Abstract
Introduction
Bardet-Biedl syndrome (BBS) is a rare genetic disease associated with hyperphagia, a pathologic insatiable hunger, due to impaired signaling in the melanocortin-4 receptor (MC4R) pathway. The impact of hyperphagia on the lives of patients with BBS and their families has not been fully characterized.
Methods
Patients with BBS or their caregivers who participated in clinical trials of the MC4R agonist setmelanotide (NCT03013543 and NCT03746522) were included in this qualitative study. Telephone interviews were conducted using a semistructured interview guide to explore patient experience and caregiver observations of hyperphagia before and during setmelanotide treatment.
Results
Nineteen interviews (8 patients, 11 caregivers) were conducted. The term “hunger” (rather than “hyperphagia”) was used in interviews to ensure common terminology. Before setmelanotide treatment, all participants described their (or their child’s) hunger as all-consuming, leading to an obsessive focus on food. Nine participants recalled intense, continuous hunger, and most participants (5 patients, 10 caregivers) reported lack of control with eating. Negative impacts on patients’ lives included difficulties with concentration, emotional and physical manifestations, and impaired relationships. All participants experienced or observed improvements in hunger and health outcomes during treatment, the most meaningful of which included weight loss and decrease in obsessive focus on food and food-seeking behaviors. All participants reported improvements in either physical and/or emotional well-being and being satisfied with setmelanotide.
Conclusions
Hyperphagia and resulting food-seeking behaviors have notable negative impacts on quality of life in patients with BBS and caregivers. Setmelanotide improved hyperphagia, reduced body weight and obsessive focus on food, and facilitated improvements in physical and emotional well-being for both patients and caregivers.
Trial Registration
NCT03013543 and NCT03746522
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Hyperphagia and early-onset obesity are hallmark clinical features of Bardet-Biedl syndrome (BBS) and place tremendous burdens on both patients and caregivers; these burdens are not fully characterized. | |
We conducted in-depth qualitative interviews with a cohort of patients with BBS or caregivers of patients with BBS who received setmelanotide treatment to understand the burden of hyperphagia and obesity in BBS and its modulation by setmelanotide. | |
Patients with BBS and their caregivers reported experiencing or observing notable hunger and associated consequences (e.g., negative emotions, strained familial relationships, impaired concentration) before setmelanotide treatment. | |
Interview participants also reported improvements in hunger during setmelanotide treatment that resulted in meaningful improvements in physical and emotional well-being for both patients and caregivers. |
Introduction
Bardet-Biedl syndrome (BBS) is a rare autosomal recessive disease caused by variants in at least 25 genes that impair primary cilia function, resulting in clinical features including visual impairment; polydactyly; hypogonadism; cognitive impairment; genital abnormalities; early-onset, severe obesity; and pathologic insatiable hunger (hyperphagia) [1,2,3,4,5,6,7]. Obesity is estimated to occur in 72–92% of patients with BBS and is associated with short- and long-term health deficits, social stigma, and psychological impacts, which affect health-related quality of life (HRQOL) [8,9,10,11,12,13,14,15,16,17]. Hyperphagia can also reduce HRQOL and be a source of stress for caregivers and families [18, 19], given that individuals with hyperphagia exhibit excessive hunger and preoccupation with food that may interfere with daily functioning [20, 21].
Despite recognition that obesity and hyperphagia have broad impacts on QOL for patients with BBS and their caregivers, limited direct research in this population exists [19, 22, 23]. Weight management strategies for individuals with BBS have traditionally been similar to those used for general obesity and include lifestyle management, antiobesity medications, and bariatric surgery, none of which specifically target hyperphagia [7, 17, 24].
The hypothalamic melanocortin-4 receptor (MC4R) pathway is a key regulator of hunger, satiety, and energy balance [7, 25]. It is hypothesized that cilia dysfunction in patients with BBS contributes to impaired MC4R pathway signaling, resulting in hyperphagia, decreased energy expenditure, and consequently obesity [25,26,27]. Setmelanotide is an MC4R agonist approved in the USA and European Union for rare MC4R pathway diseases of obesity including pro-opiomelanocortin (POMC) deficiency, leptin receptor (LEPR) deficiency, and BBS [28, 29]. Results from a multicenter randomized placebo-controlled phase 3 clinical trial (NCT03746522) showed that setmelanotide was associated with statistically significant body weight and hunger reductions among 32 patients with BBS [30].
