Abstract
Hereditary angioedema (HAE) is an autosomal dominant disorder caused by a mutation in the C1 esterase inhibitor gene. HAE affects 1/50,000 people worldwide. Three main types of HAE exist: type I, type II, and type III. Type I is characterized by a deficiency in C1-INH. C1-INH is important in the coagulation complement, contact systems, and fibrinolysis. Most HAE cases are type I. Type I and II HAE result from a mutation in the SERPING1 gene, which encodes C1-INH. Formally known as type III HAE is typically an estrogen-dependent or hereditary angioedema with normal C1-INH activity. Current guidelines now recommend subdividing hereditary angioedema with normal C1 esterase inhibitor gene (HAE-nl-C1-INH formerly known as HAE type III) based on underlying mutations such as in kininogen-1 (HAE-KNG1), plasminogen gene (PLG-HAE), myoferlin gene mutation (MYOF-HAE), heparan sulfate-glucosamine 3-sulfotransferase 6 (HS3ST6), mutation in Hageman factor (factor XII), and in angiopoietin-1 (HAE-ANGPT-1). The clinical presentation of HAE varies between patients, but it usually presents with nonpitting angioedema and occasionally abdominal pain. Young children are typically asymptomatic. Those affected by HAE usually present with symptoms in their early 20s. Symptoms can arise as a result of stress, infection, or trauma. Laboratory testing shows abnormal levels of C1-INH and high levels of bradykinin. C4 and D-dimer levels can also be monitored if an acute HAE attack is suspected. Acute treatment of HAE can include IV infusions of C1-INH, receptor antagonists, and kallikrein inhibitors. Short- and long-term prophylaxis can also be administered to patients with HAE. First-line therapies for long-term prophylaxis also include IV infusion of C1-INH. This review aims to thoroughly understand HAE, its clinical presentation, and how to treat it.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Late or inaccurate diagnosis of hereditary angioedema might cause difficulties. HAE causes subcutaneous or submucosal nonpitting angioedema. Angioedema affects the face and limbs. HAE symptoms include stomach pain and life-threatening laryngeal edema. A patient's physical exam should rule out allergic angioedema, which is treated differently than HAE. Neither fever nor infectious lesions should be present. |
Young HAE patients are often asymptomatic, yet many are under 20. Acute episodes span 2–5 days and are self-limiting. Symptoms appear after a trauma, infection, medical procedure, or stress. C1-INH protein, C1-INH functional levels, or C4 deficits can confirm HAE. Acute therapy is effective within 60 min and provides relief within 2 h, reducing the progression of attacks. pdC1-INH (Cinryze or Berinert), rhC1INH, ecallantide, and icatibant are acute treatments. |
Short-term prophylaxis has been shown to reduce the occurrence of attacks in those at risk. Short-term prophylaxis is indicated in those undergoing invasive procedures and anticipated stressful events. For those already taking long-term prophylaxis, the dosing schedule can be arranged to take the IV or SC dose immediately before the procedure or event, or a 5-day course of Androgens before the procedure and continued for two to three days following. If using pdC1INH, the standard dose should be used 1 to 12 h before the procedure. |
Long-term prophylaxis includes a plasma kallikrein inhibitor (lanadelumab), IV pdC1-INH (Cinryze), and SC pdC1-INH (Haegarda). Anabolic androgens or antifibrinolytics are second-line therapy. PdC-1INH is recommended for pregnant or breastfeeding women. Cinryze is infused every 3–4 days (dose depends on age), although it can cause infection and vein damage. Haegarda is taken every 3–4 days but does not cause IV side effects. 2020 FDA approved lanadelumab. After 6 months, the initial 300 mg SC dose every 2 weeks can be changed to 300 mg every 4 weeks. Berotralstat, a plasma kallikrein inhibitor given once daily, was also recently approved for patients 12 and older. This drug causes QT prolongation. |
Prepubescent and pregnant patients shouldn’t utilize androgens. Effective oral Danazol has dose-related adverse effects; start with a large dose and titrate down or up as needed. Tranexamic acid, an antifibrinolytic favored for pregnant women and children, may increase thrombosis risk. |
Introduction
Hereditary angioedema (HAE) is a rare inherited disorder that is caused by a dysfunctional C1 esterase inhibitor gene (C1-INH) or a lack of C1-INH [1]. It follows an autosomal dominant inheritance and affects 1/50,000 individuals [2, 3]. Some studies have shown that it affects females more severely than males, especially type III HAE [4]. C1-INH acts on many different pathways, including fibrinolysis, coagulation, contact, and complement systems (See Fig. 1). Three types of HAE exist. Type I HAE presents with a deficiency of C1-INH, type II HAE presents with a dysfunctional C1-INH, and type III HAE is generally estrogen-dependent or hereditary angioedema with normal C1-INH activity but several mutations have been described. Type I HAE makes up 85% of cases, while type II HAE makes up 15% [5]. Type III HAE (estrogen-dependent HAE) makes up a very small percentage of the cases of HAE. All three types of HAE behave similarly and present with similar symptoms [6]. Angioedema is nonpitting edema in subcutaneous and submucosal tissues that affects the lips, face, neck, extremities, the oral cavity, and the larynx [7]. All three types of HAE can be potentially life-threatening when the larynx is involved. Symptoms typically begin in childhood or young adulthood and get worse around puberty. Typically, it presents as recurrent episodes of swelling or abdominal pain [8,9,10,11,12]. Patients may present with a serpentine non-pruritic rash [7]. Most acute cases of HAE resolve in 1–2 days.
