 2019;50(1):40–53.
Srinivasan A, et al. A virtual clinic increases anti-TNF dose intensification success via a treat-to-target approach compared with standard outpatient care in Crohn’s disease. Aliment Pharmacol Ther. 2020;51(12):1342–52.
Suzuki T, et al. Adalimumab dose escalation therapy is effective in refractory Crohn’s disease patients with loss of response to adalimumab, especially in cases without previous infliximab treatment. Case Rep Gastroenterol. 2019;13(1):37–49.
Olivares D, et al. Differences in the need for Adalimumab dose optimization between Crohn’s disease and Ulcerative Colitis. Rev Esp Enferm Dig. 2019;111(11):846–51.
Olivares D, et al. Need for adalimumab dose optimization in patients with Crohn's disease and ulcerative Colitis [abstract]. In: 26th United European Gastroenterology Week. 2018: Vienna, Austria, p. A454.
Kanazawa J, et al. Long-term outcomes of adalimumab in patients with Crohn’s disease: can a doubled dose of adalimumab improve outcomes? J Crohns Colitis. 2019;13:S367–8.
Papamichael K, et al., Primary Non Response to Infliximab in Patients With Inflammatory Bowel Disease. AGA. 2014: S-457.
Castaño-Milla C, et al. Effectiveness of adalimumab in perianal fistulas in Crohn’s disease patients naive to Anti-TNF therapy. J Clin Gastroenterol. 2015;49(1):34–40.
Castaño-Milla C, et al. Effectiveness of adalimumab in the treatment of perianal fistulas in patients with Crohn's disease naïve to anti-TNF: a multicenter study [abstract]. In: 7th Congress of ECCO - the European Crohn's and Colitis Organisation. 2012: Barcelona, Spain. p. S155.
Roblin X, et al. Swapping versus dose optimization in patients losing response to adalimumab with adequate drug levels. Inflamm Bowel Dis. 2021.
Seo H, et al. Long-term outcomes of adalimumab treatment in 254 patients with Crohn’s disease: a hospital-based cohort study from Korea. Dig Dis Sci. 2017;62(10):2882–93.
Preda C, et al. Adalimumab and Infliximab in Crohn’s disease—real life data from a national retrospective cohort study. Curr Health Sci J. 2016;42(2):115–24.
Fulger LE, et al. Infliximab and adalimumab in Crohn's disease: a comparative analysis of efficacy, safety and management of secondary loss of response in a cohort Romanian study [abstract]. In: 23rd United European Gastroenterology Week. 2015: Barcelona, Spain. p. A431.
Kariyawasam VC, et al. Thiopurines dosed to a therapeutic 6-thioguanine level in combination with adalimumab are more effective than subtherapeutic thiopurine-based combination therapy or adalimumab monotherapy during induction and maintenance in patients with long-standing Crohn’s disease. Inflamm Bowel Dis. 2017;23(9):1555–65.
Viazis N, et al. Azathioprine discontinuation earlier than 6 months in Crohn’s disease patients started on anti-TNF therapy is associated with loss of response and the need for anti-TNF dose escalation. Eur J Gastroenterol Hepatol. 2015;27(4):436–41.
Archavlis E, et al. Adalimumab for patients with Crohn's disease and secondary loss of response or severe allergy to Infliximab. [abstract]. In: 6th Congress of ECCO—European Crohn’s and Colitis Organisation. 2011: Dublin, Ireland. p. S86.
Ghaly S, et al. Dose tailoring of anti-tumour necrosis factor-alpha therapy delivers useful clinical efficacy in Crohn disease patients experiencing loss of response. Intern Med J. 2015;45(2):170–7.
Ma C, et al. Adalimumab dose escalation is effective for managing secondary loss of response in Crohn’s disease. Aliment Pharmacol Ther. 2014;40(9):1044–55.
Duveau N, et al. Adalimumab dose escalation is effective and well tolerated in Crohn’s disease patients with secondary loss of response to Adalimumab. In: 11th Congress of ECCO—European Crohn's and Colitis Organisation. 2016: Amsterdam, The Netherlands. p. S320.
Guerbau L, et al. Crohn's disease patients with high body mass index present more frequent and rapid loss of response to Infliximab [abstract]. In: 11th Congress of ECCO – European Crohn´s and Colitis Organisation. 2016: Amsterdam, The Netherlands. p. S430.
Bhalme M, et al. Does weight-adjusted anti-tumour necrosis factor treatment favour obese patients with Crohn’s disease? Eur J Gastroenterol Hepatol. 2013;25(5):543–9.
Sakuraba A, et al. Combination therapy with infliximab and thiopurine compared to infliximab monotherapy in maintaining remission of postoperative Crohn’s disease. Digestion. 2015;91(3):233–8.