In this study, we characterized the impacts of hyperphagia and obesity on HRQOL and their modulation by setmelanotide in patients with BBS and their caregivers. We conducted in-depth qualitative interviews with patients or caregivers of patients with BBS who previously participated in a phase 2 or phase 3 clinical trial of setmelanotide (NCT03013543 or NCT03746522). The primary objectives were to characterize the nature, severity, and effect of hyperphagia on patient and caregiver HRQOL before and during setmelanotide treatment.
Methods
Participant Recruitment
Interview participants were identified from a pool of patients who completed previous phase 2 and 3 clinical trials (NCT03013543 or NCT03746522) of setmelanotide for the treatment of BBS and were provided information about the interview study by mail or email. Patients and caregivers who expressed interest in participating in the interview study were contacted by RTI Health Solutions (RTI-HS), a healthcare consultancy, and screened for eligibility. In addition to completion of a clinical trial of setmelanotide, patient eligibility criteria included age at least 16 years, genetic confirmation or clinical diagnosis per Beales criteria [31] of BBS, and informed consent (i.e., willingness and ability to participate in a 1-h, audio-recorded telephone interview). Study site staff obtained written informed consent for interview participation and for participant contact information to be provided to RTI-HS for scheduling and conducting interviews. Verbal consent was also obtained prior to recording interviews. To ensure patients had sufficient cognitive function, individuals with BBS were eligible to participate in an interview only if they had self-completed patient-reported hunger items (as opposed to caregivers of patients younger than 12 years of age or those with cognitive impairment completing observer-reported versions of the hunger assessments) during the prior clinical trial. Caregiver eligibility criteria included age at least 18 years, current full-time caregiver status for an individual with genetic confirmation or clinical diagnosis per Beales criteria of BBS aged at least 6 years who had previously participated in a clinical trial of setmelanotide (NCT03013543 or NCT03746522), completion of observer-reported hunger assessments in the clinical trial, and informed consent. Although not required for inclusion, patients and caregivers of patients currently enrolled in the setmelanotide open-label long-term extension study (NCT03651765; prior completion of a phase 2 or 3 trial of setmelanotide required for inclusion) were also eligible for participation.
Compliance with Ethics Guidelines
The method of obtaining and documenting informed consent complied with the International Council on Harmonisation for Good Clinical Practice and all applicable regulatory requirements. Because BBS is a rare disease, qualitative data are reported with limited identifying information to preserve patient confidentiality. This study was conducted in accordance with the Declaration of Helsinki. The institutional review board or independent ethics committee at all trial sites reviewed and approved all appropriate study documentation.
Interview Methods
Eligible participants were contacted by RTI-HS to schedule an interview at a convenient date and time. All interviews were conducted between March and June 2021 in English by members of the RTI-HS project team who have extensive qualitative research experience (CE and LN). During each interview, one researcher led the interview while the other observed and took field notes. Both patient and caregiver interviews followed semistructured interview guides specific to the type of participant; the caregiver guide was modified following the first few interviews to reduce participant burden (Supplementary Material).
The patient interview guide began with open-ended questions designed to elicit perspectives on experiences of hunger and its effect on the lives of patients before starting setmelanotide treatment. To supplement verbal descriptions and facilitate subsequent discussion about changes in hunger following treatment with setmelanotide, patients were also asked to rate their hunger on a numerical rating scale as was done during the clinical trials from which they were recruited. Caregivers were asked to report their observations related to these issues for the same time period. The term “hunger” was primarily used in interviews (rather than “hyperphagia”) to ensure common terminology. Patients and caregivers were then asked to describe changes in hunger (experienced and observed), weight, and functioning after initiating setmelanotide treatment, as well as the perceived meaningfulness of these changes. Finally, all patients and caregivers were asked about their satisfaction with setmelanotide. Interviews were audio recorded, transcribed, and deidentified.
Analysis
A thematic analysis approach was used to code and analyze the qualitative interview data, which were captured in the form of interview transcripts and Microsoft Excel-based field notes. To facilitate this process, a coding framework based on the content of the interview guide was initially developed; new codes were added throughout the analysis process to allow for emerging concepts. Similar to qualitative interviews conducted in other settings, themes or patterns in participant responses were identified by comparing relevant concepts and predominant trends across interviews [32]. All coding and qualitative analyses, including quality checks, were completed by CE and LN.
