Abstract
Introduction
This study evaluated the efficacy and safety of transarterial chemoembolization (TACE) combined with lenvatinib plus programmed death (PD)-1 inhibitor (TACE-L-P) versus TACE combined with sorafenib plus PD-1 inhibitor (TACE-S-P) in the treatment of hepatocellular carcinoma (HCC) with portal vein tumor thrombus (PVTT).
Methods
The clinical data of patients with HCC and PVTT treated with TACE-L-P or TACE-S-P from January 2018 to March 2022 were collected. The Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and modified RECIST (mRECIST) standard were used to evaluate the therapeutic effect. The progression-free survival (PFS) and overall survival (OS) of the two groups were compared. Blood samples were collected before and after treatment to detect the changes of biochemical indicators, and the adverse events (AEs) related to treatment were recorded.
Results
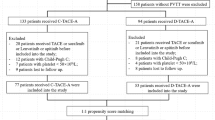
A total of 165 patients were included in the study, including 80 patients receiving TACE-L-P treatment and 85 patients receiving TACE-S-P. Patients in the TACE-L-P group had longer median OS (21.7 months vs. 15.6 months, P = 0.0027), longer median PFS (6.3 months vs. 3.2 months, P < 0.0001), higher objective response rate (41.25% vs. 30.59%, P = 0.008), and higher disease control rate (86.25% vs. 62.35%, P = 0.008) than those in the TACE-S-P group. Multivariate analysis of the TACE-L-P group showed that VP classification of PVTT, Child–Pugh grade, interleukin-17 (IL-17), vascular endothelial growth factor (VEGF), procalcitonin (PCT), and C-reactive protein (CRP) were independent factors significantly affecting patients’ OS (P < 0.05). There was no significant difference in the incidence and severity of AEs between the two groups.
Conclusion
TACE-L-P treatment can improve the survival of patients with HCC and PVTT with an acceptable safety, but higher inflammatory indicators will affect the therapeutic effect.






Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660.
Khan AR, Wei X, Xu X. Portal vein tumor thrombosis and hepatocellular carcinoma—the changing tides. J Hepatocell Carcinoma. 2021;8:1089–115. https://doi.org/10.2147/JHC.S318070.
European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. https://doi.org/10.1016/j.jhep.2018.03.019.
Liu K, Min XL, Peng J, Yang K, Yang L, Zhang XM. The changes of HIF-1α and VEGF expression after TACE in patients with hepatocellular carcinoma. J Clin Med Res. 2016;8(4):297–302. https://doi.org/10.14740/jocmr2496w.
Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–50. https://doi.org/10.1002/hep.29913.
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. https://doi.org/10.1056/NEJMoa0708857.
Kudo M. Scientific rationale for combined immunotherapy with PD-1/PD-L1 antibodies and VEGF inhibitors in advanced hepatocellular carcinoma. Cancers (Basel). 2020;12(5):1089. https://doi.org/10.3390/cancers12051089.
Cai M, Huang W, Huang J, et al. Transarterial chemoembolization combined with lenvatinib plus PD-1 inhibitor for advanced hepatocellular carcinoma: a retrospective cohort study. Front Immunol. 2022;13: 848387. https://doi.org/10.3389/fimmu.2022.848387.
Zhou J, Sun H, Wang Z, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer. 2020;9(6):682–720. https://doi.org/10.1159/000509424.
Renzulli M, Peta G, Vasuri F, et al. Standardization of conventional chemoembolization for hepatocellular carcinoma. Ann Hepatol. 2021;22: 100278. https://doi.org/10.1016/j.aohep.2020.10.006.
Prajapati HJ, Xing M, Spivey JR, et al. Survival, efficacy, and safety of small versus large doxorubicin drug-eluting beads TACE chemoembolization in patients with unresectable HCC. AJR Am J Roentgenol. 2014;203(6):W706–14. https://doi.org/10.2214/AJR.13.12308.
Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst. 2014;106(9):dju244. https://doi.org/10.1093/jnci/dju244.
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. https://doi.org/10.1055/s-0030-1247132.
Sato Y, Watanabe H, Sone M, et al. Tumor response evaluation criteria for HCC (hepatocellular carcinoma) treated using TACE (transcatheter arterial chemoembolization): RECIST (response evaluation criteria in solid tumors) version 1.1 and mRECIST (modified RECIST): JIVROSG-0602. Ups J Med Sci. 2013;118(1):16–22. https://doi.org/10.3109/03009734.2012.729104.
Yu H, Bai Y, Xie X, Feng Y, Yang Y, Zhu Q. RECIST 1.1 versus mRECIST for assessment of tumour response to molecular targeted therapies and disease outcomes in patients with hepatocellular carcinoma: a systematic review and meta-analysis. BMJ Open. 2022;12(6): e052294. https://doi.org/10.1136/bmjopen-2021-052294.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. https://doi.org/10.1016/j.ejca.2008.10.026.
Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. https://doi.org/10.1016/S0140-6736(18)30207-1.
Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62(5):1187–95. https://doi.org/10.1016/j.jhep.2015.02.010.
Yang B, Jie L, Yang T, et al. TACE plus lenvatinib versus TACE plus sorafenib for unresectable hepatocellular carcinoma with portal vein tumor thrombus: a prospective cohort study. Front Oncol. 2021;11: 821599. https://doi.org/10.3389/fonc.2021.821599.
Ding X, Sun W, Li W, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a prospective randomized study. Cancer. 2021;127(20):3782–93. https://doi.org/10.1002/cncr.33677.
Doycheva I, Thuluvath PJ. Systemic therapy for advanced hepatocellular carcinoma: an update of a rapidly evolving field. J Clin Exp Hepatol. 2019;9(5):588–96. https://doi.org/10.1016/j.jceh.2019.07.012.
Yamashita T, Kudo M, Ikeda K, et al. REFLECT-a phase 3 trial comparing efficacy and safety of lenvatinib to sorafenib for the treatment of unresectable hepatocellular carcinoma: an analysis of Japanese subset. J Gastroenterol. 2020;55(1):113–22. https://doi.org/10.1007/s00535-019-01642-1.
Zhang X, Wang K, Wang M, et al. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE for hepatocellular carcinoma with portal vein tumor thrombus: a systematic review and meta-analysis. Oncotarget. 2017;8(17):29416–27. https://doi.org/10.18632/oncotarget.15075.
Kuzuya T, Ishigami M, Ito T, et al. Sorafenib vs. lenvatinib as first-line therapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. Anticancer Res. 2020;40(4):2283–90. https://doi.org/10.21873/anticanres.14193.
Mahipal A, Tella SH, Kommalapati A, Lim A, Kim R. Immunotherapy in hepatocellular carcinoma: is there a light at the end of the tunnel? Cancers (Basel). 2019;11(8):1078. https://doi.org/10.3390/cancers11081078.
Zhang L, Mai W, Jiang W, Geng Q. Sintilimab: a promising anti-tumor PD-1 antibody. Front Oncol. 2020;10: 594558. https://doi.org/10.3389/fonc.2020.594558.
Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–52. https://doi.org/10.1016/S1470-2045(18)30351-6.
Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. https://doi.org/10.1200/JCO.19.01307.
Deng H, Kan A, Lyu N, et al. Dual vascular endothelial growth factor receptor and fibroblast growth factor receptor inhibition elicits antitumor immunity and enhances programmed cell death-1 checkpoint blockade in hepatocellular carcinoma. Liver Cancer. 2020;9(3):338–57. https://doi.org/10.1159/000505695.
Zhu J, Fang P, Wang C, et al. The immunomodulatory activity of lenvatinib prompts the survival of patients with advanced hepatocellular carcinoma. Cancer Med. 2021;10(22):7977–87. https://doi.org/10.1002/cam4.4312.