Park SH, et al. Long-term outcomes of infliximab treatment in 582 korean patients with Crohn’s disease: a hospital-based cohort study. Dig Dis Sci. 2016;61(7):2060–7.
Park SH, et al. Long-term outcome of infliximab treatment in patients with Crohn’s disease: a hospital-based cohort study from Korea [abstract]. In: 10th Congress of ECCO—European Crohn’s and Colitis Organisation. Spain: Barcelona; 2015. p. S57–8.
Dreesen E, et al. Anti-infliximab antibody concentrations can guide treatment intensification in patients with Crohn’s disease who lose clinical response. Aliment Pharmacol Ther. 2018;47(3):346–55.
Katz L, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18(11):2026–33.
Lin KK, et al. Durability of infliximab dose intensification in Crohn’s disease. Dig Dis Sci. 2012;57(4):1013–9.
Komaki Y, et al. Infliximab therapy intensification based on endoscopic activity is related to suppress treatment discontinuation in patients with Crohn disease: a retrospective cohort study. Med (Baltim). 2021;100(6): e24731.
Chaparro M, et al. Intensification of infliximab therapy in Crohn’s disease: efficacy and safety. J Crohns Colitis. 2012;6(1):62–7.
Chaparro M, et al. Long-term durability of Infliximab treatment in Crohn's disease and efficacy of dose “escalation” in patients losing response. J Clin Gastroenterol. 2011;45(2): 113–118.
Kopylov U, et al. The efficacy of shortening the dosing interval to once every six weeks in Crohn’s patients losing response to maintenance dose of Infliximab. Aliment Pharmacol Ther. 2011;33(3):349–57.
Nagata Y, et al. Therapeutic strategy for Crohn’s disease with a loss of response to infliximab: a single-center retrospective study. Digestion. 2015;91(1):50–6.
Guerbau L, et al. Patients with Crohn’s disease with high body mass index present more frequent and rapid loss of response to infliximab. Inflamm Bowel Dis. 2017;23(10):1853–9.
Maemoto A, et al. Mo1263 CRP, but Not CDAI is associated with enterocolonic mucosal healing in crohn's disease treated with infliximab; a single center cohort study. In: Gastrointestinal Endoscopy. 2012.
Moon W, et al. Efficacy and safety of certolizumab pegol for Crohn’s disease in clinical practice. Aliment Pharmacol Ther. 2015;42(4):428–40.
Moon W, et al. Effectiveness and safety of certolizumab pegol for Crohn's disease in a large cohort followed at a tertiary care center [abstract]. In: 46th Annual Digestive Disease Week (DDW). 2015:S-871.
Haider SA, et al. Ustekinumab dose escalation improves clinical responses in refractory Crohn’s disease. Therap Adv Gastroenterol. 2020;13:1756284820959245.
Ollech JE, et al. Effectiveness of ustekinumab dose escalation in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2021;19(1):104–10.
Ding Z, et al. Real-world persistence, maintenance dosing, and pre-post corticosteroid and opioid use among Crohn’s disease patients with prescription claims for ustekinumab in the USA. Drugs Real World Outcomes. 2021;8(4):565–75.
Muser E, et al. Treatment persistence and maintenance dosing for Ustekinumab and Adalumumab among patients with Crohn's disease with 2-years follow-up in the Symphony health database [abstract]. In: 2020 Digestive Disease Week (DDW) 2020: Virtual. p. S959.
Dalal RS, et al. Predictors of ustekinumab failure after dose intensification among patients with Crohn’s disease [abstract]. In: 2020 ACG Annual Meeting. Tennessee: Nashville; 2020. p. S324–5.
Nije C, Dalal RS, Allegretti JR. Factors Associated With Ustekinumab Dose Escalation in Patients With Crohn’s Disease [abstract]. In: 2020 ACG Annual Meeting, Nashville, Tennessee. p. S368. 2020
Greenup AJ, Rosenfeld G, Bressler B. Ustekinumab use in Crohn’s disease: a Canadian tertiary care centre experience. Scand J Gastroenterol. 2017;52(12):1354–9.
Kopylov U, et al. Subcutaneous Ustekinumab for the treatment of anti-TNF resistant Crohn’s disease–the McGill experience. J Crohns Colitis. 2014;8(11):1516–22.
Dalal RS, et al. Ustekinumab Dose Intensification in Patients With Crohn’s Disease: Comparing Every 4 and Every 6-Week Dosing [abstract]. In: 2020 ACG Annual Meeting.Nashville, Tennessee. p. S414. 2020
Obando C, et al. Persistence, dose titration, and health care resource utilization among Crohn’s disease patients treated with ustekinumab: a real-world analysis in the United States. Adv Ther. 2020;37(5):2127–43.