Results
Participant Characteristics
Between March and June 2021, 19 telephone interviews were conducted with patients (n = 8) and caregivers (n = 11). All patients except one were enrolled in the long-term extension study of setmelanotide at the time of their interview. Baseline weight and hunger were previously reported for all patients included in the phase 2 and 3 trial primary analyses [30, 33]. The average age of patient participants was 36 years, and the average age of patients whose caregivers were interviewed was 16 years. Most patient participants were female (n = 6; 75.0%) and all caregiver participants were female parents (i.e., the mother). The mean time since the first approximately 1-year clinical trial of setmelanotide was 29 (range 12–48) months for patient participants and 24 (range 8–40) months for patients whose caregivers were interviewed (Table 1). Exemplar quotes are from individual patients with BBS and caregivers of patients with BBS.
Symptoms and Behaviors Associated with Hyperphagia
Feelings of Hunger and Pursuit/Consumption of Food
While all 19 participants described experiences of uncontrollable hunger that were consistent with hyperphagia, none spontaneously used the term “hyperphagia” (Table 2). All participants characterized hyperphagia as all-consuming and extreme, which caused an obsessive focus on and a relentless pursuit and consumption of food. Hyperphagia was continuous, with nearly half of participants (n = 9) reporting hunger that was intense throughout the day; the remaining participants reported hunger that varied in relation to the time of day. Caregivers indicated that hyperphagia prompted incessant food-seeking behaviors from patients including queries about the next meal or constantly wanting more food.
“I was always hungry. And if I didn’t eat when I needed to, I would be very unhappy. Couldn’t wait to eat.” —Patient
“She was hungry all the time, and it was a relentless hunger. She would eat everything on her plate and everything on my plate, and anything she could find.” —Caregiver
Patients were also asked to recall the highest level of hunger they experienced before starting the clinical trial using a numerical rating scale where 0 = “not hungry at all” and 10 = “the hungriest possible.” Pretreatment peak hunger ratings ranged from 8 to 10, and peak hunger was associated with physical and emotional manifestations including fatigue, pain, frustration, irritability, and a hyperfocus on obtaining food. When caregivers were asked what behaviors signified their child was experiencing the most intense level of hunger, they reported severe tantrums, irritability, and hyperfocus on obtaining food.
“If she didn’t get food, she was just a terror…. She’d have a fit, everything except for self-harming. That’s the only thing she didn’t do.” —Caregiver
While patients consistently reported some level of satisfaction after eating, many noted the sensation was short-lived, only achieved with a large meal, and resembled fullness or satiation rather than a feeling of positive emotional valence. Conversely, most caregivers (n = 9; 81.8%) reported that their child never seemed to be full or satisfied after eating.
Eating Habits and Engagement in Food-Seeking Behaviors
All participants reported that hyperphagia influenced what and/or how much was consumed (Table 2), with carbohydrates and/or sugary foods being the most sought during periods of extreme hunger. Further, most participants (15 of 19 overall [78.9%]; 5 of 8 patients [62.5%] and 10 of 11 caregivers [90.9%]) described a lack of control with eating, and caregivers frequently remarked that their child would not stop eating without outside influence (Table 2). Food-seeking behaviors were reported by most caregivers and included begging, bargaining, and/or attempting to bribe others for food. Although hiding and stealing food is considered a hyperphagic behavior [6, 21], only 25.0% of patients reported doing this, but more than half of caregivers (n = 6; 54.5%) reported observing these behaviors.
“I was eating pretty much whatever, whenever and wasn’t able to stop myself from eating or sneaking food in the middle of the night.” —Patient
“He would eat until he was physically hurting. And then in the same breath, tell us that he was hurting and asked for a snack or something to eat. He’d sneak food…. It seemed like he was just hungry all the time.” —Caregiver
Multifactorial Consequences of Hyperphagia on Patients and Caregivers
Emotional Consequences of Hyperphagia
Participants were asked about the effects of hyperphagia experienced before beginning setmelanotide treatment. Overall, most patients (n = 7; 87.5%) and caregivers (n = 10; 90.9%) experienced negative effects directly related to hyperphagia; one patient and one caregiver attributed negative effects to increased weight instead. The most commonly reported effect pertained to patients’ emotional states (Table 2). Both patients and caregivers reported emotional effects of hyperphagia including sadness, frustration, irritability, anxiety, and guilt. These feelings often centered around food and were related to either the desire to eat (e.g., irritability, anxiety) or the patients’ inability to control their hunger or eating habits (e.g., frustration, guilt).
