Abstract
Introduction
Spinal muscular atrophy (SMA) is a rare neuromuscular disease characterized by progressive muscular atrophy and weakness. Nusinersen was the first treatment approved for SMA. Per the US label, the nusinersen administration schedule consists of three loading doses at 14-day intervals, a fourth loading dose 30 days later, and maintenance doses every 4 months thereafter. Using two large US databases, we evaluated real-world adherence to nusinersen with its unique dosing schedule among generalizable populations of patients with SMA.
Methods
Patients with SMA treated with nusinersen, likely to have complete information on date of treatment initiation, were identified in the Optum® de-identified electronic health records (EHR) database (7/2017–9/2019), and in the Merative™ MarketScan® Research Databases from commercial (1/2017–6/2020) and Medicaid claims (1/2017–12/2019). Baseline demographics, number of nusinersen administrations on time, and distribution of inter-dose intervals were summarized.
Results
Totals of 67 and 291 patients were identified in the EHR and claims databases, respectively. Most nusinersen doses were received on time (93.9% EHR, 80.5% claims). Adherence was higher during the maintenance phase (90.6%) than the loading phase (71.1%) in the claims analysis, in contrast with the EHR analysis (95.5% and 92.6%, respectively), suggesting that not all loading doses of nusinersen may be accurately captured in claims. Inter-dose intervals captured in both databases aligned with the expected dosing schedule.
Conclusion
Most nusinersen doses were received on time, consistent with the recommended schedule. Our findings also highlight the importance of careful methodological approaches when using real-world administrative databases for evaluation of nusinersen treatment patterns.
Plain Language Summary
Adherence to medicines in the real world is important for patients with chronic disease to see long-term benefits of treatment. This study shows the importance and challenges of measuring adherence using real-world administrative data sources. This is especially important for drugs given through lumbar puncture with unique dosing schedules, such as nusinersen for the treatment of spinal muscular atrophy. In this study, most patients with spinal muscular atrophy received their nusinersen doses on time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out the study? |
Real-world adherence to nusinersen is essential for long-term treatment effectiveness of spinal muscular atrophy (SMA), a rare neuromuscular disease. |
Using two large US databases, the objective of the study was to evaluate real-world adherence to nusinersen among generalizable populations of patients with SMA. |
What was learned from the study? |
Most doses of nusinersen were received on time and the inter-dose intervals captured in both databases aligned with the expected unique dosing schedule of nusinersen. |
Our findings also highlight the importance of careful methodological approaches when using real-world administrative databases for evaluation of nusinersen treatment patterns. |
Introduction
Spinal muscular atrophy (SMA) is a rare, autosomal recessive neuromuscular disease characterized by degeneration of motor neurons in the spinal cord caused by mutations, rearrangements, or deletions of the survival motor neuron 1 gene [1]. Patients present with progressive muscular atrophy and weakness, with most subtypes experiencing significant motor impairment and related comorbidities, including difficulties with feeding and breathing, and increased mortality [1, 2].
Nusinersen (SPINRAZA®), an intrathecally administered antisense oligonucleotide, was the first treatment to be approved for SMA [3, 4]. It has been available for use across pediatric and adult patient populations in the United States since December 2016 at a recommended dose of 12 mg [5]. According to the US label, the approved administration schedule of nusinersen is unique and starts with three loading doses at 14-day intervals followed by a fourth loading dose 30 days after the third, and maintenance doses every 4 months thereafter [5]. The favorable benefit/risk profile of nusinersen is well established in clinical trials, with clinically meaningful efficacy on motor function and survival across a broad spectrum of patients with SMA [6,7,8,9,10,11,12]. Furthermore, its effectiveness is supported in evidence from recent real-world studies in pediatric, adolescent, and adult patients [13,14,15,16,17,18,19].
Adherence to medications for chronic diseases is an important factor that contributes to long-term treatment effectiveness in real-world practice [20]. In a retrospective medical chart review study of nusinersen-treated adult patients conducted in nine US Muscular Dystrophy Association care centers, most nusinersen doses were received according to the dosing schedule [21]. In contrast, retrospective observational studies based on US commercial claims suggested low adherence to nusinersen [22, 23]. US commercial claims, however, often provide an incomplete picture of all medications received by a patient, which may lead to inaccuracies in calculating patient adherence or treatment-related outcomes in the absence of appropriate study methods [24, 25].