Torrens L, Montironi C, Puigvehí M, et al. Immunomodulatory effects of lenvatinib plus anti-programmed cell death protein 1 in mice and rationale for patient enrichment in hepatocellular carcinoma. Hepatology. 2021;74(5):2652–69. https://doi.org/10.1002/hep.32023.
Finn RS, Ikeda M, Zhu AX, et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–70. https://doi.org/10.1200/JCO.20.00808.
Chen SC, Huang YH, Chen MH, et al. Anti-PD-1 combined sorafenib versus anti-PD-1 alone in the treatment of advanced hepatocellular cell carcinoma: a propensity score-matching study. BMC Cancer. 2022;22(1):55. https://doi.org/10.1186/s12885-022-09173-4.
Llovet JM, Vogel A, Madoff DC, et al. Randomized phase 3 LEAP-012 study: transarterial chemoembolization with or without lenvatinib plus pembrolizumab for intermediate-stage hepatocellular carcinoma not amenable to curative treatment. Cardiovasc Interv Radiol. 2022;45(4):405–12. https://doi.org/10.1007/s00270-021-03031-9.
Kumada T, Toyoda H, Tada T, Yasuda S, Tanaka J. Changes in background liver function in patients with hepatocellular carcinoma over 30 years: comparison of Child–Pugh classification and albumin bilirubin grade. Liver Cancer. 2020;9(5):518–28. https://doi.org/10.1159/000507933.
Khabbaz RC, Lokken RP, Chen YF, et al. Albumin–bilirubin and platelet–albumin–bilirubin grades do not predict survival after transjugular intrahepatic portosystemic shunt creation. Cardiovasc Interv Radiol. 2018;41(7):1029–34. https://doi.org/10.1007/s00270-018-1923-2.
Minagawa M, Makuuchi M, Takayama T, Ohtomo K. Selection criteria for hepatectomy in patients with hepatocellular carcinoma and portal vein tumor thrombus. Ann Surg. 2001;233(3):379–84. https://doi.org/10.1097/00000658-200103000-00012.
Hiraoka A, Kumada T, Atsukawa M, et al. Important clinical factors in sequential therapy including lenvatinib against unresectable hepatocellular carcinoma. Oncology. 2019;97(5):277–85. https://doi.org/10.1159/000501281.
Stroescu C, Dragnea A, Ivanov B, et al. Expression of p53, Bcl-2, VEGF, Ki67 and PCNA and prognostic significance in hepatocellular carcinoma. J Gastrointest Liver Dis. 2008;17(4):411–7.
Gao Y, Wang PX, Cheng JW, et al. Chemotherapeutic perfusion of portal vein after tumor thrombectomy and hepatectomy benefits patients with advanced hepatocellular carcinoma: a propensity score-matched survival analysis. Cancer Med. 2019;8(16):6933–44. https://doi.org/10.1002/cam4.2556.
Jun J, Lee SR, Lee JY, et al. Pneumonitis and concomitant bacterial pneumonia in patients receiving pembrolizumab treatment: three case reports and literature review. Medicine (Baltimore). 2019;98(25): e16158. https://doi.org/10.1097/MD.0000000000016158.
Liu G, Sun J, Yang ZF, et al. Cancer-associated fibroblast-derived CXCL11 modulates hepatocellular carcinoma cell migration and tumor metastasis through the circUBAP2/miR-4756/IFIT1/3 axis. Cell Death Dis. 2021;12(3):260. https://doi.org/10.1038/s41419-021-03545-7.
Ma HY, Yamamoto G, Xu J, et al. IL-17 signaling in steatotic hepatocytes and macrophages promotes hepatocellular carcinoma in alcohol-related liver disease. J Hepatol. 2020;72(5):946–59. https://doi.org/10.1016/j.jhep.2019.12.016.
Elovic AE, Ohyama H, Sauty A, et al. IL-4-dependent regulation of TGF-alpha and TGF-beta1 expression in human eosinophils. J Immunol. 1998;160(12):6121–7.