The pathophysiology of HAE is described as bradykinin-mediated [7]. As stated previously, C1-INH is a regulator of the complement and contact systems. If C1-INH is deficient or defective, as in types I and II HAE, the contact system will be activated, resulting in continuous production of kallikrein. This will lead to the uncontrolled proteolysis of high molecular weight kininogen and bradykinin [7]. Bradykinin will increase vascular permeability, causing edema. Type III HAE leads to increased activation of factor XII by activating plasmin Table 1.
HAE can arise when a mutation in one or both alleles for the gene that encodes C1-INH (SERPING1). Deletions and missense mutations make up the majority of SERPING1 variants [13]. There are postulations that epigenetic and environmental factors can lead to the pathogenesis of HAE [14]. Type III HAE, resulting from a normal C1-INH function, is not fully understood. Type III HAE has been shown to result in elevated estrogen levels, which also increases levels of activated factor XII, and has a variety of other mutations that can be seen in this subtype [13, 15, 16]. More recent guidelines suggest classifying all HAE-nl-C1-INH based on specific mutations [17]. Use of the term HAE type III or HAE-nl-C1-INH may still be used as a broad category of these mutations but should not be used for pathological classification under current guidelines [17]. Specifically, HAE-nl-C1-INH can arise from a missense mutation, causing a gain-of-function in the F12 gene, the gene responsible for encoding coagulation factor XII [13]. This leads to increased levels of bradykinin.
By writing this review, we hope to offer a thorough clinical presentation of HAE and how practitioners can treat it. The remainder of this review will focus on the etiologies of the three types of HAE, how to diagnose and test for HAE, and a discussion on the medication to help manage HAE.
Compliance with Ethical Guidelines: This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Etiology
Angioedemas rise due to increased vascular permeability and can be grossly classified as either allergic (i.e., mast cell or IgE-mediated) or nonallergic. HAE is a subcategory of nonallergic angioedema, along with renin–angiotensin–aldosterone system blocker-induced angioedema, pseudoallergic angioedema, and acquired angioedema [18, 19]. Hereditary angioedema is commonly subclassified into three types, all of which are virtually clinically indistinguishable and largely driven by bradykinin [19].
Type 1 and 2 HAE are characterized by a mutation in the SERPING1 gene, which encodes the C1-inhibitor protein [21,22,23]. C1-inhibitor protein, as the name suggests, is a serine protease inhibitor that delicately balances proteins in important biological systems, including the coagulation cascade (factor XIa), contact activation system (also known as bradykinin formation system, factor XIIa, plasma kallikrein), fibrinolytic system (plasmin), and complement system (mannose-binding lectin-associated serine protease [MASP] 1 and 2) [15]. Though over 150 different mutations in the SERPING1 gene have been identified, the discernment between HAE types 1 and 2 can be ascertained by whether the C1-inhibitor protein is functional or not [15, 24, 25]. Patients with type 1 HAE synthesize misfolded or truncated C1-inhibitor protein and consequently possess decreased circulating levels. Though the overall level of C1-inhibitor protein may be low in the case of type 1 HAE, the protein itself is still functional. Conversely, type 2 HAE is characterized by the production of nonfunctional C1-inhibitor protein, though its secretion is unaffected and consequently the patient appears to have ‘normal’ levels. Type 1 HAE is the more common of the two (80–85% compared to 15–20%) and is associated with multiple mutations within the gene-encoding region (missense, nonsense, insertion, or deletion mutations), as opposed to type 2 HAE mutations, which are most often a missense mutation in exon 8 [23,24,25]. Regardless of the HAE subclass, the threshold for functional physiology, at least concerning the contact activation system, is thought to be 40% [26]. HAE operates in a dominant-negative fashion such that a mutation in a single gene adversely affects the wild-type gene product, explaining its presentation in heterozygous individuals [27].
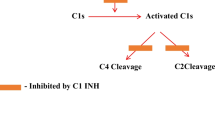
HAE is largely mediated by the pro-inflammatory mediator bradykinin and results from a deficiency in the liver-produced acute-phase reactant C1-inhibitor protein [24]. C1-inhibitor protein minimizes the activation of C1r, C1s, MASP 1, and MASP 2, thereby inhibiting the complement system. Nonfunctional or low levels of C1-inhibitor protein allow for unrestrained complement activity, facilitating vascular permeability and edema [28]. Additionally, the C1-inhibitor protein inhibits plasma kallikrein, the enzyme which mediates the conversion of high-molecular-weight kininogen (HMWK) to bradykinin. Low levels of C1-inhibitor allow plasma kallikrein levels to rise, subsequently increasing bradykinin levels. Importantly, plasma kallikrein increases the active form of factor XII, factor XIIa, which positively feedbacks to facilitate the conversion of prekallikrein to kallikrein, facilitating bradykinin production. Bradykinin is responsible for many of the symptoms in HAE patients as it functions in endothelial contraction, nociceptor activation, and bronchoconstriction, explaining the edema, pain, and dry cough typical in HAE presentation [24]. Of note, angiotensin-converting-enzyme (ACE) lowers bradykinin levels. Consequently, ACE-inhibitors, an effective anti-hypertensive therapeutic, may lead to HAE (referred to as ACE-induced HAE) [22].