The objective of the present study was to evaluate real-world adherence to nusinersen with its unique dosing schedule across all ages among generalizable patient populations, utilizing suitable methodological approaches and two large US databases, one based on US electronic health records (EHR) and the second based on commercial insurance and Medicaid claims.
Methods
Data Sources and Cohort Selection
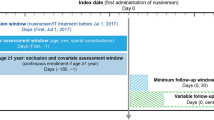
Patients with SMA treated with nusinersen were identified from each database during a similar period: for the Optum® de-identified EHR database (hereafter identified as EHR database), the period covered July 1, 2017 to September 30, 2019; for the Merative™ MarketScan® Research Databases, formerly owned by IBM® (hereafter identified as claims databases), the period spanned January 1, 2017 to June 30, 2020 for commercial insurance claims and from January 1, 2017 to December 31, 2019 for Medicaid claims. Patients with one or more codes for nusinersen treatment during the study period were identified using Healthcare Common Procedure Coding System (HCPCS) codes (C9489 and J2326) and National Drug Codes (NDC; 64406-0058-01, 64406-058-01) in both databases. Patients were additionally required to have one or more International Classification of Diseases, 10th Revision (ICD-10) diagnosis codes for SMA (G12.0, G12.1, G12.8, G12.9) during the same period.
To understand adherence to nusinersen, which has a unique dosing schedule (i.e., loading dose intervals of 14 days, 14 days, 30 days, and maintenance doses every 4 months thereafter), patients who were likely to have complete information on the date of nusinersen initiation were identified from the databases [i.e., incident users [26] (patients newly treated with nusinersen)]. Some of the initial doses that were provided through Early Access Programs or recorded using nonspecific HCPCS codes (HCPCS C9399 OR J3490 OR J3590) prior to July 1, 2017 may not be completely captured in EHR or the claims databases. In addition, doses provided through different healthcare systems or insurance plans that are not part of the EHR or claims databases are also incompletely captured (e.g., patients with changes in providers/insurance plans or those with more than one insurance coverage, which is common in patients with SMA treated with nusinersen) [24, 27]. To exclude patients with incomplete dosing history from the study cohort, patient who received any of the first four recorded doses in the databases with an inter-dose interval of 120 days or greater (i.e., an interval indicating a potential maintenance dose, not a potential loading dose per US label) were excluded. No exclusion was made based on the total duration of the loading dose phases, nor on the total number of doses received. Patients with four or fewer doses were retained if the inter-dose intervals for each of the first four recorded doses were within 120 days.
Patients in the EHR database were additionally required to have at least 6 months’ time in the database prior to the first recorded date of nusinersen use. This criterion was applied to the patients in the claims databases as an additional sensitivity analysis (except patients aged younger than 1 year at the first recorded dose, who likely would not have met this requirement), as a 6-month washout period alone is not sufficient to exclude prevalent users in the US commercial claims databases [28]. Patients were followed from the date of the first recorded nusinersen dose (baseline) until the end of the active patient time in the databases (EHR), continuous enrollment (claims), or the end of the study period (all databases), whichever was sooner. Ethics committee approval was not required for this study because the data utilized from both databases were de-identified.
Study Variables and Analyses
Baseline patient demographics and follow-up time were summarized. The dates of nusinersen administration were identified using the specific HCPCS and NDC codes and their associated procedure/administration dates (EHR database) and service dates (claims databases). Additional records of nusinersen administration that met the following criteria were included in the claims analysis: documentation of unspecific treatment (HCPCS codes C9399 OR J3490 OR J3590) AND an SMA ICD-10 diagnosis code (G12.0, G12.1, G12.8, G12.9) AND $100,000 or greater net pay cost from January 1, 2017 to December 31, 2017. This was to capture the records of nusinersen administration as completely as possible during the period when the specific HCPCS J code (J2326) for nusinersen was unavailable, and during the period when only nusinersen was approved as a disease-modifying therapy for SMA.