“I felt very agitated and very sad a lot of the time…. I just really didn’t have many friends, really even many friends to hang out with and…I don’t know. I just kind of felt alone in a sense.” —Patient
“I’d feel crappy and crabby because maybe I ate sweets and stuff I really shouldn’t have had but I wanted…. It made me mad after I ate it because I know I did want it, but then I thought, ‘Should I have really eaten that even though I really wanted it?’” —Patient
Impacts of Hyperphagia on Family Dynamics
Hyperphagia had broad negative impacts on family dynamics including impaired relationships with siblings and tensions between caregivers and patients (Table 2). For example, one caregiver described decreased interactions between siblings because the siblings “didn’t know how to handle” the consistent emotional distress of the patient. Caregivers also experienced strains on spousal and familial relationships due to regulation of food intake.
“It affected my relationship with my husband…he is a lot better at saying no than I am. And sometimes it would be so hard for me because he was just so black-and-white about it and would just say, ‘No, get out of the kitchen,’ and then I would feel bad and cry and be mad at him. I would think he was being mean to her when really, he was doing what was best for her.” —Caregiver
Caregivers experienced diminished emotional and psychological well-being due to their child’s hyperphagia, reporting feeling burdened by the need to constantly monitor eating behaviors, guilt related to their child’s health, and fear of judgment from others. For more than half of caregivers (n = 6; 54.5%), hyperphagia negatively impacted social participation because of required hypervigilance in situations involving food and reluctance to leave their child in the care of another individual.
“Yeah, we didn’t like to go places. And if you go to somebody’s house, trying to keep them away from the chips and dip is so hard, it’s easier to just not go.” —Caregiver
Productivity at Work and School
Participants reported impaired concentration and focus as a consequence of hyperphagia (2 of 8 patients [25.0%]; 8 of 11 caregivers [72.7%]). For children, this translated into challenges at school because of an obsessive focus on food.
“Looking back at it now, I think it affected his concentration a lot, especially at school. Before the trial, at school, he’d spend a lot of time out of the classroom. He’d be crying, he’d be upset about something. Most times, he wouldn’t be able to tell them why he was upset. And I just always think that it was because he was hungry. But of course, we didn’t know that at the time.” —Caregiver
A subset of caregivers (n = 2; 18.2%) also experienced reduced productivity and ability to complete tasks as a result of their child’s hyperphagic behaviors and the attendant need to plan and prepare meals.
Changes with Setmelanotide Treatment
Improvements in Hyperphagia, Food-Seeking Behaviors, and Weight with Setmelanotide Treatment
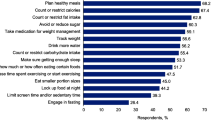
Table 3 shows the proportion of participants who reported improvements in hyperphagia and associated outcomes with setmelanotide treatment. All participants (N = 19) reported substantial improvements in hyperphagia and satiety within 2 months of initiating setmelanotide treatment. Decreases in peak hunger scores ranged from 2 to 6 points. Notably, a previous psychometric evaluation estimated that within-patient decreases in hunger score of 1–2 points are sufficient to yield improvements in clinical status and perceived meaningfulness of hunger reduction [30]. Similarly, caregivers consistently observed changes in food-seeking and eating behaviors.
“I feel great. I feel satisfied. I feel full when I’m done eating. Sometimes, I won’t eat everything that’s on my plate.” —Patient
“After we started this setmelanotide trial, she told me for the first time that she had no idea what it was not to be hungry. So that was really sad. So before the trial, I guess, according to her, she had never experienced not being hungry before….” —Caregiver
Consistent with improved hunger, all participants reported a large reduction in food consumption, greater control over how much food was consumed, and consumption of or requests for healthier foods (Table 4).
“I feel like I’ve had a lot more willpower since I’ve been on the medication, and I feel like I haven’t really been tempted to grab really anything during meals, which has felt good.” —Patient
Patients and caregivers also reported fewer hyperphagic behaviors (e.g., eating in secrecy, eating foods that others would find undesirable) after treatment initiation. None of the participants who reported these behaviors before setmelanotide (2 of 8 patients [25.0%] and 6 of 11 caregivers [54.5%]) experienced or observed them after initiating setmelanotide treatment. Additionally, all 11 caregivers noted the ability to give their child greater autonomy around food choices and consumption and a reduced need to monitor their child’s food intake.