The third HAE, also known as HAE with normal C1 activity, is an extremely rare subclass only described at the turn of the second millennium [29, 30]. Though the pathophysiological mechanism has not been thoroughly elucidated, type 3 HAE is strongly associated with increases in estrogen levels and is resultantly called estrogen-dependent HAE [21,22,23,24,25,26,27,28,29,30,31,32,33]. Accordingly, type 3 HAE presents most commonly in females and has been shown to initiate presentation and/or exacerbate symptoms in pregnant patients or hormonal-contraceptive users [30, 32, 34, 35]. Elevated estrogen levels correspond with increased factor XIIa levels which, using the previously mentioned positive feedback loop, increase bradykinin levels [36]. Current guidelines now recommend subdividing Hereditary Angioedema with Normal C1 esterase inhibitor gene (HAE-nl-C1-INH formerly known as HAE type III) based on underlying mutations such as in kininogen-1 (HAE-KNG1), plasminogen gene (PLG-HAE), myoferlin gene mutation (MYOF-HAE), heparan sulfate-glucosamine 3-sulfotransferase 6 (HS3ST6), a mutation in Hageman factor (factor XII), and in angiopoietin-1 (HAE-ANGPT-1) [16,17,18].
Diagnosis
Clinical Presentation
Hereditary Angioedema can clinically present with patient differences in symptomology and disease-inducing triggers. HAE typically presents with acute episodic cutaneous or submucosal angioedema and accompanied abdominal pain [37]. In HAE, angioedema can affect most areas of the body, but most frequently involves the face and extremities [37]. The cutaneous angioedema should be nonpitting and present with ill-defined margins [38]. In addition to cutaneous angioedema, the gastrointestinal tract and respiratory tracts are the most commonly involved visceral structures [39]. While the cutaneous lesions may be most apparent, the visceral angioedema can be just as bothersome to the patient and key in diagnosing the disease. While not a common initial presenting complaint, laryngeal edema is one of the deadly visceral disease complications patients with HAE may encounter [37]. There is no specified time frame for which the complication evolves, but it usually increases in severity several hours after onset [37]. This complication is especially important to recognize and address quickly as it can lead to a rapid death related to airway obstruction if not adequately treated.
In addition to clinical symptoms, the age of symptom onset is also considered when diagnosing HAE. Most HAE patients present with symptomatic cases before age 20 [37]. It is uncommon, however, for the disease to present in young children as they are often asymptomatic [39]. The use of patient age should not be the main reason for the inclusion or exclusion of the disease but can be used to help guide a differential diagnosis and applicable treatment options. Acute symptom time frame is also important to consider when making an HAE diagnosis.
In most cases, the acute angioedema episodes should last between 2 and 5 days and additionally resolve spontaneously without the need for medical intervention [39]. This typical disease progression time frame can help exclude patients with chronic, non-episodic symptoms. Knowing the progression and typical disease pattern can also help appropriately diagnose these recurring episodes when combined with other clinical symptoms.
Symptom onset is another key part of diagnosing HAE and providers should ask all patients with a suspected HAE diagnosis about preceding events prior to the increase in symptoms which may clue providers of a non-HAE cause such as drug-induced causes. In the case of HAE, some attacks may have a preceding trigger, but often many attacks have no trigger at all [39]. While triggers vary for each patient, common triggers can include trauma, medical procedures, stress, oral contraceptives (estrogen), infectious processes, and ACE inhibitors [39]. Additionally, patients with HAE have different disease response levels to the same triggers [39]. Interestingly, there seems to be a prevalence of epilepsy among patients with HAE. One article by Kuwahara S, Fukunaga A, Ohata M, et al., found that in a group of 18 patients with HAE (with more normal C1-INH HAE than C1-INH HAE), 16.7% had epilepsy [40]. A 2019 study by Mugisho OO, Robilliard LD, Nicholson LFB, Graham ES, O’Carroll SJ., demonstrated increased vascular permeability in human cerebral microvascular endothelial cells with activation of bradykinin 1 receptor; a similar mechanism has been suggested for transient cerebral edema, which is closely associated with hypertension and epileptic seizures. [40,41,42,43,44]. While a specific trigger may cause a severe disease response in one patient, that same trigger may only result in a minor disease response in another patient.
When constructing a differential diagnosis list, urticaria is an important finding to consider for the exclusion of an HAE diagnosis [38]. Patients presenting with an HAE flare should not have urticaria on physical exam and the lesions present should not indicate an infectious process (warm or painful) [38, 39]. In addition to the lesions requiring no evidence of an infectious process, the patient should also be fever free for a diagnosis of acute HAE [4]. One clinical test that can help aid in either making or excluding a diagnosis of HAE is the administration of an ACE inhibitor. If a patient is suspected to have Hereditary Angioedema, their symptoms should increase in intensity with an ACE inhibitor [38]. If the patient does not show a change in the disease process with the administration of the ACE inhibitor, the diagnosis of HAE is not as likely.
In a subset of HAE, clinical findings are the most predictive and diagnostic of HAE [38]. The subset of HAE relying the most on a patient’s clinical presentation is HAE with normal C1-INH levels. In this subset of HAE, patients will have identifiable clinical symptoms, normal lab values (including C1-INH), and either an F12 mutation of a positive family history and a known treatment failure to chronic high-dose antihistamine therapy [38]. The combination of these different factors can help establish a diagnosis of HAE with normal C1-INH levels through clinical presentation.