To calculate real-world adherence and treatment patterns of nusinersen [29], the percentage of doses on time was determined using grace periods of 7 days for loading doses and 30 days for maintenance doses, based on US expert clinical opinion and pharmacokinetic modeling [30]. Distributions of inter-dose intervals from the previous dose were calculated for the second dose and all subsequent doses for patients who received at least two doses.
Results
Baseline Characteristics and Follow-up
During the study, 67 and 291 patients who met the inclusion criteria were identified from the EHR and claims databases, respectively (Fig. 1). Of the patients included, 58.2% in the EHR database and 47.1% in the claims databases were female. The median (range) age of patients at first recorded dose was 19 (1–72) years and 15 (0–63) years in the EHR and claims databases, respectively (Table 1). Adults comprised more than half (53.7%) of the EHR database and nearly half (45.4%) of the claims databases. In the claims cohort, 58.4% of patients were identified from the commercial insurance database and 41.6% were from the Medicaid database (Table 1). Median (range) follow-up time from the first recorded dose of nusinersen was 14.0 (1–27) months in the EHR database cohort and 11.5 (0–41) months in the claims databases cohort.
Cohort selection from Optum® EHR (EHR database) and Merative™ MarketScan® Research databases (claims databases). EHR electronic health record, HCPCS Healthcare Common Procedure Coding System, ICD-10 International Classification of Diseases, 10th Revision, SMA spinal muscular atrophy. aTreated between July 1, 2017 and September 30, 2019 (EHR database), or between January 1, 2017 and June 30, 2020 (commercial claims database) or December 31, 2019 (Medicaid claims database); nusinersen treatment codes include HCPCS codes J2326 and C9489 and NDC codes 64406-0058-01 and 64406-058-01; SMA ICD-10 diagnosis codes include G12.0, G12.1, G12.8, and G12.9. bPatients who received any of the first four recorded nusinersen doses in ≥ 120-day intervals (which would indicate maintenance doses, not loading doses, per US label) were excluded. Patients with four or fewer doses were retained as long as the inter-dose intervals for each of the first four recorded doses were within 120 days, respectively. cPatients younger than 1 year of age were included regardless of prior insurance enrollment
Patterns of Real-World Use of Nusinersen
Patients in the EHR database had a mean (SD) of 5.4 (2.7) nusinersen doses. Patients in the claims databases had a mean (SD) of 5.0 (3.5) nusinersen doses. Of the overall study cohort, 60 (89.6%) patients in EHR database and 230 (79.0%) patients in claims databases had received two or more doses during the study period and contributed to the dose-level adherence analysis (Table S1 in the Supplementary Material). In both data sources, most nusinersen doses were received on time: 93.9% and 80.5% in the EHR and claims databases, respectively. The percentage of doses received on time was similar between the loading (92.6%) and maintenance (95.5%) doses in the analysis of the EHR database. In contrast, adherence was substantially higher during the maintenance phase (90.6%) than during the loading phase (71.1%) in the analysis of the claims databases (Table 2). Similar results were observed when patients without 6 months’ prior enrollment were additionally excluded as the sensitivity analysis for the claims databases cohort (Table S2).
The calculated inter-dose intervals for both databases aligned with the expected dosing schedule. In the EHR database, the median [quartile (Q)1, Q3] days from the previous dose were 14 (14, 14) for the second and third doses, 35 (30, 35) for the fourth dose, and 126 (122, 127) for the combined maintenance doses (Fig. 2). Similarly in the claims databases, the median (Q1, Q3) days from the previous dose were 14 (14, 28) for the second and third loading doses, 30 (28, 35) for the fourth loading dose, and 124 (119, 131) for the combined maintenance doses (Fig. 3).