Hanzel J, et al. Ustekinumab concentrations shortly after escalation to monthly dosing may identify endoscopic remission in refractory Crohn’s disease. Eur J Gastroenterol Hepatol. 2021;33(1S):e831–6.
Kiat C, et al. Ustekinumab in Crohn’s disease: experience from a single tertiary centre. Ir J Med Sci. 2015;184:S245.
Kopylov U, et al. Effectiveness of Ustekinumab dose escalation in Crohn’s disease patients with insufficient response to standard-dose subcutaneous maintenance therapy. Aliment Pharmacol Ther. 2020;52(1):135–42.
Kopylov U, et al. Effectiveness of dose escalation in Crohn’s disease patients with insufficient response to standard-dose subcutaneous Ustekinumab maintenance therapy: a multicentre international cohort study [abstract]. In: 15th Congress of ECCO—European Crohn’s and Colitis Organisation. Austria: Vienna; 2020. p. S111–2.
Iborra M, et al. Real-world long-term effectiveness of Ustekinumab in Crohn's disease: Results from the ENEIDA registry [abstract]. In: Digestive disease week (DDW) 2020. Virtual. p. S953. 2020
Townsend T, et al. Comparative effectiveness of Ustekinumab or Vedolizumab after one year in 130 patients with anti-TNF-refractory Crohn’s disease. Aliment Pharmacol Ther. 2020;52(8):1341–52.
Biemans V, et al. Ustekinumab for Crohn’s disease: results of the ICC registry, a nationwide prospective observational cohort study. J Crohns Colitis. 2019;14(1):33–45.
European Medicines Agency. Janssen Biologics B.V. STELARA (ustekinumab) [package insert]. 2013 [cited 2022 February 15]. Available from: https://www.ema.europa.eu/en/documents/product-information/stelara-epar-product-information_en.pdf
Wice M, et al. Efficacy and safety of vedolizumab in patients with inflammatory bowel disease in a large tertiary medical center. Am J Gastroenterol. 2016;111:S270.
Reinglas J, et al. Low rate of drug discontinuation, frequent need for dose adjustment, and no association with development of new arthralgia in patients treated with vedolizumab: results from a tertiary referral IBD center. Dig Dis Sci. 2020;65(7):2046–53.
Williet N, et al. Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses within 6 months. Clin Gastroenterol Hepatol. 2017;15(11): 1750–1757 e3
Lee SD, Singla A, Clark-Snustad K. Standard and modified Vedolizumab dosing is effective in achieving clinical and endoscopic remission in moderate-severe Crohn’s disease. GastroHep. 2021;3(1):26–36.
Dreesen E, et al. Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018;16(12): 1937–1946 e8.
Lee SD, et al. Real-world experience: clinical and endoscopic effectiveness of standard Vedolizumab dosing and modified maintenance dosing in patients with moderate to severe Crohn's disease [abstract]. In: 2020 Digestive Disease Week (DDW). Virtual. p. S-961–S-962. 2020
Restellini S, et al. Therapeutic drug monitoring guides the management of Crohn’s patients with secondary loss of response to adalimumab. Inflamm Bowel Dis. 2018;24(7):1531–8.
Peters CP, et al. Adalimumab for Crohn’s disease: long-term sustained benefit in a population-based cohort of 438 patients. J Crohns Colitis. 2014;8(8):866–75.
Monfared AAT, Fan T, Sen S. Dose Escalation of Adalimumab (Humira1)in Patients with Crohn’s Disease in Routine Clinical Practice in Quebec [abstract], in 27th International Conference on Pharmacoepidemiology and Therapeutic Risk Management, Chicago, Illinois. p. S180. 2011
Mantzaris GJ, et al. Factors influencing the time to and outcome of dose escalation of Adalimumab in Crohn’s disease patients who achieved steroid-free remission on Adalimumab. In: 9th Congress of ECCO—the European Crohn’s and Colitis Organisation. Denmark: Copenhagen; 2014. p. S269–70.
Aguas M, et al. Adalimumab in prevention of postoperative recurrence of Crohn’s disease in high-risk patients. World J Gastroenterol. 2012;18(32):4391–8.
Fortea-Ormaechea JI, et al. Adalimumab is effective in long-term real life clinical practice in both luminal and perianal Crohn’s disease. The madrid experience. Gastroenterol Hepatol. 2011;34(7):443–8.
Mastronardi M, et al. Administration timing is the best clinical outcome predictor for adalimumab administration in Crohn’s disease. Front Med (Lausanne). 2019;6:234.
Verstockt B, et al. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naive Crohn’s disease patients: the usefulness of rapid testing. Aliment Pharmacol Ther. 2018;48(7):731–9.