Laboratory Tests
HAE can be detected with laboratory testing as a deficiency of C1 esterase inhibitor, leading to an abnormally high bradykinin level [38]. Patients with HAE can also be detected through low C4, C1-INH protein, and C1-INH functional levels [38]. Low C1-INH levels occur due to a deficiency without the SERPING1 gene [38]. If an HAE patient presents with a low circulating C1-INH level, they likely have type 1 HAE, whereas a normal circulating C1-INH level indicates type 2 HAE.
Additionally, laboratory tests to monitor C4 levels should be done with suspected HAE but only in episodes of an acute attack [38]. Randomly checking an HAE patient’s C4 level alone only has a sensitivity of 80% [45]. D-dimer level monitoring may also be useful in diagnosing an acute HAE attack as the value may rise in this setting [45]. In addition to monitoring C4 and D-dimer levels, a physician may choose to check C1 and/or C3 levels [46]. If any lab values are less than or equal to 50% of the normal values, labs should be repeated in 1–3 months to ensure values are accurate and not related to a more acute illness [38]. It is recommended that to make a diagnosis of HAE, the patient should have both clinical symptoms and positive laboratory findings [38].
Genetic testing is another test that can diagnose HAE, but the limitation presented with this testing is predicting disease progression. A positive result on genetic screening for HAE cannot indicate a patient’s future symptomology or severity since the same genotypic variation can present differently in multiple patients [38].
Treatment and Management
Acute Treatment
In the setting of acute attacks, swelling typically resolves within 3 to 5 days in the absence of treatment [37]. The main complications of immediate concern are laryngeal edema, possibly fatal asphyxiation, and decapitating pain secondary to gastrointestinal attacks [47]. The main goal of acute treatment is to prevent rapid progression to these sequelae as peak symptom severity is reached within hours. It has been proven that early recognition of symptoms and treatment administration within 6 h of onset has been shown to have better outcomes than delayed treatment [48]. Therefore, treatment should be made readily available to be initiated “on-demand” by the patient. The acute treatment options include plasma-derived C1-INH (pdC1-INH), recombinant human C1-INH (rhC1-INH), ecallantide, and icatibant [49]. Proper education on self-administration techniques and two home doses should be made available to the patient to improve disease management [48]. A subcutaneous (SC) option may relieve many of the challenges associated with the self-administration of IV formulations at home. These medications become effective within 60 min and relief is usually seen within 2 h [17]. A second dose may be warranted if the attack begins to worsen. If the on-demand medications are unavailable, fresh frozen plasma (FFP) containing C1-INH can be used however it is not recommended as the level of evidence is very low. No randomized controlled trials have proven FFP’s efficacy, but retrospective studies have shown effectiveness thus this treatment should be reserved in the absence of other available treatments [50]. Longer resolution times and more adverse effects were seen. Therefore, precautions should be taken to protect the patient’s airway. Because angioedema and vascular permeability are due to bradykinin-mediated effects rather than histamine, epinephrine, antihistamines, and glucocorticoids are ineffective [17]. Likewise, ACE inhibitors should be discontinued due to their effects on increased bradykinin and angioedema.
The intravenous (IV) options include pdC1-INH and rhC1-INH [17]. IV pdC1-INH is a plasma concentrate available in vials of 500 units under the brand name Cinryze or Berinert. Haegarda is a brand name that is only approved for prophylaxis. The recommended dosing is 20 units/kg and rounded up to each vial size [17]. To avoid denaturation, caution must be utilized to avoid shaking the solution. The most common adverse effect reported is headache. Thrombotic events have been associated with pdC1INH1 [51]. RhC1-INH is pooled from the milk of transgenic rabbits. Because of this, allergy to rabbits is a contraindication to its use. It has a similar efficacy, but shorter half-life. Therefore, higher doses are needed to achieve therapeutic levels for acute treatment and is not used for prevention [52]. The recommended dose is 50 units/kg, with a maximum of 4200 units per dose [17]. Both may also be used in children aged 5 and older.
Icatibant is a bradykinin B2-receptor antagonist approved for those aged 18 and older in the United States [17]. The dosing is weight-based by SC injection. The dosing is 10 mg for 12–25 kg, 15 mg for 26–40 kg, 20 mg for 41–50 kg, 25 mg for 51–65 kg, and 30 mg for those greater than 65 kg [17]. A second dose may be recommended if symptoms do not improve within 6 h, with a maximum of three doses within 24 h. For those with angina or coronary artery disease, icatibant should be used with caution because studies have shown the effect of reduced coronary blood flow [53].
Another SC option is ecallantide, a genetically engineered recombinant plasma kallikrein inhibitor [17]. This antagonism blocks the production of bradykinin. The FDA approved this for treating attacks in those aged 12 and older in the U.S. Due to the most common and severe adverse reaction being anaphylaxis and allergic reactions, it may only be administered in a healthcare setting equipped to manage this complication [54]. The adult dose is 30 mg and is available in 1 mg vials of 10 mg [17]. Therefore, the dose should be administered as three separate injections in the abdomen, upper arm, or thigh and distinct from the site of angioedema. A second dose of 30 mg may be given as early as 1 h after the first dose [17].
Substantially increased mortality has been associated with laryngeal attacks [47]. Subsequently, emergency care should be sought for those experiencing laryngeal, throat or tongue swelling. Elective intubation should be considered to protect the airway for those experiencing respiratory distress despite the administration of treatment. If intubation has failed, emergent cricothyrotomy may be required [47]. Gastrointestinal attacks may range from mild to severe and usually resolve without treatment. On-demand treatment should be sufficient, but rehydration and symptomatic treatment with antiemetics, anti-diarrheal, and constipation medications may be warranted.