Distribution of nusinersen inter-dose intervals for patients identified in the Optum® EHR Database (EHR database). Duplicated observations from prescription and procedure files were removed (e.g., duplicated dates of ≤ 7 days for loading doses and ≤ 14 days for maintenance doses). Data points exceeding a distance of 1.5 times the interquartile range below the first quartile (Q1) or above the third quartile (Q3) were considered an outlier in the box and whisker plot. Larger circle symbols indicate mean values. Data for doses with sample sizes < 10 are not shown in the box and whisker plots due to small sample size. Of the 67 patients, 60 had two or more doses in the EHR database and contributed to the dose-level adherence analysis. EHR electronic health record
Distribution of nusinersen inter-dose intervals for patients identified in the Merative™ MarketScan® Research Databases (claims databases). Duplicated observations from inpatient admissions, inpatient services, and outpatient services files were removed (e.g., duplicated dates of ≤ 7 days for loading doses and ≤ 31 days for maintenance doses). Data points exceeding a distance of 1.5 times the interquartile range below the first quartile (Q1) or above the third quartile (Q3) were considered an outlier in the box and whisker plot. Crossmark symbols indicate mean values. Data for doses with sample sizes < 10 are not shown in the box and whisker plots due to small sample size. Of the 291 patients, 230 had two or more doses in the claims databases and contributed to the dose-level adherence analysis
Discussion
This study examined real-world adherence and treatment patterns of nusinersen using two large US data sources. Most nusinersen doses were received on time according to the recommended dosing schedule: 93.9% of doses recorded in the EHR database and 80.5% doses recorded in the claims databases. Our findings based on a broad patient population with SMA are aligned with a recent medical chart review study of 86 adults treated with nusinersen: 92% (454/493) of doses were received on time using similar grace periods of ± 7 days for loading doses and ± 28 days for maintenance doses [21].
The observed levels of adherence were higher for maintenance doses than for loading doses in our claims databases analyses. In contrast, similar patterns of adherence across loading and maintenance doses were observed in the EHR database analysis and in the previous medical chart review study [21], where records of nusinersen administration are more likely to be complete. The different findings in the claims analyses suggest that, despite the methodology employed in this study, not all loading doses of nusinersen may be accurately captured in the claims databases. Identifying incident users in administrative claims databases has challenges [28]. As patients may enter the database at any time during the course of their treatment, the first four doses recorded in these databases may not always be the first four loading doses. Prevalent users (or existing users of nusinersen) who are already on maintenance doses may be misclassified as incident users (or new users of nusinersen) on loading doses, resulting in lower reported adherence during the loading dose phase. US commercial claims have also been reported to often fail in capturing all medications received by patients [24], especially for patients with multiple-insurance coverage. Indeed, around 40% of patients with SMA in the United States reported having multiple-insurance coverage [27], as they may be additionally eligible for Medicare, Medicaid, or Children’s Health Insurance Program due to disability or age. Additional reasons why doses of nusinersen were likely incompletely captured in the claims databases include the following: (1) patients were part of the Early Access Program, and (2) providers used nonspecific HCPCS codes prior to July 1, 2017 (due to specific codes not being in existence at that time).
In this study, 60 (89.6%) of the 67 patients in the EHR database and 230 (79.0%) of the 291 patients in the claims databases had received two or more doses and contributed to the dose-level adherence analysis. The percentages of patients with only one dose in the databases were relatively high (10.4% and 21.0%, respectively), especially for the claims databases. Although these patients do not contribute to the dose-level adherence analysis, they were retained in the study to provide a comprehensive overview of the treatment patterns observed in the real-world administrative databases, and to avoid applying cohort selection criteria based on a post-index event [31]. In clinical practice, it is unlikely that patients only receive one dose of nusinersen; prior multicenter observational studies of 42 and 86 adult patients on nusinersen reported that all patients completed at least the four loading doses in the United States during a mean and median follow-up of 1.0 and 1.5 years, respectively [15, 21]. These findings further suggest that the full dosing history of patients on nusinersen may be incompletely captured in real-world administrative databases. Because of the relatively high proportion of patients with only one dose captured in the databases [15, 21], discontinuation or persistence was not evaluated as an outcome in this study to avoid potential misclassification of outcomes due to incomplete dosing history [29].
Our findings highlight the challenges of obtaining valid findings in real-world adherence or treatment patterns research when the full dosing history is not completely captured in administrative databases. The magnitude of missing records in claims, which has been reported to vary from 10 to 36% [24], can introduce substantial misclassification bias of real-world adherence and treatment patterns without appropriate analytic measures. Since nusinersen has a unique dosing schedule, if some of the initial dosing records are missing, all the subsequent adherence measurements will be inaccurate. Limited methods exist to account for such missing records in claims analyses, especially when records are missing due to patients having multiple-insurance coverage. Nevertheless, examining the distribution of observed dosing intervals, as in this study, can help evaluate the accuracy and plausibility of the nusinersen administration records in real-world administrative databases. The implication of potential exposure misclassification on study findings should also be carefully evaluated.