Reenaers C, et al. Does co-treatment with immunosuppressors improve outcome in patients with Crohn’s disease treated with adalimumab? Aliment Pharmacol Ther. 2012;36(11–12):1040–8.
Baert F, et al. Adalimumab dose escalation and dose de-escalation success rate and predictors in a large national cohort of Crohn’s patients. J Crohns Colitis. 2013;7(2):154–60.
Orlando A, et al. Adalimumab in steroid-dependent Crohn’s disease patients: prognostic factors for clinical benefit. Inflamm Bowel Dis. 2012;18(5):826–31.
Barberio B, et al. Infliximab originator, infliximab biosimilar, and adalimumab are more effective in Crohn’s disease than ulcerative colitis: a real-life cohort study. Clin Transl Gastroenterol. 2020;11(5): e00177.
O’Donnell S, et al. Higher Rates of Dose Optimisation for Infliximab Responders in Ulcerative Colitis than in Crohn’s disease. J Crohns Colitis. 2015;9(10):830–6.
O'Donnell S, Stempak JM, Silverberg MS. Is there a higher rate of infliximab dose optimization in initial responders between UC and CD cases? [abstract]. In: 2014 Digestive Disease Week (DDW). Chicago, Illinois. p. S-462–S-463. 2014
Tkacz J, et al. Infliximab dosing patterns in a sample of patients with Crohn’s disease: results from a medical chart review. Am Health Drug Benefits. 2014;7:87–93.
Waters H, et al. Stability of infliximab dosing in inflammatory bowel disease: results from a multicenter US chart review. J Med Econ. 2011;14(4):397–402.
Clark-Snustad KD, Singla A, Lee SD. Efficacy of Infliximab in Crohn’s disease patients with prior primary-nonresponse to tumor necrosis factor antagonists. Dig Dis Sci. 2019;64(7):1952–8.
Kato M, et al. Long-term outcomes of infliximab therapy for patients with Crohn's disease at different time of initiation of infliximab administration: a single-centre cohort study in Japan [abstract]. In: United European Gastroenterology Week. Barcelona, Spain. p. 351. 2019
Tanaka H, et al. Long-term outcomes of infliximab therapy for japanese patients with Crohn’s disease at different times of initiation of infliximab administration [abstract]. In: 2019 Digestive Disease Week (DDW). San Diego, California. p. S-884–S-885. 2019
Mizoshita T, et al. Long-term clinical remission in biologically naive Crohn’s Disease Patients With Adalimumab Therapy, Including Analyses Of Switch From Adalimumab To Infliximab. Case Rep Gastroenterol. 2016;10(2):283–91.
Paul S, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2013;19(12):2568–76.
Brandse JF, et al. Re-introduction of infliximab after consecutive failure of infliximab and adalimumab is beneficial in refractory Crohn’s disease [abstract]. In: 8th Congress of ECCO—European Crohn’s and Colitis Organisation. Austria: Vienna; 2013. p. S165–6.
Perry C, et al. Vedolizumab dose escalation improves therapeutic response in a subset of patients with ulcerative colitis. Dig Dis Sci. 2021;66(6):2051–8.
Perry C, et al. An evaluation of Vedolizumab “partial responders”: Quantifying benefit of shortened dosing invervals in ulverative Colitis [abstract]. In: 2020 Crohn’s and Colitis Congress. Texas: Austin; 2020. p. S70–1.
Perry C, et al. Vedolizumab dose escalation improves disease activity for “partial responders” in Ulcerative Colitis [abstract]. In: Digestive Disease Week (DDW) 2020. Virtual. p. S-965. 2020
Taxonera C, et al. Adalimumab maintenance treatment in ulcerative colitis: outcomes by prior anti-TNF use and efficacy of dose escalation. Dig Dis Sci. 2017;62(2):481–90.
Ferrante M, et al. Long-term outcome of infliximab therapy in patients with ulcerative colitis: identification of predictors of relapse-free and colectomy-free survival [abstract]. In: 2013 Digestive Disease Week (DDW). Orlando, Florida. p. S-426. 2013
Dumitrescu G, et al. The outcome of infliximab dose doubling in 157 patients with ulcerative colitis after loss of response to infliximab. Aliment Pharmacol Ther. 2015;42(10):1192–9.
Taxonera C, et al. Infliximab dose escalation as an effective strategy for managing secondary loss of response in ulcerative colitis. Dig Dis Sci. 2015;60(10):3075–84.
Taxonera C, et al. Short- and long-term outcomes of infliximab dose intensification in patients with ulcerative colitis. In: 2012 Digestive Disease Week (DDW). San Diego, California. p. S-355. 2012
Cesarini M, et al. Dose optimization is effective in ulcerative colitis patients losing response to infliximab: a collaborative multicentre retrospective study. Dig Liver Dis. 2014;46(2):135–9.