“I’ll let her eat what she wants. I guess that’s the biggest deal is that. Do you want to eat more brussels sprouts? Eat more brussels sprouts because I know you’re not going to eat 10 servings of macaroni and cheese or a whole jar of peanut butter today.” —Caregiver
In addition to improvements in hyperphagia and related behaviors, all participants reported weight loss following setmelanotide treatment initiation. While patients self-reported an average weight loss of 52 pounds (range 25–130 pounds; n = 8), three reported regaining some weight, which they attributed to the coronavirus disease 2019 (COVID-19) pandemic (e.g., constant access to food, lack of physical activity). Caregivers reported that patients lost an average of 36 pounds (range 5–100 pounds; n = 11), which underestimates reductions in body mass index, because many noted that their child’s height had also increased (range 2–12 inches).
Emotional and Physical Impacts of Improved Hyperphagia in Patients and Caregivers
Most patients (n = 7; 87.5%) reported improvements in how they felt physically while receiving setmelanotide (e.g., had more energy, could be more active). Caregivers also observed physical improvements, frequently noting that their child was more readily able to participate in recreational activities and sports. Participants consistently reported significant improvements in patients’ moods or emotions with treatment; improvements in hunger led to decreased anxiety, stress, and irritability, as well as increased happiness, self-esteem, and confidence (Table 4). Caregivers also reported reduced stress, worry, and/or anxiety stemming from the need to monitor and control their child’s food intake after their child initiated setmelanotide treatment.
“It made me more positive and more peppy, my voice and thinking and wanting to be out more with people.” —Patient
Improvements in Family and Social Dynamics with Setmelanotide Treatment
Both patients (n = 4; 50.0%) and caregivers (n = 6; 54.5%) noted that improvements in hunger had benefited family dynamics including better sibling interactions, and some participants (2 of 8 patients [25.0%]; 3 of 11 caregivers [27.3%]) noted their (or their child’s) social life and friendships improved because of improved hunger (Table 3). Further, for some caregivers, reduced stress and worry led to improved family relationships, better sleep, and a more positive outlook.
“And he has friends this year. He’s never really had friends, probably because he was always crying…. So, yeah, he’s got some buddies this year.” —Caregiver
“Well, it’s just a lot easier because I don’t have to be the food police anymore…. It’s like everything in the house is more laid back instead of being on edge about food.” —Caregiver
Improvements in Concentration and Productivity at School and Home with Setmelanotide Treatment
All participants reported improved focus and concentration related to reductions in obsessive behaviors associated with food. A subset of participants (2 of 8 patients [25.0%]; 7 of 11 caregivers [63.6%]) noted that this increased ability to focus resulted in improved task performance at school or home (Table 3).
Perceived Meaningfulness and Overall Satisfaction with Treatment
Participants were asked to identify the most important changes attributable to improvements in their (or their child’s) hunger (open-ended question; Table 5). The most frequently reported changes (5 of 8 patients [62.5%]; 6 of 11 caregivers [54.5%]) were weight loss and associated health benefits (e.g., reduced blood pressure) and reduced obsessive focus on food (3 of 8 patients [37.5%]; 5 of 11 caregivers [45.5%]) and associated life impact. Patients and caregivers commonly described the improvements in hyperphagia as “very meaningful” and “life changing” (Table 6).
“Oh, totally meaningful. This is who he gets to be for the rest of his life. Somebody who’s conscious of what food does to your body and be healthy and not die young. This is everything.” —Caregiver
All but one participant reported that they were “very satisfied” with setmelanotide (Table 6). Similar to perceived meaningfulness, participant satisfaction was attributed to improvements in hyperphagia and associated behavioral changes, as well as weight loss and associated health benefits. Participants also commonly reported feeling less worry about their (or their child’s) health in the future as a result of treatment. The patient who indicated being less than very satisfied with setmelanotide noted being satisfied with the improvement in hunger, but was disappointed with weight regain during the COVID-19 pandemic.
At the conclusion of each interview, participants were asked to describe how they would feel if patients could no longer take setmelanotide (Table 6). Patients commonly used the terms “very upset,” “disappointed,” and “distraught.” Caregivers used more dramatic language such as “terrifying,” “devastating,” and “a death sentence.”
Discussion
Hyperphagia and obesity are salient clinical features associated with BBS [1, 6]. The present study adds to existing evidence and highlights the considerable burden that hyperphagia and obesity impose on patients with BBS and their families. Further, these interviews exemplify the usefulness of “patient experience data,” as recommended by the US Food and Drug Administration for patient-centered drug development, given that such data elucidate how setmelanotide treatment is important to patients with BBS and could potentially aid in the development of robust HRQOL measures in this patient population [34]. Currently available patient-reported outcome measures used to assess hyperphagia focus on the severity of hyperphagia and associated behaviors but do not address their impacts on the lives of patients and caregivers [20, 21]. Our results suggest that improving hyperphagia through pharmacologic intervention can minimize its negative impact on productivity, family dynamics, overall patient health, and emotional well-being.