Recent retrospective observational studies using US claims databases [22, 23] (e.g., Symphony Health’s Integrated Dataverse, IQVIA PharMetrics Plus Adjudicated Claims) have suggested that real-world adherence to nusinersen is lower than that observed in the present study or in the medical chart review study [21]. However, the inclusion/exclusion criteria used in these claims-based studies were likely insufficient to identify the patients with complete nusinersen dosing history. The study by Chen et al. [22] only used the 6-month pre-index eligibility criterion to identify incident users of nusinersen, which may insufficiently identify incident users. In another methodological study by authors at the University of North Carolina, it was found that half of the individuals remained prevalent users after applying a 6-month pre-index eligibility criterion using US commercial claims data [28]. In addition, this criterion cannot account for patients with incomplete dosing history due to having multiple-insurance coverage [24]. Another study by Gauthier-Loiselle et al. [23] included patients if they received at least four doses (i.e., potential loading doses) and had < 120 days between their first and second dose. However, the latter criterion would still retain patients who are incompletely captured from their second or third loading doses.
In the event that patients do miss a dose of nusinersen, population pharmacokinetic modeling has shown that nusinersen levels can be rapidly restored following a dose delay of up to 4 months in the maintenance phase of treatment. This is done by administering the delayed dose as soon as possible; then, the subsequent dose is administered according to the original scheduled date, as long as these two doses are administered at least 14 days apart, followed by a dose every 4 months thereafter [30, 32]. For a second or third loading dose delayed by 30–90 days, it is recommended to resume the loading dose as soon as feasible and shift all remaining doses by the same number of days delayed [30].
Limitations
There are several limitations to this study. First, despite the rigorous study inclusion criteria, the different patterns of loading dose adherence in the claims databases suggested that some patients may still likely have inaccurate information on the date of nusinersen initiation. The results of the sensitivity analysis were similar to those of the main analysis, suggesting that the addition of a 6-month prior enrollment period is not sufficient to accurately capture the date of nusinersen initiation in administrative claims databases. Second, reasons for nonadherence, such as clinical, logistical, or administrative factors, are unknown in EHR or claims databases. Third, our analyses were largely conducted in the pre-COVID era and did not assess any impact of the COVID-19 pandemic to the adherence to nusinersen treatment. Fourth, it is possible that there may have been an overlap of patients in both data sources, which could not be examined in the current analysis. Fifth, our study focused on understanding the level of adherence while remaining on treatment; discontinuation was not examined in the study due to limitations of real-world administrative databases. Further studies are needed that evaluate real-world adherence with longer follow-up times and larger sample sizes.
Strengths
Our study has several strengths. The analyses from this study utilized comprehensive real-world databases in the United States that included broad and generalizable patient populations with SMA. Consistent analytic methods were applied across the EHR database and claims databases to accurately identify incident users of nusinersen for adherence analysis. Moreover, the measures of adherence (percentage of doses received on time and distribution of inter-dose intervals) were based on the recommended taxonomy for adherence [29] and are suitable for nusinersen and its unique dosing intervals.
Conclusions
In this real-world sample of patients with SMA treated with nusinersen, the vast majority of nusinersen doses were received on time and the calculated inter-dose intervals for both EHR and claims databases aligned with the expected dosing schedule. Our findings also highlight the importance of careful methodological approaches when using real-world administrative databases to obtain valid findings in research studies evaluating treatment patterns.
References
Lunn MR, Wang CH. Spinal muscular atrophy. Lancet. 2008;371(9630):2120–33.
Finkel R, Bertini E, Muntoni F, Mercuri E, ENMC SMA Workshop Study Group. 209th ENMC International Workshop: Outcome Measures and Clinical Trial Readiness in Spinal Muscular Atrophy 7-9 November 2014, Heemskerk, The Netherlands. Neuromuscul Disord. 2015;25(7):593–602.