Arias MT, et al. A panel to predict long-term outcome of infliximab therapy for patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2015;13(3):531–8.
Stefanovic S, et al. Endoscopic remission can be predicted by golimumab concentrations in patients with ulcerative colitis treated with the changed label. Eur J Gastroenterol Hepatol. 2021;33(1):54–61.
Taxonera C, et al. Clinical outcomes of golimumab as first, second or third anti-tnf agent in patients with moderate-to-severe ulcerative colitis. Inflamm Bowel Dis. 2017;23(8):1394–402.
Taxonera C, et al. Real-life experience with golimumab in ulcerative colitis patients according to prior anti-TNF use [abstract]. In: 11th Congress of ECCO - European Crohn's and Colitis Organisation. Amsterdam, The Netherlands. p. S303. 2016
Ollech JE, et al. Efficacy and safety of induction therapy with calcineurin inhibitors followed by Vedolizumab maintenance in 71 patients with severe steroid-refractory Ulcerative Colitis. Aliment Pharmacol Ther. 2020;51(6):637–43.
Ollech J, et al. Efficacy and safety of induction therapy with calcineurin inhibitors followed by maintenance therapy with Vedolizumab in severe Ulcerative Colitis: a large patient cohort with long-term follow-up [abstract]. In: 15th Congress of ECCO - European Crohn's and Colitis Organisation. Vienna, Austria. p. S401. 2020
European Medicines Agency. Pfizer service company BVBA. XELJANZ (tofacitinib) [package insert] 2017 March 2017. Available from: https://www.ema.europa.eu/en/documents/product-information/xeljanz-epar-product-information_en.pdf
Chaparro M, et al. Tofacitinib in ulcerative colitis: real world evidence from ENEIDA registry [abstract]. In: 28th United European Gastroenterology Week. Virtual. p. 419–420. 2020
Hong S, et al. Real-world effectiveness of ustekinumab in ulcerative colitis, in 2020 Crohn's & Colitis Congress. 2020: Austin, Texas. p. S120.
Dalal RS, et al. Predictors and Outcomes of Ustekinumab Dose Intensification in Ulcerative Colitis: A Multicenter Cohort Study. Clin Gastroenterol Hepatol. 2021.
Dalal R, et al. Predictors and outcomes of ustekinumab dose escalation to every 4 or every 6 weeks in ulcerative colitis: a multicenter cohort study in the united states [abstract]. In: 16th Congress of ECCO—European Crohn's and Colitis Organisation. Virtual. p. S296–S298. 2021
Chaparro M, et al. Effectiveness and safety of ustekinumab in ulcerative colitis: real-world evidence from the ENEIDA registry. J Crohns Colitis. 2021;15(11):1846–51.
Ecker D, et al. Ustekinumab for Ulcerative Colitis a real-world experience- Retrospective data analysis of the IBD cohort Ordensklinikum Linz. In: 29th United European Gastroenterology Week. Virtual. p. 490. 2021
Almutairdi A, et al. Clinical and endoscopic remission with vedolizumab treatment in ulcerative colitis. J Crohns Colitis. 2018;12:S430–1.
Dignass A, et al. Living with ulcerative colitis in germany: estimating the frequency and extent of dose escalations among patients with moderate to severe ulcerative colitis. United Eur Gastroenterol J. 2019;7(8):341–2.
European Medicines Agency. Janssen biologics B.V. SIMPONI (golimumab) [package insert]. 2014 June 2014 [cited 2022 February 15]. Available from: https://www.ema.europa.eu/en/documents/product-information/simponi-epar-product-information_en.pdf
Kumar A, et al. Golimumab in ulcerative colitis: a multi-centre real world experience [abstract]. In: International Digestive Disease Forum (IDDF). Hong Kong. p. A83. 2018
Iborra M, et al. Real-life experience with 4 years of golimumab persistence in ulcerative colitis patients. Sci Rep. 2020;10(1):17774.
Iborra M, et al. Real-life experience with long-term maintenance of Golimumab in Ulcerative Colitis patients [abstract]. In: 14th Congress of ECCO—European Crohn’s and Colitis Organisation. Denmark: Copenhagen; 2019. p. S393–4.
Taxonera C, et al. Early dose optimization of golimumab induces late response and long-term clinical benefit in moderately to severely active ulcerative colitis. Curr Med Res Opin. 2019;35(7):1297–304.
Baert F, et al. Prior response to infliximab and early serum drug concentrations predict effects of adalimumab in ulcerative colitis. Aliment Pharmacol Ther. 2014;40(11–12):1324–32.