The results across the interviews were highly consistent, with all participants describing very similar experiences of hyperphagia. To our knowledge, these are the first detailed descriptions of hyperphagia and related effects provided by both patients with BBS and their caregivers. These results complement findings from the phase 3 trial of setmelanotide in BBS, where patients experienced improved hunger, reduced weight, and improvements in HRQOL following 1 year of setmelanotide treatment [35, 36]. Specifically, 85% of patients reported clinically meaningful improvements (i.e., change in Impact of Weight on Quality of Life Questionnaire-Lite (IWQOL-Lite) or Pediatric Quality of Life Inventory (PedsQL) scores of 7.7–12 or more than 4.4 points, respectively) in HRQOL or preserving their nonimpaired HRQOL status after 1 year of setmelanotide treatment [36].
No new themes or concepts important to participants (i.e., regarding symptoms and impacts associated with hyperphagia) were reported while the interviews progressed, supporting concept saturation [37]. In addition, the overall sample size herein is supported by previously conducted research [38]. Nonetheless, this study has several limitations. Participants were patients and mothers of patients who chose to continue treatment, which may reflect bias toward setmelanotide. Further, no fathers or other caregivers were included in the sample. No sex- or gender-specific analyses for patients or caregivers were performed because of these limitations. Another limitation is the variable duration of time that patients were treated with setmelanotide and length of time since the first clinical trial, which may have contributed to baseline recall bias and potentially accounted for some differences between patient and caregiver reports. This point is highlighted by varying perceptions of hyperphagia severity and associated behaviors between patients and caregivers. Patients may not have realized the extent of their condition because of the time elapsed since and/or their age before they initiated treatment, whereas these factors may not have impacted caregiver perceptions. Further, it is possible that patients had never conceptualized what life without hyperphagia was like before participating in the clinical trial and may have not believed it was abnormal or particularly uncomfortable because this was their personal usual state. One additional consideration when interpreting the results is that patients were interviewed during the COVID-19 pandemic, during which three participants reported patient weight gain.
Conclusions
This study confirms that hyperphagia imposes a substantial burden on patients with BBS and their families. Additionally, this study provides insights into less obvious consequences of living with insatiable hunger such as the inability to focus on tasks or build relationships. Treatment with setmelanotide facilitated improvements in hunger and promoted beneficial changes in patient and caregiver experiences. Thus, the impact of setmelanotide treatment in patients with BBS extends beyond clinically beneficial outcomes (e.g., weight reduction) and includes HRQOL improvements that are deeply meaningful to patients and their families. These findings are consistent with previous results from similar interviews of patients with POMC or LEPR deficiency who showed substantial QOL improvements following setmelanotide treatment [39]. Our results support further research on the long-term consequences of hyperphagia and obesity and treatment interventions that can alleviate their impact on HRQOL in patients with BBS.
References
Forsythe E, Beales PL. Bardet-Biedl syndrome. Eur J Hum Genet. 2013;21(1):8–13.
Geets E, Meuwissen MEC, Van Hul W. Clinical, molecular genetics and therapeutic aspects of syndromic obesity. Clin Genet. 2019;95(1):23–40.
Lindstrand A, Frangakis S, Carvalho CM, et al. Copy-number variation contributes to the mutational load of Bardet-Biedl syndrome. Am J Hum Genet. 2016;99(2):318–36.
Niederlova V, Modrak M, Tsyklauri O, Huranova M, Stepanek O. Meta-analysis of genotype-phenotype associations in Bardet-Biedl syndrome uncovers differences among causative genes. Hum Mutat. 2019;40(11):2068–87.
Shamseldin HE, Shaheen R, Ewida N, et al. The morbid genome of ciliopathies: an update. Genet Med. 2020;22(6):1051–60.
Sherafat-Kazemzadeh R, Ivey L, Kahn SR, et al. Hyperphagia among patients with Bardet-Biedl syndrome. Pediatr Obes. 2013;8(5):e64–7.
Huvenne H, Dubern B, Clément K, Poitou C. Rare genetic forms of obesity: clinical approach and current treatments in 2016. Obes Facts. 2016;9(3):158–73.
Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16(1):36–46.
Ford ES, Moriarty DG, Zack MM, Mokdad AH, Chapman DP. Self-reported body mass index and health-related quality of life: findings from the Behavioral Risk Factor Surveillance System. Obes Res. 2001;9(1):21–31.
Jia H, Lubetkin EI. The impact of obesity on health-related quality-of-life in the general adult US population. J Public Health (Oxf). 2005;27(2):156–64.
Ramos Salas X, Forhan M, Caulfield T, Sharma AM, Raine KD. Addressing internalized weight bias and changing damaged social identities for people living with obesity. Front Psychol. 2019;10:1409.
Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. 2003;289(14):1813–9.
Centers for Disease Control and Prevention. Childhood obesity causes & consequences. https://www.cdc.gov/obesity/basics/causes.html. Accessed 21 Dec 2022.
Katz DA, McHorney CA, Atkinson RL. Impact of obesity on health-related quality of life in patients with chronic illness. J Gen Intern Med. 2000;15(11):789–96.
Nigatu YT, Bültmann U, Reijneveld SA. The prospective association between obesity and major depression in the general population: does single or recurrent episode matter? BMC Public Health. 2015;15:350.
Nigatu YT, Reijneveld SA, de Jonge P, van Rossum E, Bültmann U. The combined effects of obesity, abdominal obesity and major depression/anxiety on health-related quality of life: the LifeLines Cohort Study. PLoS ONE. 2016;11(2): e0148871.
Forsythe E, Kenny J, Bacchelli C, Beales PL. Managing Bardet-Biedl syndrome-now and in the future. Front Pediatr. 2018;6:23.
Butler MG, Manzardo AM, Heinemann J, Loker C, Loker J. Causes of death in Prader-Willi syndrome: Prader-Willi Syndrome Association (USA) 40-year mortality survey. Genet Med. 2017;19(6):635–42.
Pomeroy J, Krentz AD, Richardson JG, Berg RL, VanWormer JJ, Haws RM. Bardet-Biedl syndrome: weight patterns and genetics in a rare obesity syndrome. Pediatr Obes. 2021;16(2): e12703.
Heymsfield SB, Avena NM, Baier L, et al. Hyperphagia: current concepts and future directions proceedings of the 2nd international conference on hyperphagia. Obesity (Silver Spring). 2014;22(suppl 1):S1-17.
Dykens EM, Maxwell MA, Pantino E, Kossler R, Roof E. Assessment of hyperphagia in Prader-Willi syndrome. Obesity (Silver Spring). 2007;15(7):1816–26.
Hamlington B, Ivey LE, Brenna E, Biesecker LG, Biesecker BB, Sapp JC. Characterization of courtesy stigma perceived by parents of overweight children with Bardet-Biedl syndrome. PLoS ONE. 2015;10(10):e0140705.
Haws RM, Gordon G, Han JC, Yanovski JA, Yuan G, Stewart MW. The efficacy and safety of setmelanotide in individuals with Bardet-Biedl syndrome or Alström syndrome: phase 3 trial design. Contemp Clin Trials Commun. 2021;22:100780.
Kenny J, Forsythe E, Beales P, Bacchelli C. Toward personalized medicine in Bardet-Biedl syndrome. Per Med. 2017;14(5):447–56.
Yazdi FT, Clee SM, Meyre D. Obesity genetics in mouse and human: back and forth, and back again. PeerJ. 2015;3:e856.
Davenport JR, Watts AJ, Roper VC, et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17(18):1586–94.
Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18(7):1323–31.
IMCIVREE™ [package insert]. Boston, MA: Rhythm Pharmaceuticals, Inc.; 2022.
IMCIVREE™ [summary of product characteristics]. Amsterdam, Netherlands: Rhythm Pharmaceuticals, Inc.; 2022.
Haqq AM, Chung WK, Dollfus H, et al. Efficacy and safety of setmelanotide, a melanocortin-4 receptor agonist, in patients with Bardet-Biedl syndrome and Alström syndrome: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial with an open-label period. Lancet Diabetes Endocrinol. 2022;10:859–68.
Beales PL, Elcioglu N, Woolf AS, et al. New criteria for improved diagnosis of Bardet-Biedl syndrome: results of a population survey. J Med Genet. 1999;36(6):437–46.
Ervin C, Joish VN, Evans E, et al. Insights into patients’ experience with type 1 diabetes: exit interviews from phase III studies of sotagliflozin. Clin Ther. 2019;41(11):2219–30.e6.