Short-term Prophylaxis
Short-term prophylaxis has been shown to reduce the occurrence of attacks in those at-risk. Trauma and stress have been shown to incite acute attacks [55]. Short-term prophylaxis is indicated in those undergoing invasive procedures such as major dental work, oral surgery, endotracheal intubation, endoscopies, and anticipated stressful events. For those already taking long-term prophylaxis, the dosing schedule can be arranged to take the IV or SC dose immediately before the procedure or event, or a 5-day course of Androgens before the procedure and continued for two to three days following [17]. If using pdC1INH, the standard dose should be used 1 to 12 h before the procedure. Due to their short half-life, ecallantide and icatibant are not used for prevention. Tranexamic acid (TXA) is a common antifibrinolytic medication used to treat excessive bleeding caused by surgeries, pregnancies/menorrhagia, clotting disorders, and trauma. TXA is used to treat HAE both acutely and prophylactically, despite its disputed use. TXA displaces plasmin to prevent clot resolution and lower bradykinin [56]. Particularly, a 2021 case report showed that tranexamic acid may be particularly useful in treating ACE inhibitor-induced angioedema and in short-term prophylaxixuse [57].
Long-Term Prophylaxis
The criteria for deciding whether long-term prophylaxis is indicated is subjectively based on the patient. The practitioner should consider the severity and frequency of attacks, impact on quality of life, access to treatment and comorbid conditions [17]. First line therapies include monoclonal plasma kallikrein inhibitor named Lanadelumab, IV pdC1-INH (Cinryze), and SC pdC1-INH (Haegarda). Alternatively, anabolic androgens or antifibrinolytics may be used as second-line agents.
Cinryze is approved for children and adults to reduce the occurrence of attacks [58]. An infusion is given every 3 to 4 days as 2500 or 1000 units based on age [17]. Careful consideration should be given as to whether long-term IV access is appropriate, ability to infuse appropriately, and risk for infection. Repeated IV administration may lead to damaged veins and loss of access. Indwelling ports may be placed but there is an increased risk for infection and placement is not currently recommended. The SC option, Haegarda, is another first-line option that does not pose these same risks. It follows the same schedule, but with doses of 60 units/kg [59]. PdC1-INH is the preferred option for acute treatment and prevention in pregnant and lactating patients [60].
A monoclonal antibody to plasma kallikrein, Lanadelumab, was recently FDA approved in 2020 for those ages 12 and older [61]. The medication’s much longer duration of action allows for long-term prophylactic therapy and once or twice monthly dosing. The initial recommended dose is 300 mg SC every two weeks [17]. This may be de-escalated to 300 mg every 4 weeks after 6 months if attacks have been well-controlled. Berotralstat is a recently approved oral plasma kallikrein inhibitor for those 12 and older [62]. Dosing is usually 150 mg once daily with food [17]. A 110 mg option is available for those taking BCRP or P-gp inhibiting medications. The main concern is its increased risk of QT prolongation [62]. Future studies are still required to prove the long-term safety and effectiveness.
The precise mechanism contributing to attenuated androgens efficacy is not entirely understood. Danazol is an oral option available for prophylaxis widely throughout the US. Although effective, there are more dose-related side effects and are considered second-line alternatives [63]. Therefore, the lowest effective dose may be used. Dosing may range from 50 to 200 mg daily or every other day. When initiating, there are two strategies recommended [17]. Beginning with a high dose, 400 or 600 mg daily, and gradually tapering by tolerability may be preferred for those requiring quick attack control of attacks. Generally, it is recommended to titrate down by 100 mg per day each month. If another attack breaks through, the last effective dose may be resumed until attack resolution. Alternatively, one can begin with a low dose, such as 100 mg daily, and titrate up as necessary. This regimen may be best utilized in those not needing immediate resolution of attacks and limits the exposure to dose-related side effects. Androgens should be avoided in prepubertal adolescents and pregnant patients.
Another second-line off-label option is an antifibrinolytic tranexamic acid [64]. It is typically preferred over androgens in pregnant patients and children where other first-line options are unavailable. Dosing typically begins at 500 mg orally two to three times daily and is titrated up to a maximum tolerated dose of 3 g [17]. The risk for thrombosis should be monitored while using this medication.
Conclusions
It is important to recognize and treat HAE promptly related to complications of a late or improper diagnosis. HAE commonly presents with acute episodes of subcutaneous or submucosal nonpitting angioedema. Angioedema affects areas near the face and extremities. Associated symptoms for HAE may include abdominal discomfort and laryngeal edema, a life-threatening complication that should raise immediate concern. The absence of urticaria should be confirmed on a patient’s physical exam to rule out the possibility of allergic angioedema, which has a different treatment plan than HAE. Additionally, the patient should show no signs of fever or any infectious lesions. Young children with HAE tend to be asymptomatic, but many symptomatic cases occur in patients under 20. Laboratory testing for deficiencies of C1-INH protein, C1-INH functional levels, or C4 can be used to confirm an HAE diagnosis.
Acute treatment plays a significant role in preventing the progression of attacks due to the medication becoming effective within 60 min and providing relief within 2 h. Options for acute treatment include pdC1-INH (Cinryze or Berinert), rhC1-INH, ecallantide, and icatibant. Short- or long-term prophylaxis can be administered to reduce the occurrence of HAE attacks. Management via long-term prophylaxis involves first-line therapies such as IV pdC1-INH (Cinryze), SC pdC1-INH (Haegarda), and a monoclonal plasma kallikrein inhibitor (lanadelumab).