Van de Vondel S, et al. Incidence and predictors of success of adalimumab dose escalation and de-escalation in ulcerative colitis: a real-world belgian cohort study. Inflamm Bowel Dis. 2018;24(5):1099–105.
Van de Vondel S, et al. Adalimumab dose escalation and de-escalation in ulcerative colitis: incidence and predictors of success. A real life Belgian cohort study [abstract]. In: 12th Congress of ECCO—European Crohn's and Colitis Organisation. Barcelona, Spain. p. S299. 2017
Garcia-Bosch O, et al. Observational study on the efficacy of adalimumab for the treatment of ulcerative colitis and predictors of outcome. J Crohns Colitis. 2013;7(9):717–22.
Taxonera C, et al. Adalimumab induction and maintenance therapy for patients with ulcerative colitis previously treated with infliximab. Aliment Pharmacol Ther. 2011;33(3):340–8.
Pollinger B, et al. Costs of dose escalation among ulcerative colitis patients treated with adalimumab in Germany. Eur J Health Econ. 2019;20(2):195–203.
Salomonsson S, et al. Dose escalation among ulcerative colitis patients treated with adalimumab in Sweden [abstract]. In: ISPOR 18th Annual European Congress, Milan, Italy. p. A633. 2015
Balint A, et al. Efficacy and safety of adalimumab in ulcerative colitis refractory to conventional therapy in routine clinical practice. J Crohns Colitis. 2016;10(1):26–30.
Shin Shin S, et al. The efficacy and safety of adalimumab for patients with moderately to severely active ulcerative colitis and predictors of response in Korea [abstract]. In: 15th COngress of ECCO - European Crohn's and Colitis Organisation, Vienna, Austria. p. S432. 2020
Choi CH, et al. The efficacy of adalimumab for the treatment of Korean patients with ulcerative colitis and predictors of response: Preliminary results [abstract]. In: 13th Congress of ECCO - European Crohn's and Colitis Organisation, Vienna, Austria. p. P477. 2018
Shah SC, et al. Accelerated infliximab dosing increases 30-day colectomy in hospitalized ulcerative colitis patients: a propensity score analysis. Inflamm Bowel Dis. 2018;24(3):651–9.
Kosaraju RS, et al. Dose-intensified infliximab rescue therapy for severe ulcerative colitis does not reduce short-term colectomy rates or increase postoperative complications [abstract]. In: The American Society of Colon and Rectal Surgeons’ Annual Meeting. Washington: Seattle; 2022. p. 1232–40.
Balint A, et al. Infliximab biosimilar CT-P13 therapy is effective in maintaining endoscopic remission in ulcerative colitis—results from multicenter observational cohort. Expert Opin Biol Ther. 2018;18(11):1181–7.
Farkas K, et al. Infliximab biosimilar CT-P13 therapy is effective in maintaining endoscopic remission in Ulcerative Colitis- Results from multicentre observational cohort [abstract]. In: 25th United European Gastroenterology Week, Barcelona, Spain. p. A743. 2017
Magro F, et al. Calprotectin and the magnitude of antibodies to infliximab in clinically-stable ulcerative colitis patients are more relevant than infliximab trough levels and pharmacokinetics for therapeutic escalation. EBioMedicine. 2017;21:123–30.
Fernández-Salazar L, et al. Frequency, predictors, and consequences of maintenance infliximab therapy intensification in ulcerative colitis. Rev Esp Enferm Dig. 2015;107:527–33.
Fernández-Salazar L, et al. Infliximab use in ulcerative colitis from 2003 to 2013: clinical practice, safety and efficacy [abstract]. In: 9th Congress of ECCO—the European Crohn’s and Colitis Organisation. Denmark: Copenhagen; 2014. p. S259–60.
CADTH. Biologics dose escalation for the treatment of inflammatory bowel disease: a review of clinical effectiveness, cost-effectiveness, and guidelines. Chao Y-S, Visintini S (eds) Ottawa. 2018
van der Valk ME, et al. Evolution of costs of inflammatory bowel disease over two years of follow-up. PLoS One. 2016;11(4): e0142481.
Danese S, et al. Unmet medical needs in ulcerative colitis: an expert group consensus. Dig Dis. 2019;37(4):266–83.
Gordon JP, et al. Characterizing unmet medical need and the potential role of new biologic treatment options in patients with ulcerative colitis and Crohn’s disease: a systematic review and clinician surveys. Eur J Gastroenterol Hepatol. 2015;27(7):804–12.
Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33(9):987–95.
Lee S, et al. Rates of treatment failure among patients with Ulcerative Colitis treated with first-line advanced treatments [abstract]. In: 29th United European Gastroenterology Week. Virtual. p. 545. 2021
Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106(4):685–98.