Haws R, Brady S, Davis E, et al. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet-Biedl syndrome. Diabetes Obes Metab. 2020;22:2133–40.
U.S. Department of Health and Human Services Food and Drug Administration. Patient-Focused Drug Development: Collecting Comprehensive and Representative Input Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/patient-focused-drug-development-collecting-comprehensive-and-representative-input. Accessed 20 Dec 2022.
Haws R, Clément K, Dollfus H, et al. Efficacy and safety of open-label setmelanotide in Bardet-Biedl syndrome: a phase 3 trial. Presented at ObesityWeek 2021; November 1–5, 2021; Virtual.
Forsythe E, Haws R, Argente J, et al. Quality of life in patients with Bardet-Biedl syndrome in a setmelanotide phase 3 trial. Presented at ObesityWeek; November 1–5, 2021; Virtual.
Guest G, Bunce A, Johnson L. How many interviews are enough? Field Methods. 2016;18(1):59–82.
Turner-Bowker DM, Lamoureux RE, Stokes J, et al. Informing a priori sample size estimation in qualitative concept elicitation interview studies for clinical outcome assessment instrument development. Value Health. 2018;21(7):839–42.
Wabitsch M, Fehnel S, Mallya UG, et al. Understanding the patient experience of hunger and improved quality of life with setmelanotide treatment in POMC and LEPR deficiencies. Adv Ther. 2022;39(4):1772–83.
Acknowledgements
Funding
Funding for this study was provided by Rhythm Pharmaceuticals, Inc. The journal's Rapid Service and Open Access Fees were also funded by Rhythm Pharmaceuticals, Inc.
Medical Writing and Editorial Assistance
Writing and editorial assistance were provided under the direction of the authors by Rachel Haake, PhD, and David Boffa, ELS, of MedThink SciCom, and funded by Rhythm Pharmaceuticals, Inc.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Usha G. Mallya, Sheri Fehnel, Robert S. Mittleman, Matthew Webster, and Andrea M. Haqq substantially contributed to the study concept or design. Claire Ervin, Lindsey Norcross, Usha G. Mallya, Sheri Fehnel, Andrea M. Haqq, and Robert M. Haws acquired, analyzed, and/or interpreted the data. All authors critically revised this manuscript for important intellectual content, approved the final version, and agreed to be accountable for all aspects of the work.
Disclosures
Claire Ervin, Lindsey Norcross, and Sheri Fehnel’s institution received funding from Rhythm Pharmaceuticals, Inc. for this research. Usha G. Mallya, Robert S. Mittleman, and Matthew Webster were or are full-time employees at the time of this research. As full-time employees, they received stock or stock options. Andrea M. Haqq served on an advisory board for Rhythm Pharmaceuticals, Inc. and Pfizer, is a speaker for Pfizer Canada, and is a principal investigator for clinical trials with Rhythm Pharmaceuticals, Inc. and Levo Therapeutics. Robert M. Haws was contracted by Rhythm Pharmaceuticals, Inc. to conduct study trials; received personal and institutional payments from Rhythm Pharmaceuticals, Inc. and Axovia Therapeutics, LLC for consulting, research, lectures, travel, and lodging; and participated on a data safety monitoring board for Rhythm Pharmaceuticals, Inc.
Compliance With Ethics Guidelines
Informed consent was obtained for participation in the clinical trials of setmelanotide (NCT03013543 and NCT03746522); the trials were conducted in accordance with International Council on Harmonisation for Good Clinical Practice and the Declaration of Helsinki with institutional review board or independent ethics committee approval obtained at all trial sites. Study site staff obtained written informed consent for interview participation and for participant contact information to be provided to RTI Health Solutions for scheduling and conducting interviews. Verbal consent was also obtained prior to recording interviews. All patients provided informed consent for data to be reported in aggregate and anonymously.
Data Availability
The data sets generated and/or analyzed during the current study are not publicly available. Public data sharing was not included in the study consent as the rarity of this condition prohibits (or greatly reduces) the ability to ensure participant anonymity.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Ervin, C., Norcross, L., Mallya, U.G. et al. Interview-Based Patient- and Caregiver-Reported Experiences of Hunger and Improved Quality of Life with Setmelanotide Treatment in Bardet-Biedl Syndrome. Adv Ther 40, 2394–2411 (2023). https://doi.org/10.1007/s12325-023-02443-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02443-y