Myojin Y, Kodama T, Sakamori R, et al. Interleukin-6 is a circulating prognostic biomarker for hepatocellular carcinoma patients treated with combined immunotherapy. Cancers (Basel). 2022;14(4):883. https://doi.org/10.3390/cancers14040883.
Lazzarotto C, Ronsoni MF, Fayad L, et al. Acute phase proteins for the diagnosis of bacterial infection and prediction of mortality in acute complications of cirrhosis. Ann Hepatol. 2013;12(4):599–607.
Bota DP, Van Nuffelen M, Zakariah AN, Vincent JL. Serum levels of C-reactive protein and procalcitonin in critically ill patients with cirrhosis of the liver. J Lab Clin Med. 2005;146(6):347–51. https://doi.org/10.1016/j.lab.2005.08.005.
Maruna P, Nedelníková K, Gürlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49(Suppl 1):S57–61.
Meisner M. Pathobiochemistry and clinical use of procalcitonin. Clin Chim Acta. 2002;323(1–2):17–29. https://doi.org/10.1016/s0009-8981(02)00101-8.
Connert S, Stremmel W, Elsing C. Procalcitonin is a valid marker of infection in decompensated cirrhosis. Z Gastroenterol. 2003;41(2):165–70. https://doi.org/10.1055/s-2003-37314.
Nardone V, Giannicola R, Bianco G, et al. Inflammatory markers and procalcitonin predict the outcome of metastatic non-small-cell-lung-cancer patients receiving PD-1/PD-L1 immune-checkpoint blockade. Front Oncol. 2021;11: 684110. https://doi.org/10.3389/fonc.2021.684110.
Nardone V, Giannicola R, Giannarelli D, et al. Distinctive role of the systemic inflammatory profile in non-small-cell lung cancer younger and elderly patients treated with a PD-1 immune checkpoint blockade: a real-world retrospective multi-institutional analysis. Life (Basel). 2021;11(11):1235. https://doi.org/10.3390/life11111235.
Kang SJ, Kim UJ, Kim SE, et al. Predictive value of procalcitonin for bacterial infection after transarterial chemoembolization or radiofrequency ablation for hepatocellular carcinoma. Dis Markers. 2018;2018:9120878. https://doi.org/10.1155/2018/9120878.
Han S, Ye Y, Wu J, et al. Procalcitonin levels in post TACE infection. Cancer Manag Res. 2020;12:12197–203. https://doi.org/10.2147/CMAR.S281667.
Scheiner B, Pomej K, Kirstein MM, et al. Prognosis of patients with hepatocellular carcinoma treated with immunotherapy—development and validation of the CRAFITY score. J Hepatol. 2022;76(2):353–63. https://doi.org/10.1016/j.jhep.2021.09.035.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. https://doi.org/10.1038/nature07205.
Acknowledgements
The authors thank all participants for their involvement in the study.
Funding
The interventional radiology research project of Jiangsu Medical Association (210012021081) supported this study and provided funding for the journal’s Rapid Service fee.
Author Contributions
Writing and editing, Xinhua Zou, Qingyu Xu and Guowen Yin; Formal analysis, Xinhua Zou; Conceptualization, Guowen Yin; Methodology, Xinhua Zou and Ran You; Data curation and validation, Qingyu Xu and Guowen Yin. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Disclosures
The authors Xinhua Zou, Qingyu Xu, Ran You and Guowen Yin declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
Compliance with Ethics Guidelines
This study was conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent to participate in the study, and the trial was approved by the ethics committee of Jiangsu Institute of Cancer Research (No. 2022LC0165).
Data Availability
The data sets analyzed during the present study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zou, X., Xu, Q., You, R. et al. Evaluating the Benefits of TACE Combined with Lenvatinib Plus PD-1 Inhibitor for Hepatocellular Carcinoma with Portal Vein Tumor Thrombus. Adv Ther 40, 1686–1704 (2023). https://doi.org/10.1007/s12325-023-02449-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02449-6