Parente V, Corti S. Advances in spinal muscular atrophy therapeutics. Ther Adv Neurol Disord. 2018;11:1756285618754501.
Hoy SM. Nusinersen: a review in 5q spinal muscular atrophy. CNS Drugs. 2018;32(7):689–96.
Food and Drug Administration. Spinraza prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/209531s003s004lbl.pdf.
Darras BT, Farrar MA, Mercuri E, et al. An integrated safety analysis of infants and children with symptomatic spinal muscular atrophy (SMA) treated with nusinersen in seven clinical trials. CNS Drugs. 2019;33(9):919–32.
Darras BT, Chiriboga CA, Iannaccone ST, ISIS-396443-CS2/ISIS-396443-CS12 Study Groups, et al. Nusinersen in later-onset spinal muscular atrophy: long-term results from the phase 1/2 studies. Neurology. 2019;92(21):e2492–506.
Finkel RS, Mercuri E, Darras BT, ENDEAR Study Group, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723–32.
Mercuri E, Darras BT, Chiriboga CA, CHERISH Study Group, et al. Nusinersen versus sham control in later-onset spinal muscular atrophy. N Engl J Med. 2018;378(7):625–35.
De Vivo DC, Bertini E, Swoboda KJ, NUTURE Study Group, et al. Nusinersen initiated in infants during the presymptomatic stage of spinal muscular atrophy: interim efficacy and safety results from the Phase 2 NURTURE study. Neuromuscul Disord. 2019;29(11):842–56.
Finkel RS, Chiriboga CA, Vajsar J, et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388(10063):3017–26.
Acsadi G, Crawford TO, Müller-Felber W, et al. Safety and efficacy of nusinersen in spinal muscular atrophy: The EMBRACE study. Muscle Nerve. 2021;63(5):668–77.
Hagenacker T, Wurster CD, Gunther R, et al. Nusinersen in adults with 5q spinal muscular atrophy: a non-interventional, multicentre, observational cohort study. Lancet Neurol. 2020;19(4):317–25.
Veerapandiyan A, Eichinger K, Guntrum D, et al. Nusinersen for older patients with spinal muscular atrophy: a real-world clinical setting experience. Muscle Nerve. 2020;61(2):222–6.
Duong T, Wolford C, McDermott MP, et al. Nusinersen treatment in adults with spinal muscular atrophy. Neurol Clin Pract. 2021;11(3):e317–27.
Maggi L, Bello L, Bonanno S, et al. Nusinersen safety and effects on motor function in adult spinal muscular atrophy type 2 and 3. J Neurol Neurosurg Psychiatry. 2020;91(11):1166–74.
Pera MC, Coratti G, Bovis F, iSMAC group, et al. Nusinersen in pediatric and adult patients with type III spinal muscular atrophy. Ann Clin Transl Neurol. 2021;8(8):1622–34.
Pane M, Coratti G, Sansone VA, Italian EAP Working Group, et al. Type I SMA “new natural history”: long-term data in nusinersen-treated patients. Ann Clin Transl Neurol. 2021;8(3):548–57.
Pane M, Coratti G, Pera MC, Italian ISMAC group, et al. Nusinersen efficacy data for 24-month in type 2 and 3 spinal muscular atrophy. Ann Clin Transl Neurol. 2022;9(3):404–9.
Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97.
Elman L, Youn B, Proud CM, et al. Real-world adherence to nusinersen in adults with spinal muscular atrophy in the US: a multi-site chart review study. J Neuromuscul Dis. 2022;9(5):655–60.
Chen E, To T, Seetasith A, Tan R, Merida M, Iannaccone S. Nusinersen adherence among patients with spinal muscular atrophy in the real world. J Manag Care Spec Pharm. 2020;26:S39–40.
Gauthier-Loiselle M, Cloutier M, Toro W, et al. Nusinersen for spinal muscular atrophy in the United States: findings from a retrospective claims database analysis. Adv Ther. 2021;38(12):5809–28.
Cepeda MS, Fife D, Denarié M, Bradford D, Roy S, Yuan Y. Quantification of missing prescriptions in commercial claims databases: results of a cohort study. Pharmacoepidemiol Drug Saf. 2017;26(4):386–92.