Second-line therapies include anabolic androgens or antifibrinolytics. Haegarda is also given every 3–4 days but does not involve the side effects of repeated IV administration. Lanadelumab was recently FDA-approved in 2020. This medication is used for patients 12 years and older with an initial 300 mg dose SC every 2 weeks, which can be changed after 6 months to 300 mg every 4 weeks. Berotralstat, also recently approved for patients 12 years and older, is an oral plasma kallikrein inhibitor given once daily as a 150 mg dose but as a 110 mg dose for those taking BCRP. Danazol is an effective oral option with dose-related side effects; therefore, it is recommended to start with a high dosage and titrate down or start with a low dose and titrate up as needed. Antifibrinolytic tranexamic acid is another second-line option preferred for pregnant patients and children, but it may increase the risk of thrombosis.
References
Wilkerson RG, Moellman JJ. Hereditary Angioedema. Emerg Med Clin. 2022;40(1):99–118.
Andrejević S, Korošec P, Šilar M, Košnik M, Mijanović R, Bonači-Nikolić B, et al. Hereditary angioedema due to C1 inhibitor deficiency in Serbia: two novel mutations and evidence of genotype–phenotype association. PLoS ONE. 2015;10(11): e0142174.
Nasr IH, Manson AL, Al Wahshi HA, Longhurst HJ. Optimizing hereditary angioedema management through tailored treatment approaches. Expert Rev Clin Immunol. 2016;12(1):19–31.
Nordenfelt P, Nilsson M, Björkander JF, Mallbris L, Lindfors A, Wahlgren CF. Hereditary angioedema in Swedish adults: report from the national cohort. Acta Derm Venereol. 2016;96(4):540–5.
Patel G, Pongracic JA. Hereditary and acquired angioedema. Allergy Asthma Proc. 2019;40(6):441–5. https://doi.org/10.2500/aap.2019.40.4267. PMID: 31690390.
Zhang Y, Tortorici MA, Pawaskar D, Pragst I, Machnig T, Hutmacher M, et al. Exposure-response model of subcutaneous C1-inhibitor concentrate to estimate the risk of attacks in patients with hereditary angioedema. CPT Pharmacomet Syst Pharmacol. 2018;7(3):158–65.
Memon RJ, Tiwari V. Angioedema. [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538489/.
Moellman JJ, Bernstein JA, Lindsell C, Banerji A, Busse PJ, Camargo CA Jr, et al. A consensus parameter for the evaluation and management of angioedema in the emergency department. Acad Emerg Med. 2014;21(4):469–84.
Zuraw BL. Hereditary angioedema. N Engl J Med. 2008;359(10):1027–36.
Gábos G, Dobru D, Mihály E, Bara N, Dumitrache C, Popa R, et al. Recurrent ascites: a need to evaluate for hereditary angio-oedema. The Lancet. 2017;390(10107):2119–20.
Keeney S, Halalau A. Anchoring bias in a case of recurrent abdominal pain. BMJ Case Rep. 2017;2017:2017221027. https://doi.org/10.1136/bcr-2017-221027. PMID: 28978589; PMCID: PMC5652391.
Elenburg SN, Assa’ad AH, Bernstein JA, Nanda M. Clinical features of pediatric hereditary angioedema. J Allergy Clin Immunol. 2014;133(2):AB32.
Santacroce R, D’Andrea G, Maffione AB, Margaglione M, d’Apolito M. The genetics of hereditary angioedema: a review. J Clin Med. 2021;10(9):2023.
Caccia S, Suffritti C, Carzaniga T, Berardelli R, Berra S, Martorana V, et al. Intermittent C1-inhibitor deficiency associated with recessive inheritance: functional and structural insight. Sci Rep. 2018;8(1):1–14.
Busse PJ, Christiansen SC. Hereditary angioedema. N Engl J Med. 2020;382(12):1136–48.
Farkas H, Dóczy A, Szabó E, Varga L, Csuka D. Screening for plasminogen mutations in hereditary angioedema patients. Genes (Basel). 2021;12(3):402. https://doi.org/10.3390/genes12030402.
Busse PJ, Christiansen SC, Riedl MA, Banerji A, Bernstein JA, Castaldo AJ, et al. US HAEA medical advisory board 2020 guidelines for the management of hereditary angioedema. J Allergy Clin Immunol Pract. 2021;9(1):132–50.
Bas M, Adams V, Suvorava T, Niehues T, Hoffmann T, Kojda G. Nonallergic angioedema: role of bradykinin. Allergy. 2007;62(8):842–56.
Bork K, Gül D, Hardt J, Dewald G. Hereditary angioedema with normal C1 inhibitor: clinical symptoms and course. Am J Med. 2007;120(11):987–92.
Misra L, Khurmi N, Trentman TL. Angioedema: Classification, management and emerging therapies for the perioperative physician. Indian J Anaesth. 2016;60(8):534–41. https://doi.org/10.4103/0019-5049.187776.
Davis AE III. C1 inhibitor and hereditary angioneurotic edema. Annu Rev Immunol. 1988;6(1):595–628.
Bissler JJ, Aulak KS, Donaldson VH, Rosen FS, Cicardi M, Harrison RA, et al. Molecular defects in hereditary angioneurotic edema. Proc Assoc Am Physicians. 1997;109(2):164–73.