Kothari MM, Nguyen DL, Parekh NK. Strategies for overcoming anti-tumor necrosis factor drug antibodies in inflammatory bowel disease: case series and review of literature. World J Gastroint Pharmacol Therap. 2017;8(3):155–61.
Fine S, Papamichael K, Cheifetz AS. Etiology and management of lack or loss of response to anti-tumor necrosis factor therapy in patients with inflammatory bowel disease. Gastroenterol Hepatol. 2019;15(12):656–65.
Roda G, et al. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7(1): e135.
Moss AC. Optimizing the use of biological therapy in patients with inflammatory bowel disease. Gastroenterol Rep (Oxf). 2015;3(1):63–8.
Strand V, et al. Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs. 2017;31(4):299–316.
Rutgeerts P, et al. Comparison of scheduled and episodic treatment strategies of infliximab in Crohn’s disease. Gastroenterology. 2004;126(2):402–13.
Atreya R, Neurath MF, Siegmund B. Personalizing treatment in IBD: hype or reality in 2020? Can we predict response to anti-TNF? Front Med (Lausanne). 2020;7:517.
Reves J, Ungaro RC, Torres J. Unmet needs in inflammatory bowel disease. Curr Res Pharmacol Drug Discov. 2021;2: 100070.
Turner D, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-target strategies in IBD. Gastroenterology. 2021;160(5):1570–83.
Colombel J-F, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. The Lancet. 2017;390(10114):2779–89.
Panaccione R, et al. Tight control for Crohn’s disease with adalimumab-based treatment is cost-effective: an economic assessment of the CALM trial. Gut. 2020;69(4):658–64.
Gonczi L, et al. Clinical efficacy, drug sustainability and results from therapeutic drug monitoring in Crohn’s disease patients treated with ustekinumab—1-year follow-up of a prospective, nationwide, multicenter cohort from Hungary [abstract]. In: 16th Congress of ECCO—European Crohn's and Colitis Organisation, Virtual. p. S441. 2021
Gonczi L, et al. Clinical efficacy, drug sustainability and serum drug levels in Crohn’s disease patients treated with ustekinumab—a prospective, multicenter cohort from Hungary. Dig Liver Dis. 2022;54(2):207–13.
Gubbiotti A, et al. The anti-IL-23/IL-12 agent ustekinumab is an effective and safe induction therapy in patients with crohn’s disease refractory or intolerant to anti-TNF: a multicentre Italian study [abstract]. In: 15th Congress of ECCO - European Crohn's and Colitis Organisation. Vienna, Austria. p. S379. 2020
Hendler SA, et al. High-dose infliximab therapy in Crohn’s disease: clinical experience, safety, and efficacy. J Crohns Colitis. 2015;9(3):266–75.
Hendler S, et al. High dose Infliximab in Crohn's disease: clinical experience, safety, and efficacy [abstract]. In: 21st United European Gastroenterology Week, Berlin, Germany. p. A522. 2013
Seo H, et al. P641 Long-term outcome of Adalimumab therapy and predictors of response in 254 patients with Crohn’s disease: a hospital-based cohort study from Korea. In: 12th Congress of ECCO—European Crohn’s and Colitis Organisation. Spain: Barcelona; 2017. p. S408–S408.
Calvo Moya M, et al. Effectiveness and evolution in the use of ustekinumab in real-life clinical practice: a single center experience with intravenous induction therapy in refractory Crohn's disease patients. In: 27th UEG Week, Barcelona, Spain. p. 636. 2019
Saiz Chumillas RM, et al. Efficacy and safety of ustekinumab in patients with Crohn’s disease refractory to anti-tumour necrosis factor: real clinical practice [abstract]. In: 16th Congress of ECCO - European Crohn's and Colitis Organisation, Virtual. p. S400–S401. 2021
Mcgregor C, et al. Real world effectiveness of ustekinumab for refractory Crohn’s disease: a regional experience [abstract]. In: 2021 British Society of Gastroenterology Annual Meeting. p. A108-A108. 2021
Gadhok R, et al. Ustekinumab: medium-term outcomes from a uk multi-centre real-world cohort [abstract]. In: 2021 British Society of Gastroenterology Annual Meeting, p. A94-A95. 2021.
Loftus EV Jr, et al. Adalimumab real-world dosage pattern and predictors of weekly dosing: patients with Crohn’s disease in the United States. J Crohns Colitis. 2011;5(6):550–4.
Kiss LS, et al. Early clinical remission and normalisation of CRP are the strongest predictors of efficacy, mucosal healing and dose escalation during the first year of adalimumab therapy in Crohn’s disease. Aliment Pharmacol Ther. 2011;34(8):911–22.