Wade RL, Patel JG, Hill JW, De AP, Harrison DJ. Estimation of missed statin prescription use in an administrative claims dataset. J Manag Care Spec Pharm. 2017;23(9):936–42.
Johnson ES, Bartman BA, Briesacher BA, et al. The incident user design in comparative effectiveness research. Pharmacoepidemiol Drug Saf. 2013;22(1):1–6.
Chen E, Dixon S, Naik R, et al. Early experiences of nusinersen for the treatment of spinal muscular atrophy: results from a large survey of patients and caregivers. Muscle Nerve. 2021;63(3):311–9.
Roberts AW, Dusetzina SB, Farley JF. Revisiting the washout period in the incident user study design: why 6–12 months may not be sufficient. J Comp Eff Res. 2015;4(1):27–35.
Vrijens B, De Geest S, Hughes DA, ABC Project Team, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73(5):691–705.
MacCannell D, Berger Z, East L, et al. Population pharmacokinetics-based recommendations for a single delayed or missed dose of nusinersen. Neuromuscul Disord. 2021;31(4):310–8.
Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(4):492–9.
MacCannell D, Berger Z, Kirschner J, et al. Restoration of nusinersen levels following treatment interruption in people with spinal muscular atrophy: simulations based on a population pharmacokinetic model. CNS Drugs. 2022;36(2):181–90.
Acknowledgements
We thank Susan Hall, PhD, for her contributions in the analysis of the Optum® EHR portion of this study in the initial study design.
Funding
This study was sponsored by Biogen (Cambridge, MA, USA). The study sponsor is also responsible for the journal’s Rapid Service and Open Access fees. Authors, who are employees of Biogen, were involved in the study design, collection, analysis, and interpretation of the data.
Medical Writing, Editorial and Other Assistance
Biogen provided funding for medical writing support in the development of this report; Alison Gagnon and Vanessa Ducas from Excel Scientific Solutions provided writing assistance in the development of the first and subsequent drafts based on input from authors, and Cara Farrell from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements. The authors had full editorial control of the paper and provided their final approval of all content.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Bora Youn, Emma Viscidi, Susan Eaton, Angela D. Paradis, and Nicole B. Johnson participated in the design of the analyses; Bora Youn, Nasha Wang, Qiang Hou, and Bridget A. Neville conducted the statistical analyses. All authors participated in the analysis and interpretation of data and in drafting and critically revising the manuscript.
Disclosures
At the time of the study, Bora Youn, Nasha Wang, Qiang Hou, Emma Viscidi, Susan Eaton, Angela D. Paradis, Bridget A. Neville, Nicole B. Johnson: employees of and hold stock/stock options in Biogen. Crystal M. Proud: advisory boards and consultant for AveXis/Novartis Gene Therapies, Biogen, Genentech/Roche, Sarepta, and Scholar Rock; speaker for AveXis/Novartis Gene Therapies and Biogen; Principal Investigator of studies sponsored by Astellas, AveXis/Novartis Gene Therapies, Biogen, Catabasis, CSL Behring, FibroGen, Pfizer, PTC, Sarepta, and Scholar Rock. Qiang Hou is an employee of Vertex Pharmaceutics and Emma Viscidi is an employee of Moderna during the completion of the manuscript.
Compliance with Ethics Guidelines
Data for these analyses were made available to the authors through third-party licenses from Optum® EHR and MerativeTM. Ethics committee approval was not required for this study due to the use of secondary, de-identified data.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available due to the data use agreements with Optum® EHR and Merative™ MarketScan® Research databases.
Prior Presentation
These results have previously been presented in part at the Academy of Managed Care Pharmacy 33rd annual meeting, April 12–16, 2021, and at the International Society for Pharmacoeconomics and Outcomes Research 24th Annual European Congress, November 30 to December 3, 2021.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Youn, B., Proud, C.M., Wang, N. et al. Examining Real-World Adherence to Nusinersen for the Treatment of Spinal Muscular Atrophy Using Two Large US Data Sources. Adv Ther 40, 1129–1140 (2023). https://doi.org/10.1007/s12325-022-02414-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02414-9