Verpy E, Biasotto M, Brai M, Misiano G, Meo T, Tosi M. Exhaustive mutation scanning by fluorescence-assisted mismatch analysis discloses new genotype–phenotype correlations in angiodema. Am J Hum Genet. 1996;59(2):308.
Zuraw BL, Christiansen SC. HAE pathophysiology and underlying mechanisms. Clin Rev Allergy Immunol. 2016;51(2):216–29.
Zahedi R, Aulak KS, Eldering E, Davis AE. Characterization of C1 inhibitor-Ta: a dysfunctional C1INH with deletion of lysine 251. J Biol Chem. 1996;271(39):24307–12.
Longhurst H, Cicardi M, Craig T, Bork K, Grattan C, Baker J, et al. Prevention of hereditary angioedema attacks with a subcutaneous C1 inhibitor. N Engl J Med. 2017;376(12):1131–40.
Haslund D, Ryø LB, Majidi SS, Rose I, Skipper KA, Fryland T, et al. Dominant-negative SERPING1 variants cause intracellular retention of C1 inhibitor in hereditary angioedema. J Clin Invest. 2019;129(1):388–405.
Kaplan AP, Ghebrehiwet B. The plasma bradykinin-forming pathways and its interrelationships with complement. Mol Immunol. 2010;47(13):2161–9.
Bork K, Gül D, Hardt J, Dewald G. Hereditary angioedema with normal C1 inhibitor: clinical symptoms and course. Am J Med. 2007;120(11):987–92.
Binkley KE, Davis A III. Clinical, biochemical, and genetic characterization of a novel estrogen-dependent inherited form of angioedema. J Allergy Clin Immunol. 2000;106(3):546–50.
Lunn M, Banta E. Ecallantide for the treatment of hereditary angiodema in adults. Clin Med Insights Cardiol. 2011. https://doi.org/10.4137/CMC.S4434.
Bork K, Barnstedt SE, Koch P, Traupe H. Hereditary angioedema with normal C1-inhibitor activity in women. The Lancet. 2000;356(9225):213–7.
Miranda AR, Ue APF de, Sabbag DV, Furlani W de J, Souza PK de, Rotta O. Hereditary angioedema type III (estrogen-dependent) report of three cases and literature review. An Bras Dermatol. 2013;88:578–84.
Serrano C, Guilarte M, Tella R, Dalmau G, Bartra J, Gaig P, et al. Oestrogen-dependent hereditary angio-oedema with normal C1 inhibitor: description of six new cases and review of pathogenic mechanisms and treatment. Allergy. 2008;63(6):735–41.
Frank MM. Hereditary angioedema. J Allergy Clin Immunol. 2008;121(2):S398-401.
Binkley KE. Factor XII mutations, estrogen-dependent inherited angioedema, and related conditions. Allergy Asthma Clin Immunol. 2010;6(1):1–7.
Henao MP, Kraschnewski JL, Kelbel T, Craig TJ. Diagnosis and screening of patients with hereditary angioedema in primary care. Ther Clin Risk Manag. 2016;12:701.
Nzeako UC, Frigas E, Tremaine WJ. Hereditary angioedema: a broad review for clinicians. Arch Intern Med. 2001;161(20):2417–29.
Bernstein JA. Severity of hereditary angioedema, prevalence, and diagnostic considerations. Am J Manag Care. 2018;24(14 Suppl):S292–8.
Kuwahara S, Fukunaga A, Ohata M, et al. High prevalence of epilepsy in HAE with normal C1-INH. Allergol Int. 2020;69(4):630–2. https://doi.org/10.1016/j.alit.2020.04.008.
Wang S, Zhang Q, Wang P, et al. Clinical features of hypertensive patients with COVID-19 compared with a normotensive group: Single-center experience in China. Open Med (Wars). 2021;16(1):367–74. https://doi.org/10.1515/med-2021-0225.
Gasparini S, Ferlazzo E, Sueri C, et al. Hypertension, seizures, and epilepsy: a review on pathophysiology and management. Neurol Sci. 2019;40(9):1775–83. https://doi.org/10.1007/s10072-019-03913-4.
Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036–42. https://doi.org/10.3174/ajnr.A0928.
Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008;29(6):1043–9. https://doi.org/10.3174/ajnr.A0929.
Jindal AK, Bishnoi A, Dogra S. Hereditary angioedema: Diagnostic algorithm and current treatment concepts. Indian Dermatol Online J. 2021;12(6):796.
Johnston DT. Diagnosis and management of hereditary angioedema. J Osteopath Med. 2011;111(1):28–36.
Bork K, Hardt J, Witzke G. Fatal laryngeal attacks and mortality in hereditary angioedema due to C1-INH deficiency. J Allergy Clin Immunol. 2012;130(3):692–7.
Murphy E, Donahue C, Omert L, Persons S, Tyma TJ, Chiao J, et al. Training patients for self-administration of a new subcutaneous C1-inhibitor concentrate for hereditary angioedema. Nurs Open. 2019;6(1):126–35.
Gompels M, Lock R, Abinun M, Bethune C, Davies G, Grattan C, et al. C1 inhibitor deficiency: consensus document. Clin Exp Immunol. 2005;139(3):379–94.
Wentzel N, Panieri A, Ayazi M, Ntshalintshali SD, Pourpak Z, Hawarden D, et al. Fresh frozen plasma for on-demand hereditary angioedema treatment in South Africa and Iran. World Allergy Organ J. 2019;12(9): 100049.