Kiss LS, et al. Predictors of efficacy, mucosal healing and need for dose intensification at 12-months of adalimumab therapy in patients with Crohn's disease. National data from Hungary. [abstract]. J Crohn's Colitis. 2011;5: S73
Juillerat P, et al. Characteristics of patients with durable use of infliximab for 5 years. Eur J Med Sci. 2012;142:11S.
Juillerat P, et al. Factors associated with durable response to infliximab in Crohn's disease 5 years and beyond: a multicenter international cohort [abstract]. In: 21st United European Gastroenterology (UEG) Week. Berlin, Germany. p. A33. 2013
Rubin D, et al. Assessment of therapy changes and drug discontinuation among Crohn’s disease patients using health claims data. Am J Gastroenterol. 2012: S681.
Gomes CF, et al. Use of bowel ultrasound to assess disease activity in Crohn’s disease patients after induction therapy with infliximab [abstract]. In: 15th Congress of ECCO - European Crohn's and Colitis Organisation, Vienna, Austria. p. S431. 2020
Osamura A, Suzuki Y. Fourteen-year anti-TNF therapy in Crohn’s disease patients: clinical characteristics and predictive factors. Dig Dis Sci. 2018;63(1):204–8.
Hanzel J, D’Haens GR. Anti-interleukin-23 agents for the treatment of ulcerative colitis. Expert Opin Biol Ther. 2020;20(4):399–406.
Taxonera C, et al. Real-life experience with golimumab in ulcerative colitis patients according to prior anti-TNF use. Gastroenterology. 2016;150:S979.
Taxonera C, et al. Adalimumab dose escalation is effective for managing loss of response in ulcerative colitis [abstract]. In: 2015 Digestive Disease Week (DDW). 2015:S-789.
Pugliese D, et al. Results of interim analysis of a retrospective igibd study on adalimumab use in real practice in italy: the real-life clinical effectiveness of adalimumab in ulcerative colitis (REALADA-UC) Study [abstract],.In: 2019 Digestive Disease Week® (DDW) 2019: San Diego, California. p. S-887.
Acknowledgements
Funding
This review, medical writing support, and journal fees were funded by AbbVie.
Medical Writing and/or Editorial Assistance
Writing and editing support for the manuscript was provided by Michon Jackson, PhD, and Elizabeth Hubscher, PhD, of Cytel, Inc. Sharada Harricharan provided support as the third reviewer to resolve any disagreements and/or make final decisions on data extraction. This support was funded by AbbVie.
Author Contributions
Wan-Ju Lee and Ryan Clark were involved in the planning/conduct of study, Rhiannon I. Campden and Stacy Grieve were involved in collecting data, all authors were involved in interpreting data, Remo Panaccione, Wan-Ju Lee, Ryan Clark, and Kristina Kligys were involved in drafting and/or critically revising the manuscript, and all authors provided approval to submit this work for publication.
Disclosures
Wan-Ju Lee, Ryan Clark and Kristina Kligys are employees of AbbVie and may own AbbVie stock and/or options. Rhiannon Campden and Stacy Grieve are employees of Cytel, Inc, which was a paid consultant on the project. Tim Raine has received research/educational grants and/or speaker/consultation fees from AbbVie, Arena, Aslan, AstraZeneca, Boehringer-Ingelheim, BMS, Celgene, Ferring, Galapagos, Gilead, GSK, Heptares, LabGenius, Janssen, Mylan, MSD, Novartis, Pfizer, Roche, Sandoz, Takeda, and UCB. Remo Panaccione serves on advisory boards for and has received research/educational support and/or speaker/consultation fees from AbbVie, Abbott, Alimentiv (formerly Robarts), Amgen, Arena Pharmaceuticals, AstraZeneca, Biogen, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Eli Lilly, Ferring, Galapagos, Genentech, Gilead Sciences, Glaxo-Smith Kline, Janssen, Merck, Mylan, Oppilan, Pandion Pharma, Pfizer, Progenity, Protagonist Therapeutics, Roche, Satisfai Health, Sandoz, Shire, Sublimity Therapeutics, Theravance Biopharma, UCB, Takeda Pharmaceuticals.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Panaccione, R., Lee, WJ., Clark, R. et al. Dose Escalation Patterns of Advanced Therapies in Crohn’s Disease and Ulcerative Colitis: A Systematic Literature Review. Adv Ther 40, 2051–2081 (2023). https://doi.org/10.1007/s12325-023-02457-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02457-6




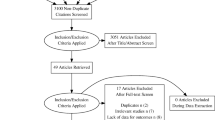


 Studies that did not record the type of dose escalation were excluded from the analysis
Studies that did not record the type of dose escalation were excluded from the analysis