Gandhi PK, Gentry WM, Bottorff MB. Thrombotic events associated with C1 esterase inhibitor products in patients with hereditary angioedema: investigation from the United States Food and Drug Administration adverse event reporting system database. Pharmacother J Hum Pharmacol Drug Ther. 2012;32(10):902–9.
Li HH, Moldovan D, Bernstein JA, Reshef A, Porebski G, Stobiecki M, et al. Recombinant human-C1 inhibitor is effective and safe for repeat hereditary angioedema attacks. J Allergy Clin Immunol Pract. 2015;3(3):417–23.
Deeks ED. Icatibant Drugs. 2010;70(1):73–81.
Craig TJ, Li HH, Riedl M, Bernstein JA, Lumry WR, MacGinnitie AJ, et al. Characterization of anaphylaxis after ecallantide treatment of hereditary angioedema attacks. J Allergy Clin Immunol Pract. 2015;3(2):206–12.
Magerl M, Frank M, Lumry W, Bernstein J, Busse P, Craig T, et al. Short-term prophylactic use of C1-inhibitor concentrate in hereditary angioedema: findings from an international patient registry. Ann Allergy Asthma Immunol. 2017;118(1):110–2.
Horiuchi T, Hide M, Yamashita K, Ohsawa I. The use of tranexamic acid for on-demand and prophylactic treatment of hereditary angioedema: a systematic review. J Cutan Immunol Allergy. 2018;1(4):126–38.
Wang K, Geiger H, McMahon A. Tranexamic acid for ACE inhibitor induced angioedema. Am J Emerg Med. 2021;43:292.e5-292.e7. https://doi.org/10.1016/j.ajem.2020.10.029.
Aygören-Pürsün E, Soteres D, Moldovan D, Christensen J, Van Leerberghe A, Hao J, et al. Preventing hereditary angioedema attacks in children using Cinryze®: interim efficacy and safety phase 3 findings. Int Arch Allergy Immunol. 2017;173(2):114–9.
Lumry WR, Craig T, Zuraw B, Longhurst H, Baker J, Li HH, et al. Health-related quality of life with subcutaneous C1-inhibitor for prevention of attacks of hereditary angioedema. J Allergy Clin Immunol Pract. 2018;6(5):1733–41.
Caballero T, Farkas H, Bouillet L, Bowen T, Gompel A, Fagerberg C, et al. International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibitor deficiency. J Allergy Clin Immunol. 2012;129(2):308–20.
Banerji A, Riedl MA, Bernstein JA, Cicardi M, Longhurst HJ, Zuraw BL, et al. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018;320(20):2108–21.
Hwang JR, Hwang G, Johri A, Craig T. Oral plasma kallikrein inhibitor BCX7353 for treatment of hereditary angioedema. Immunotherapy. 2019;11(17):1439–44.
Zuraw BL, Davis DK, Castaldo AJ, Christiansen SC. Tolerability and effectiveness of 17-α-alkylated androgen therapy for hereditary angioedema: a re-examination. J Allergy Clin Immunol Pract. 2016;4(5):948–55.
Wintenberger C, Boccon-Gibod I, Launay D, Fain O, Kanny G, Jeandel P, et al. Tranexamic acid as maintenance treatment for non-histaminergic angioedema: analysis of efficacy and safety in 37 patients. Clin Exp Immunol. 2014;178(1):112–7.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Study concept and design: Evan S. Sinnathamby, Peter P. Issa, Logan Roberts, Haley Norwood, Kevin Malone, Harshitha Vemulapalli, Shahab Ahmadzadeh, Elyse M. Cornett, Sahar Shekoohi, Alan D. Kaye. Analysis and interpretation of data: Evan S. Sinnathamby, Peter P. Issa, Logan Roberts, Haley Norwood, Kevin Malone, Harshitha Vemulapalli, Shahab Ahmadzadeh, Elyse M. Cornett, Sahar Shekoohi, Alan D. Kaye. Drafting of the manuscript: Evan S. Sinnathamby, Peter P. Issa, Logan Roberts, Haley Norwood, Kevin Malone, Harshitha Vemulapalli, Shahab Ahmadzadeh, Elyse M. Cornett, Sahar Shekoohi, Alan D. Kaye critical revision of the manuscript for important intellectual content: Evan S. Sinnathamby, Peter P. Issa, Logan Roberts, Haley Norwood, Kevin Malone, Harshitha Vemulapalli, Shahab Ahmadzadeh, Elyse M. Cornett, Sahar Shekoohi, Alan D. Kaye statistical analysis: Evan S. Sinnathamby, Peter P. Issa, Logan Roberts, Haley Norwood, Kevin Malone, Harshitha Vemulapalli, Shahab Ahmadzadeh, Elyse M. Cornett, Sahar Shekoohi, Alan D. Kaye.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Disclosures
Evan S. Sinnathamby has nothing to disclose. Peter P. Issa has nothing to disclose. Logan Roberts has nothing to disclose. Haley Norwood has nothing to disclose. Kevin Malone has nothing to disclose. Harshitha Vemulapalli has nothing to disclose. Shahab Ahmadzadeh has nothing to disclose. Elyse M. Cornett has nothing to disclose. Sahar Shekoohi has nothing to disclose. Alan D. Kaye has nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Sinnathamby, E.S., Issa, P.P., Roberts, L. et al. Hereditary Angioedema: Diagnosis, Clinical Implications, and Pathophysiology. Adv Ther 40, 814–827 (2023). https://doi.org/10.1007/s12325-022-02401-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02401-0